Abstract
Background
Previous studies showed that the mitral inter-commissural (IC) distance differed by a few millimeters between the systolic and diastolic cardiac cycles. However, sizing of the mitral annuloplasty ring with a ring sizer, which should be performed in the systole, is performed in diastole during hyperkalemic cardioplegic arrest. The aim of this study was to investigate whether three-dimensional transesophageal echocardiography (3D-TEE) measurements of the mitral valve in end-systole are effective to determine the size of the annuloplasty ring.
Methods
This study retrospectively reviewed 92 patients who underwent mitral annuloplasty for degenerative. The IC distance and anterior leaflet height of the A2 segment of the mitral valve were measured by 3D-TEE at the end-systole. The annuloplasty ring size was measured by the surgeons using specific ring sizers. We compared the IC distance measured by 3D-TEE with the implanted annuloplasty size. We also investigated differences in IC distance, A2 height, and ratio of A2 height to IC distance in patients with and without recurrent mild to moderate MR for 36 months.
Results
There was a significant correlation between the IC distance by 3D-TEE and the implanted ring size (R2 = 0.7023, p < 0.001). Eight cases had mild or greater recurrent MR. There was a significant difference in the ratio of A2 height to IC distance between patients with and without recurrent MR (p = 0.006). A2 height was greater in patients with recurrent MR, but this difference was not significant (p = 0.059).
Conclusions
Our results demonstrated a larger ratio of A2 height to IC distance in patients with recurrent MR. 3D-TEE could be useful for the ring sizing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mitral valve repair is preferred to valve replacement in cases of degenerative mitral regurgitation (MR) due to the lower risk of valve-related complications and operative mortality [1,2,3]. In mitral valve repair in this context, annuloplasty is associated with better clinical outcomes [4,5,6,7,8]. The annuloplasty ring size is generally determined with a ring sizer based on the inter-commissural (IC) distance or the inter-trigonal distance, and the anterior leaflet height or area.
Sizing of the annuloplasty ring with a ring sizer, which should be performed in the end-systolic phase, is performed in diastole during hyperkalemic cardioplegic arrest. Previous studies showed that the IC distance differed by a few millimeters between the systolic and diastolic cardiac cycles [9,10,11]. As a result, the methodology to decide the optimal annuloplasty ring size remains to be established. Three-dimensional transesophageal echocardiography (3D-TEE) allows measurement of the mitral valve while the heart is beating, which is beneficial since the mitral valve size changes throughout the cardiac cycle.
Several reports showed that the annuloplasty ring size was predicted by 3D-transthoracic echocardiography (TTE) and 3D-TEE [12,13,14]. However, commercially available software cannot precisely analyze the mitral valve structure using 3D echocardiography due to a low frame rate, which results in an inability to accurately choose the frame at the end of the systolic phase. Recently, 3D-TEE was developed with a higher frame rate and better temporal, axial, and lateral resolutions, all of which enable more accurate intraoperative analysis of the mitral valve structure. We analyzed the details of the mitral valve structure using 3D-TEE intraoperatively and compared implanted ring sizes between patients with and without recurrent MR to investigate whether 3D-TEE measurements were effective to determine the size of annuloplasty ring.
Methods
This study retrospectively reviewed consecutive 139 patients who underwent mitral valve repair with annuloplasty for moderate to severe degenerative MR between January 2015 and December 2016. Our institutional ethics committee (reference no. M28-142) approved this study and waived the need for informed consent since the research was retrospective in nature.
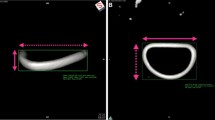
Using an iE33 or EPIQ 3D-TEE ultrasound system and an X2-7t matrix-array transducer (Philips Medical System, Andover, MA, USA), the mitral valve was visualized using a three-dimensional (3D) enface view. We directly measured the IC distance and the height of the middle anterior leaflet (A2 height) on the 3D view at mid-systole by reviewing the recorded images because these parameters were used to determine the size of the annuloplasty ring we used (Fig. 1a). The IC distance of the sizer of the Cosgrove-Edwards flexible band™ (Edwards Life Sciences, Irvine, CA, USA) and the Carpentier-Edwards Physio II ring™ (Edwards Life Sciences) was same according to the direct measurement data of both the sizers.
