Abstract
Background
Left ventricular non-compaction (LVNC) cardiomyopathy in adults has primarily been studied with a phenotypic expression of low ejection fraction (EF) and dilated cardiomyopathy; however, data on LVNC with preserved EF is scarce. The present study aimed to evaluate cardiac geometry and mechanics in LVNC patients with preserved EF.
Methods
A retrospective cohort study of patients diagnosed with LVNC and a preserved EF between 2008 and 2019 was performed. LVNC was defined according to the presence of established transthoracic 2D echocardiographic (TTE) criteria as follows: (1) prominent LV trabeculations with deep recesses; (2) bi-layered myocardial appearance; and, (3) systolic non-compacted:compacted ratio≥ 2. Subjects were matched 1:1 to controls without LVNC referred for routine TTE. Geometric, functional and mechanics parameters were analyzed in the two cohorts using 2D and speckle-tracking TTE.
Results
Seventeen patients with LVNC and preserved EF were identified. Compared with controls, patients with LVNC had similar LV systolic function and chamber dimensions, but a larger mass and relative wall thickness, and more abnormal LV geometry (76% vs. 18%, p = 0.002), LA remodeling, and pulmonary hypertension. Global longitudinal strain was significantly decreased (-15.4 ± 3.2 vs. -18.9 ± 2.8%, p = < 0.01) and the prevalence of rigid body rotation was significantly increased (57% vs. 14%, p = 0.05) in the LVNC population. The peak twist values were comparable in both cohorts.
Conclusions
Impaired LV geometry and longitudinal mechanics, as well as increased myocardial stiffness as expressed by rigid body rotation, characterize LVNC with preserved EF when compared with controls.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-compaction of the left ventricular myocardium (LVNC) is characterized by excessive myocardial trabeculations, and is generally believed to arise from a developmental arrest in normal embryogenesis [1,2,3,4]. It typically affects the inferior segments of the myocardium with a predilection for the LV apex. The phenotypic manifestations are various, widely ranging from asymptomatic to heart failure with associated arrhythmias and thromboembolic events [5,6,7,8]. While most patients with LVNC have significant LV systolic dysfunction, there is a proportion of LVNC patients who maintain a normal ejection fraction (EF), which has led some to question the identification of LVNC as a distinct cardiomyopathy. This argument is based on an observed association between the development of trabeculations and physiologic states, such as pregnancy or athletic training, or chronic conditions, including hypertension, chronic kidney disease or hematologic disorders [9,10,11,12].
The controversy surrounding the pathology of LVNC is reflected in its classification as a genetic cardiomyopathy by the American Heart Association [1] vs. a non-classified entity by the European Society of Cardiology [13]. Nevertheless, the emergence of myocardial strain, torsion, and twist mechanics as a discipline within cardiovascular imaging has expanded our knowledge of cardiac physiology in health and disease, and has allowed for detection of subclinical myocardial dysfunction in a variety of disease states including LVNC [14,15,16]. Prior analyses of cardiac mechanics in this population has revealed significant impairment prior to the development of clinical symptoms; however, these investigations mostly focused on patients with systolic dysfunction and reduced EF [17,18,19,20]. We hypothesize that notwithstanding a preserved EF, LVNC patients may show early signs of myocardial dysfunction through abnormal myocardial geometry and mechanics. The present study aimed to evaluate cardiac geometry and mechanics in LVNC patients with preserved EF.
Materials and methods
Patient selection
This is a retrospective cohort study approved by the Mount Sinai Medical Center (Miami Beach, FL, USA) Institutional Review Board of adult patients with LVNC cardiomyopathy followed at our institution. Patients were identified by a detailed search of our digital echocardiography database between June 2008 and December 2019. A review of the echocardiograms was performed by two level III board-certified echocardiographers for confirmation of the diagnosis. In accordance with the Jenni criteria [21], LVNC was defined by the presence of all the following 2D echocardiographic findings: (1) prominent LV trabeculations with deep recesses; (2) bi-layered myocardial appearance; and (3) end-systolic non-compacted-to-compacted myocardial ratio ≥ 2. Clinical and demographic variables were collected from each patient’s electronic medical record to include the presence of cardiovascular comorbidities (Table 1). Each patient meeting inclusion criteria for the study was matched in a 1:1 fashion with controls without LVNC referred for routine TTE based on age, gender, and LV ejection fraction.