a Direct measurements by three-dimensional echocardiography at the end of systole under direct view of the surgeon. Inter-commissural (IC) distance and height of the A2 segment of the anterior leaflet (A2 height) were measured directly. b To avoid the parallax error, we constructed a vertical line from the top of the fulcrum of the 3D volume into the center of the mitral valve (yellow dot line) and the left ventricle in the two orthogonal planes before 3-dimensional (3D) zoom image acquisition. A perpendicular line to this vertical line (yellow solid line) was aligned between the most posterior and most anterior points of the mitral annulus to permit exact annular measurements
Exact distance measurements in the 3D enface views are not encouraged as these will often result in imprecise measurements due to the parallax error [15]. To address this problem and to avoid the parallax error, we constructed a vertical line from the top of the fulcrum of the 3D volume into the center of the mitral valve and the left ventricle in the two orthogonal planes before 3-dimensional (3D) zoom image acquisition. A perpendicular line to this vertical line was aligned between the most posterior and most anterior points of the mitral annulus to permit exact annular measurements (Fig. 1b). Echocardiographic examination was performed by an expert certified by the Japanese Society of Cardiovascular Anesthesiologists due to the retrospective nature of this study.
The mitral annuloplasty ring size was determined using a proprietary specific ring sizer in the cardioplegia-arrested heart under direct view of the surgeon. The annuloplasty ring size and type were selected by the surgeon. The annuloplasty ring types used in this observation period were the Cosgrove-Edwards flexible band™, a Tailor Flexible Annuloplasty Ring (Abbott, IL, USA) and the Carpentier-Edwards Physio II ring™.
Patients were followed up postoperatively for 36 months, and transthoracic echocardiography (TTE) was performed by an independent laboratory technologist. The grades of mitral regurgitation were reported by cardiac echo laboratory according to the guideline of American Society of Echocardiography, “Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation,” mild (grade 1), mild to moderate (grade 2), moderate to severe (grade 3), and severe (grade 4) [16]. Recurrent mitral regurgitation was defined as follows: mitral regurgitation which developed more than mild grade on and after 6–36 months postoperatively with no or trivial regurgitation just after the operation. In regard to mild grade, qualitative evaluation was performed in case of difficulty of proximal isovelocity surface area method. We compared size difference between IC distance measured by 3DTEE in the enface view of the mitral valve and the implanted annulus ring size, in patients with and without recurrent MR.
Statistical analysis
The mitral annuloplasty ring size was compared using the IC distance measured by 3D-TEE. Baseline characteristics and intraoperative TEE measurements were compared between patients with and without MR during each observational period. Patients were followed for more than 12 months postoperatively to evaluate the degree of post-repair recurrent MR using TTE.
Data are expressed as the mean (standard deviation) or median [interquartile range], and samples are expressed as the number or percentage. Continuous values were analyzed by the t-test, and categorical values were analyzed by the chi-squared test. In regard to the difference between patients with and without recurrent MR, univariate logistic regression analysis was performed. Simple regression analysis was performed to test the association between IC distance and implanted ring size to obtain R2. A p value below 0.05 was considered to be statistically significant. All statistical analyses were performed using STATA SE15 (StataCorp, College Station, TX, USA).
Results
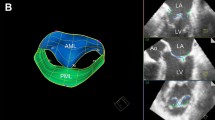
Totally 139 patients were reviewed in this study. Patients with missing TEE data (n = 24) were excluded. To focus our analysis on the mid-term effects of implanted annulus rings, patients who had mild to moderate MR just after coming off cardiopulmonary bypass were excluded (n = 5). Since intraoperative mild MR is a risk factor for recurrent postoperative MR [12], we excluded obvious intraoperative causes. Patients with a Tailor Flexible Annuloplasty Ring (Abbott, Chicago, IL, USA) were excluded (n = 18) because ring size was determined by measuring the inter-trigon distance, not the IC distance (Fig. 2).
The characteristics of the 92 patients are summarized in Table 1. Preoperative severity of MR was 3 (n = 19) or 4 (n = 73). Details of the implanted rings are shown in Table 2. Eight patients, preoperative MR grade 3 (n = 2) and grade 4 (n = 6), had postoperative MR for 36 months. There was a significant correlation between the size of the mitral annuloplasty ring and the IC ring size (R2 = 0.7023, p < 0.001). As shown in Fig. 3, IC distance values of the 28-mm annuloplasty ring ranged from 27.1 to 32.1 mm (interquartile range: IQR 27.8–29.2 mm, median = 28.3 mm), those of the 30-mm annuloplasty ring from 28.1 to 34.3 mm (IQR 30.1–31.4 mm, median = 30.5 mm), those of the 32-mm annuloplasty ring from 29.6 to 34.8 mm (IQR 31.5–32.9 mm, median = 32.3 mm), and those of the 34-mm annuloplasty ring ranged from 31.9 to 35.4 mm (IQR 33.7–34.7 mm, median = 34.4 mm).