Echocardiographic analysis
All transthoracic echocardiograms were performed using a GE Vivid cardiovascular ultrasound system (General Electric Healthcare, Waukesha, WI, USA). The assessment of LV systolic function and LVEF was performed using the biplane method of disks (modified Simpson’s rule) in accordance with the American Society of Echocardiography chamber quantification guidelines [22]. LV mass was calculated and indexed to body surface area (BSA) and geometry was classified as normal, concentric remodeling, concentric hypertrophy or eccentric hypertrophy. The location of non-compacted segments was reported according to the LV 16-segment model. The LV shape was expressed as the sphericity index (%), which was calculated as the LV volume divided by the volume of a hypothetical sphere with a diameter equal to the LV long axis, and multiplied by 100.
The right ventricle was also examined for the presence of pathologic trabeculations with systolic impairment. Right ventricular systolic function was assessed by the tricuspid lateral annular velocity (S’). The tricuspid valve annulus was measured in the apical four chamber view, and tricuspid regurgitation severity was graded by color flow Doppler. The pulmonary artery systolic pressure (PASP) was estimated from both the peak tricuspid regurgitation velocity (using the modified Bernoulli equation) and the right atrial pressure. The mean pulmonary artery pressure (MPAP) was derived from the PASP using the formula: MPAP = 0.61 × PASP + 1.95 [23]. Pulmonary hypertension was defined as MPAP > 20 mmHg [24].
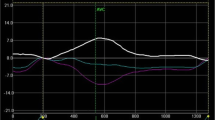
Cardiac mechanics were analyzed using the two-dimensional speckle tracking technique via the General Electric Echo PAC Q-Analysis software. As previously described, trabeculations were excluded from the endocardial tracings [25, 26]. Global longitudinal strain measurements were obtained in the apical four, three and two chamber views. Peak systolic LV twist was obtained by subtracting LV basal rotation from apical rotation, using the manually calculated aortic valve closure time as a reference point for the end of systole. [27] The presence of rigid body rotation (RBR) as a marker of myocardial stiffness, which indicates that LV apex and base rotate in the same direction (as opposed to rotating in opposite directions as in normal physiology) was also assessed (Fig. 1) [19]. The subtypes of RBR were classified as follows: type 1, holosystolic clockwise RBR; type 2, holosystolic counterclockwise RBR; type 3, initial clockwise followed by counterclockwise RBR; type 4, initial counterclockwise followed by clockwise RBR [28].
Normal left ventricular twist and rigid body rotation as assessed by speckle-tracking 2-dimensional echocardiography. Panel A, Normal LV Twist: The light green line represents apical counterclockwise rotation (positive twist), the purple line represents basal clockwise rotation (negative twist), and the white line shows the net and peak twist at aortic valve closure. Note the apical and basal tracings are in opposite directions. Panel B, Rigid Body Rotation: With identical light green and purple lines representing apical and basal rotation, it is noted that rotation occurs in the same counterclockwise direction throughout the majority of systole indicating LV myocardial stiffness and rigid body rotation. AVC aortic valve closure, LV left ventricle
Statistical analysis
Data was analyzed using IBM SPSS Statistics version 21 (IBM Corporation, Armonk, NY). Categorical variables were expressed as number (frequency), while continuous variables were expressed as mean (standard deviation) or median (interquartile range) dependent upon their Gaussian distribution. Categorical variables were compared using a chi-square or Fisher’s exact test. Continuous variables were analyzed using the student t test or the Mann–Whitney U test, as appropriate. A p value < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 17 patients with LVNC and preserved EF were identified. The mean age was 45.7 ± 13.8 years, 5 (29%) were female, and 9 (53%) were of African–American ethnicity. Compared with controls, LVNC patients did not differ significantly with respect to age, sex, incidence of systemic hypertension, coronary artery disease, atrial fibrillation or kidney function (Table 1).
Cardiac geometry and function
Although chamber dimensions (LV internal diameter and volume) were similar in LVNC patients and controls, the interventricular septal and posterior walls were found to be significantly thickened in LVNC patients (IVSd: 0.8 ± 0.2 vs. 1.1 ± 0.4 cm, p = 0.003 and PWd: 0.8 ± 0.1 vs. 1.2 ± 0.5 cm, p = 0.002). This resulted in significant differences in LV mass indices (67 ± 15.3 vs. 119.1 ± 62.5 g/m2, p = 0.002) and a greater prevalence of abnormal LV geometry amongst LVNC patients (18% vs. 76%, p = 0.002). LA volume indices were also found to be significantly increased in the LVNC population (23.4 ± 7.2 vs. 28.9 ± 8.9 mL/m2, p = 0.05) (Table 2).
The mean ratio of noncompacted:compacted myocardium thickness was 2.5 ± 0.4 in the LVNC population. The non-compacted morphology involved the apical–lateral and apical–inferior LV segments in all 17 (100%) patients, none of the basal LV segments, and the mid-ventricular LV segments variably (Table 3).
Analysis of the right ventricular parameters showed a 35% prevalence of pulmonary hypertension (6/17 patients) in the LVNC population compared with none in the control population (p = 0.02). The RV dimensions and RV function were comparable and within normal limits for both groups (Table 4).
Cardiac mechanics
Patients with LVNC were found to have decreased global longitudinal strain when compared to sex and age-matched controls ( – 15.4 ± 3.2 vs. – 18.9 ± 2.8, p = 0.002). The peak twist measurements were comparable in both cohorts; however, the prevalence of RBR was significantly higher in the LVNC population (57% vs. 14%, p = 0.05) (Table 2). Of the 8 LVNC patients with RBR, a type 3 pattern was observed in 7 individuals and a type 4 pattern in 1, respectively. In the control population there were 2 with RBR, 1 case each of type 1 and type 3 pattern.
Discussion
The present study evaluated cardiac geometry and mechanics in LVNC patients with preserved EF and compared them with a 1:1 matched cohort of patients without LVNC referred for routine TTE. The following important findings were noted: (1) both groups had similar demographics and clinical risk factors with the exception of a greater prevalence of African–American ethnicity in LVNC; (2) the non-compacted morphology consistently involved the apical segments of the LV; (3) LVNC was associated with structural changes of the LV in the form of increased wall thickness, mass, and abnormal geometry; and, 4) LVNC was associated with impaired global longitudinal strain and a higher rate of rigid body rotation, but a preserved LV twist.
The structural myocardial changes, namely, increased wall thickness and LV mass, observed in our LVNC population could be explained by a compensatory mechanism aimed at maintaining a normal LVEF and wall stress in the face of dysfunctional non-compacted segments. It is less likely that those changes are due to hypertension as the prevalence of hypertension was found to be similar between the two cohorts. Likewise, the left atrium appears to remodel in LVNC, which potentially could be explained by the same compensatory mechanism. These findings should be interpreted within the context of a greater prevalence of African American ethnicity amongst the LVNC group, and as such may be predisposed to left ventricular wall thickening and hypertrophy, as was shown in the Dallas Heart Study [29]. It is noteworthy that LVNC patients also had a higher prevalence of pulmonary hypertension; whether this is part of the genetic noncompaction cardiomyopathy syndrome or rather the result of altered left heart physiology remains to be determined.
In a prior cardiac mechanics study of ten LVNC patients with preserved EF, Bellavia et al. observed reduced longitudinal strain and LV torsion in LVNC patients compared with age and sex-matched controls [17]. In the present cohort, LVNC patients with preserved EF also experienced impaired longitudinal strain; however, the LV twist remained within normal limits. This may be explained by an earlier stage of dysfunction primarily affecting the longitudinal subendocardial myocardial fibers [27]. In normal physiology, longitudinal subendocardial fibers arranged in a right-handed helix and longitudinal subepicardial fibers arranged in a left-handed helix rotate the myocardium in opposite directions; the subendocardial fibers in a negative clockwise direction, and the subepicardial in a positive counterclockwise direction. Because of their larger radius and torque, the subepicardial fibers dominate the overall LV rotation with a net positive twist. Thus, in the presence of subendocardial fiber dysfunction, the twist increases in the direction imposed by the subepicardial fibers. This phenomenon potentially represents an initial compensatory mechanism to maintain diastolic filling and stroke volume. Eventually, the subepicardial layer also fails, leading to a decreased LV twist and overall decompensation of LV performance. It is possible that the different results pertaining to LV twist between the study by Bellavia and colleagues and the present cohort reflect LVNC patients at different transitional stages of the disease process. This discrepancy thus highlights the dynamic nature of this pathology, especially in its early stages.