The box-and-whisker plot shows the difference in inter-commissural (IC) distance according to implanted annulus ring. The box represents the values from the lower to upper quartile (25th–75th percentile), and the middle line represents the median. A line extends from the minimum to the maximum value
There was a significant difference between patients with and without postoperative MR in terms of the ratio of A2 height to IC distance. Table 3 shows that the ratio of A2 height to IC distance was associated with recurrent MR, which could indicate the compensation of left ventricle dilatation. Area under the curve of receiver operating curve of the logistic regression analysis was 0.805. However, there was no significant difference in the ratio of A2 height to IC distance between patients with preoperative MR grades 3 and 4, 0.653 ± 0.004 and 0.673 ± 0.004, respectively. The A2 height was greater in patients with postoperative MR than in those without, but this difference was not significant.
Discussion
There was a significant correlation between IC distance and annuloplasty ring size. The ratio of A2 height to IC distance was significantly larger in patients with recurrent MR.
Regarding the Physio II and Cosgrove-Edwards rings, it is recommended that the sizer could be attached to the anterior and posterior commissures under cardioplegic arrest. The IC distance corresponds to the mitral annulus ring size, but it changes during the cardiac cycle [9,10,11]. Although there was a significant correlation between the IC distance and the implanted annuloplasty ring, IC distance varied, as shown in Fig. 3. The difference in IC distance between systole and diastole depends on cardiac function [9], resulting in the variety of IC distances for each implanted ring size.
The ratio of A2 height to IC distance was significantly larger in patients with recurrent MR, but the reason for this is unknown. The manufacturer of the Physio II and Cosgrove-Edwards rings recommends measuring IC distance and the area of the anterior leaflet to decide the ring size. The mitral annuloplasty ring we implanted in patients with recurrent MR might have been larger than the recommended size for the following reasons: The mitral anteroposterior (AP) diameter and IC distance were significantly larger in patients with MR than in those without MR [17]. More advanced MR was associated with larger mitral AP and IC distances. In addition, the mitral leaflet area was enlarged as the mitral annulus expanded to maintain cardiac output [18]. Although there was no significant difference in the ratio of A2 height to IC distance between patients with preoperative MR grades 3 and 4, patients with a larger ratio of A2 height to IC distance might lead to the recurrent MR.
Furthermore, Chen and colleagues [9] used 3D-TEE to show that the mitral AP distance and lateromedial (LM) distance significantly changed between systole and diastole in patients with MR. In patients without MR, there was no significant difference in the AP or LM distance between systole and diastole. On the other hand, with worsening MR, the difference in the LM distance between systole and diastole increased significantly, from 1.8 to 2.5 mm. In this study, IC distance (corresponding to LM distance) was measured by 3D-TEE in systole, but sizing of the mitral annulus ring was measured during diastole during hyperkalemic cardioplegic arrest. Therefore, the difference between IC diameter measured by 3D-TEE and the size of the mitral annulus ring might increase in patients with advanced MR. Thus, larger rings can be implanted in patients with advanced MR than in patients with shorter A2 height, and these larger rings may lead to recurrent MR.
Three-dimensional TEE sizing may be a better approach than that using a ring sizer because mitral valve geometry changes dramatically throughout the cardiac cycle and regurgitation occurs during ventricular systole. The ratio of A2 height to IC distance of mitral valve would be important for annuloplasty ring size determination. For larger ratios of A2 height to IC distance, we should consider a smaller ring size.
This study had several limitations. First, this was a retrospective study. Prospective studies may be necessary to match patient backgrounds and detailed mitral lesion sites. Also, patient number was small to evaluate factors of recurrent MR. Therefore, we performed univariable logistic regression analysis to avoid overfitting of the analysis. Second, we did not evaluate the outcomes of different types of surgical procedures. Mitral valve repair can be performed in several ways, such as by quadrangular resection, triangular resection, the sliding technique for posterior leaflet lesions, and by chordal reconstruction for anterior leaflet lesions. Third, 3D-TEE measurements were performed by only one certified expert. We could not evaluate the inter- or intravariability of measurements due to the nature of the retrospective study. Furthermore, direct measurement in the 3D images still might have an error after adequately oriented considering parallax. Direct measurement on 3D image was easy to perform, but multi planer reconstruction would be better.