An interesting finding observed was the presence of LV RBR in 57% of LVNC patients, which is defined by rotation (‘twisting’) of the subendocardial and subepicardial layers in the same direction. This results in varying degrees of uncoupled diastolic–systolic mechanics of the LV, impaired myocardial shear deformation and energy storage, attenuation of diastolic suction and recoil, and a greater prevalence of advanced heart failure symptoms and limited functional capacity [20, 27, 30]. Types 1 and 4 RBR are thought to be markers of more advanced mechanics dysfunction with clockwise RBR dominating LV twist, significantly attenuating net torsion, and resulting in a low or negative twist value. Types 2 and 3, the latter of which was most observed in the present study, are characterized by counterclockwise RBR. It is hypothesized that the type 3 RBR subtype signals subclinical twist dysfunction as the larger radius subepicardial fiber continues to dominate rotation, albeit at the expense of the subepicardial basal segments, with a fairly positive and preserved twist value [17, 19, 28].
In a prior study by Van Dalen et al. 36 LVNC patients were compared with 52 age and gender-matched patients with no cardiovascular co-morbidities [28]. The incidence of RBR was 83.3% in the LVNC group, with none observed in healthy patients. In addition, the LVNC group had a lower peak LV twist (3.9 ± 2.2 vs. 10.1 ± 2.3 degrees) and ejection fraction (42 ± 14 vs. 62 ± 7%), and a larger chamber size (end-diastolic diameter: 57 ± 8 vs. 50 ± 6 mm), consistent with LV remodeling when compared with healthy controls. In contrast to the present study, these investigators also reported a higher prevalence of RBR with a type 1 or 4 pattern (90%), which involves partial or holosystolic clockwise RBR. The importance of this finding is in the fact that normal ejection is characterized by clockwise basal and counterclockwise apical rotation, suggesting a more advanced dysfunction of the myocardial helices in the Van Dalen study. The prevalence of type 3 counterclockwise RBR in our cohort of LVNC and preserved EF, with a greater absolute LV twist and normal chamber geometry, supports the observed RBR as an earlier onset of decompensated LV mechanics that may be a sensitive preclinical marker of disease.
There are several limitations to the present study that merit discussion. First, our study is limited by its retrospective nature, which introduces an inherent selection bias. Second, the diagnostic inclusion criteria utilized have been thoroughly studied and applied in both clinical and research practice; however, due to its rarity no clear societal guidelines on LVNC exist. Misclassification of ventricular geometry and function, particularly as normal vs. pathologic in the presence of preserved EF, is a concern. This is especially true given the higher prevalence of African American subjects in the study, since ‘hypertrabeculation’ (i.e., trabeculations in otherwise healthy individuals) has been described in athletes of African and Afro-Caribbean origin.12 Third, cardiac mechanics are impacted by physiologic and hemodynamic variables, albeit to a lesser extent than LVEF. Changes in preload, afterload, inotropy, and chronotropy impact indices of LV strain and twist [27]. Finally, the small sample size limits the statistical power to detect differences in measured clinical and echocardiographic variables, as well as in the analyses that may be applied. In addition, the effect of RBR on prognosis in LVNC is unknown given the small number of published studies on the topic, and limited follow-up. Nevertheless, the present data may have clinical implications as to the diagnosis and management of LVNC patients with preserved EF, wherein earlier and closer monitoring may be warranted despite the absence of symptoms.
In conclusion, impaired LV geometry and longitudinal mechanics, as well as increased myocardial stiffness as expressed by rigid body rotation, characterize LVNC with preserved EF when compared with controls. This may signal early subclinical myocardial dysfunction warranting close monitoring of LVNC patients regardless of their LVEF phenotypic expression. Larger prospective studies and registry data are important in validating these findings and furthering our understanding of LVNC.
References
Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies. Circulation. 2006;113:1807–16.
Varnava AM. Isolated left ventricular non-compaction: A distinct cardiomyopathy? Heart. 2001;86:599–600.
Oechslin E, Jenni R. Left ventricular non-compaction revisited: A distinct phenotype with genetic heterogeneity? Eur Heart J. 2011;32:1446–56.
Wessels A, Sedmera D. Developmental anatomy of the heart: A tale of mice and man. Physiol Genom. 2004;15:165–76.
Towbin JA, Jefferies JL. Cardiomyopathies due to left ventricular noncompaction, mitochondrial and storage diseases, and inborn errors of metabolism. Circ Res. 2017;121:838–54.
Lofiego C, Biagini E, Pasquale F, et al. Wide spectrum of presentation and variable outcomes of isolated left ventricular non-compaction. Heart. 2007;93:65–71.
van Waning JI, Caliskan K, Hoedemaekers YM, et al. Genetics, clinical features, and long-term outcome of noncompaction cardiomyopathy. J Am Coll Cardiol. 2018;71:711–22.
Li S, Zhang C, Liu N, et al. Genotype-positive status is associated with poor prognoses in patients with left ventricular noncompaction cardiomyopathy. J Am Heart Assoc. 2018;7: e009910.
Gati S, Papadakis M, Papamichael ND, et al. Reversible de novo left ventricular trabeculations in pregnant women: Implications for the diagnosis of left ventricular noncompaction in low-risk populations. Circulation. 2014;130:475–83.
Arbustini E, Favalli V, Narula N, Serio A, Grasso M. Left ventricular noncompaction: a distinct genetic cardiomyopathy? J Am Coll Cardiol. 2016;68:949–66.
Anderson RH, Jensen B, Mohun TJ, et al. Key questions relating to left ventricular noncompaction cardiomyopathy: is the emperor still wearing any clothes? Can J Cardiol. 2017;33:747–57.
Gati S, Chandra N, Bennett RL, et al. Increased left ventricular trabeculation in highly trained athletes: Do we need more stringent criteria for the diagnosis of left ventricular non-compaction in athletes? Heart. 2013;99:401–8.
Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: A position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2008;29:270–6.
Biering-Sørensen T, Biering-Sørensen SR, Olsen FJ, et al. Global longitudinal strain by echocardiography predicts long-term risk of cardiovascular morbidity and mortality in a low-risk general population: The Copenhagen City Heart Study. Circ Cardiovasc Imag. 2017;10:1–11.
Opdahl A, Helle-Valle T, Skulstad H, Smiseth OA. Strain, strain rate, torsion, and twist: echocardiographic evaluation. Curr Cardiol Rep. 2015;17:1–14.
Stokke TM, Hasselberg NE, Smedsrud MK, et al. Geometry as a confounder when assessing ventricular systolic function: comparison between ejection fraction and strain. J Am Coll Cardiol. 2017;70:942–54.
Bellavia D, Michelena HI, Martinez M, et al. Speckle myocardial imaging modalities for early detection of myocardial impairment in isolated left ventricular non-compaction. Heart. 2010;96:440–7.
Arenas IA, Mihos CG, DeFaria YD, Yucel E, Elmahdy HM, Santana O. Echocardiographic and clinical markers of left ventricular ejection fraction and moderate or greater systolic dysfunction in left ventricular noncompaction cardiomyopathy. Echocardiography. 2018;35:941–8.
van Dalen BM, Caliskan K, Soliman OII, et al. Left ventricular solid body rotation in non-compaction cardiomyopathy: A potential new objective and quantitative functional diagnostic criterion? Eur J Heart Fail. 2008;10:1088–93.
Peters F, Khandheria BK, Libhaber E, et al. Left ventricular twist in left ventricular noncompaction. Eur Heart J Cardiovasc Imag. 2014;15:48–55.
Jenni R. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart. 2001;86:666–71.
Lang RM, Badano LP, Victor MA, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.
Steckelberg RC, Tseng AS, Nishimura R, Ommen S, Sorajja P. Derivation of mean pulmonary artery pressure from noninvasive parameters. J Am Soc Echocardiogr. 2013;26:464–8.
Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913.
Arunamata A, Stringer J, Balasubramanian S, et al. Cardiac segmental strain analysis in pediatric left ventricular noncompaction cardiomyopathy. J Am Soc Echocardiogr. 2019;32:763–73.
Badano LP, Muraru D. Twist mechanics of the left ventricle. Circ Cardiovasc Imag. 2019;12: e009085.
Omar AMS, Vallabhajosyula S, Sengupta PP. Left ventricular twist and torsion. Circ Cardiovasc Imag 2015;8:1–10.
van Dalen BM, Caliskan K, Soliman OI, et al. Diagnostic value of rigid body rotation in noncompaction cardiomyopathy. J Am Soc Echocardiogr. 2011;24:548–55.
Drazner MH, Dries DL, Peshock RM, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: The Dallas heart study. Hypertension. 2005;46:124–9.
Nawaytou HM, Montero AE, Yubbu P, et al. A preliminary study of left ventricular rotational mechanics in children with noncompaction cardiomyopathy: do they influence ventricular function? J Am Soc Echocardiogr. 2018;31:951–61.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guigui, S.A., Horvath, S.A., Arenas, I.A. et al. Cardiac geometry, function and mechanics in left ventricular non-compaction cardiomyopathy with preserved ejection fraction. J Echocardiogr 20, 144–150 (2022). https://doi.org/10.1007/s12574-021-00560-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12574-021-00560-7