In conclusion, this study demonstrated a high ratio of A2 height to IC distance in patients with post-repair recurrent MR during mitral annuloplasty. 3D-TEE could be useful for sizing the mitral annulus ring.
References
Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91.
Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017;70:252–89.
Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438–88.
Carpentier A, Deloche A, Dauptain J, et al. A new reconstructive operation for correction of mitral and tricuspid insufficiency. J Thorac Cardiovasc Surg. 1971;61:1–13.
Chang BC, Youn YN, Ha JW, et al. Long-term clinical results of mitral valvuloplasty using flexible and rigid rings: a prospective and randomized study. J Thorac Cardiovasc Surg. 2007;133:995–1003.
Marwick TH, Stewart WJ, Currie PJ, et al. Mechanisms of failure of mitral valve repair: an echocardiographic study. Am Heart J. 1991;122:149–56.
Mihaileanu S. Outflow tract obstruction and failed mitral repair. Circulation. 1994;90:1107–8.
Nardi P, Pellegrino A, Olevano C, et al. Mitral valve repair for the treatment of degenerative mitral valve disease with or without prosthetic ring annuloplasty: long-term outcomes. J Cardiovasc Surg (Torino). 2013;54:305–12.
Chen TE, Ong K, Suri RM, et al. Three-dimensional echocardiographic assessment of mitral annular physiology in patients with degenerative mitral valve regurgitation undergoing surgical repair: comparison between early- and late-stage severe mitral regurgitation. J Am Soc Echocardiogr. 2018;31:1178–89.
Leng S, Zhang S, Jiang M, et al. Imaging 4D morphology and dynamics of mitral annulus in humans using cardiac cine MR feature tracking. Sci Rep. 2018;8:81.
van Wijngaarden SE, Kamperidis V, Regeer MV, et al. Three-dimensional assessment of mitral valve annulus dynamics and impact on quantification of mitral regurgitation. Eur Heart J Cardiovasc Imaging. 2018;19:176–84.
Ender J, Eibel S, Mukherjee C, et al. Prediction of the annuloplasty ring size in patients undergoing mitral valve repair using real-time three-dimensional transoesophageal echocardiography. Eur J Echocardiogr. 2011;12:445–53.
Ender J, Koncar-Zeh J, Mukherjee C, et al. Value of augmented reality-enhanced transesophageal echocardiography (TEE) for determining optimal annuloplasty ring size during mitral valve repair. Ann Thorac Surg. 2008;86:1473–8.
Labib DO, Heshmat H, Gaafar AH, et al. Real-time three-dimensional transthoracic echocardiography for predicting mitral annuloplasty ring size. J Heart Valve Dis. 2014;23:583–90.
Mahmood F, Jeganathan J, Saraf R, et al. A practical approach to an intraoperative three-dimensional transesophageal echocardiography examination. J Cardiothorac Vasc Anesth. 2016;30:470–90.
Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. 2017;30:303–71.
Grewal J, Suri R, Mankad S, et al. Mitral annular dynamics in myxomatous valve disease: new insights with real-time 3-dimensional echocardiography. Circulation. 2010;121:1423–31.
Chaput M, Handschumacher MD, Guerrero JL, et al. Mitral leaflet adaptation to ventricular remodeling: prospective changes in a model of ischemic mitral regurgitation. Circulation. 2009;120:S99–103.
Funding
Funding was provided by Department fundings (Grant No. Basic-9)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tasuku Fujii, Kenji Yoshitani, Eiki Kanemaru, Michikazu Nakai, Kunihiro Nishimura, Yoshihiko Ohnishi, and Kimitoshi Nishiwaki declare that they have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later revisions.
Informed consent
Informed consent was obtained from all patients for being included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fujii, T., Yoshitani, K., Kanemaru, E. et al. Sizing of mitral annuloplasty rings using real-time three-dimensional transesophageal echocardiography and the difference between patients with and without recurrent mitral regurgitation: retrospective cohort study. J Echocardiogr 18, 169–174 (2020). https://doi.org/10.1007/s12574-020-00465-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12574-020-00465-x