Abstract
Introduction
The diagnosis of cardiac amyloidosis (CA) is usually performed by endomyocardial biopsy; however, possible sampling errors and procedural risks such as cardiac tamponade, malignant arrhythmias and bleeding risk, limit its use. Therefore, a non-invasive diagnostic method appears to be necessary.
Materials and methods
Echocardiography plays an important role in this need. Conventional two-dimensional echocardiography appears able to detect some specific and distinguishing signs of cardiac amyloid infiltration.
Results and conclusions
Of these, thickened right and left ventricular (LV) myocardium, normal or small LV cavity size in contrast to enlarged biatrial cavities, diffuse hyper-refractile ‘granular sparkling’ appearance and ‘mismatch’ ECG/ECHO are the most specific findings. The magnitude of cyclic variation recorded with integrated backscatter reflects structural changes in the myocardium. In patients with CA, this magnitude is reduced because myocardial amyloid infiltration is characterized by a reduction of number of “contractile” fibers. Other informations concerning LV dysfunction CA-related can be obtained by Tei index. Finally, new echocardiographic imaging modalities, such as tissue Doppler, Doppler-based strain, speckle tracking imaging and three-dimensional echocardiography, can provide some findings regarding the preclinical stages of LV dysfunction when other echocardiographic measurements are showing normal; however, these are unable to provide a non-invasive diagnosis of cardiac amyloidosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amyloidosis consists of the extracellular deposition of misfolding fibrillar proteins, representing an expression of diverse pathologies [1]. It is diagnosed by the tinctorial properties of binding Congo red dye and green birefringence under polarized light [2]. Amyloid is a generic term and includes all forms of systemic or local amyloidosis. Primary amyloidosis, also known as immunoglobulin light chain amyloidosis (AL amyloidosis), is the most common type and is associated with the worst prognosis and may be associated with multiple myeloma. Secondary amyloidosis is associated with chronic inflammation or infectious disease. Other variants include senile systemic amyloidosis, isolated amyloidosis, and hemodialysis-related amyloidosis [3].
Cardiac amyloidosis
Cardiac amyloidosis (CA), also called ‘stiff heart syndrome’, is a non-frequent manifestation of amyloidosis, responsible for cardiac dysfunction evolving in restrictive cardiomyopathy and concluding in overt heart failure, resulting in sudden death [4]. Its pathogenesis is due to the infiltration of amyloid proteins into the myocardial interstitium, as fibrous nodular deposits. In the early stages, this is responsible for left ventricular (LV) diastolic dysfunction and preserved LV systolic function [5]. In the long-term, the amyloid infiltration reducing the LV dilatation induces elevation of filling pressures which leads to two-atrial dilatation. With disease progression, amyloid infiltration is responsible for the reduction in LV performance with significant fall of ejection fraction (EF). In fact, the peculiar finding of cardiac amyloid is a continuum between the diastolic dysfunction that progresses from impaired relaxation to a restrictive pattern and concomitant reduction of EF and stroke volume.
The most common form is AL amyloidosis (primary amyloidosis) resulting from light chain immunoglobulin infiltration [6]. Other forms that frequently affect the heart include transthyretin (TTR) amyloidosis (familiar, hereditary form) due to deposition of TTR involved in the transport of thyroxine [7] and senile systemic amyloidosis (secondary amyloidosis) also induced by TTR infiltration [8]. Cardiac involvement in amyloidosis can be secondary to some inflammatory disease such as rheumatoid arthritis, Crohn’s disease or tuberculosis but is extremely rare [9]. Dialysis-related amyloidosis also occasionally only involves the heart [10].
Clinical symptoms
Many of the features found in CA, such a lower extremity edema, progressive dyspnea, hepatomegaly, ascites and distension of jugular veins can be attributed to right-sided heart failure. Amyloid deposition into the atrial walls can cause atrial dysfunction and conduction abnormalities. The conduction system may also be involved. In fact, cardiac arrhythmias are a frequent cause of death in these patients. Amyloid infiltration of the pericardium and of the pleural space is often present and can be considered a result of amyloid deposition secondary to either limitation of lymphatic drainage or increased fluid secretion [11]. Weight loss is frequently present in these patients and is an effect of cardiac cachexia.
Invasive diagnosis
An endomyocardial biopsy is usually used to diagnose both systemic amyloidosis and cardiac involvement; this procedure is virually 100 % sensitive because the amyloid is widely deposited throughout the cardiac cavities. It demonstrates apple-green birefringence when stained with Congo red and viewed under a polarizing microscope [12]. Nevertheless, possible sampling errors and procedural risks such as cardiac tamponade, arrhythmias induction, and bleeding, limit its use. Therefore, noninvasive methods that can recognize the signs revealing cardiac involvement may be employed.
Conventional echocardiography
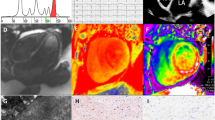
M-Mode echocardiography shows increased LV wall thickeness, with normal or small LV cavity size. In addition, biatrial enlargement and, occasionally, pericardial effusion are seen. Standard 2-D echocardiography (2-DE) has focused on the assessment of structural anatomy and ventricular function of cardiac muscle infiltrated by amyloid. All cardiac valves, interatrial septum and right ventricular walls also appear to be thickened [13]. Structural changes in CA are due to interstitial infiltration of amyloid in the myocardium that appears hypertrophied, but the LV walls are really pseudohypertrophied for intramyocardial amyloid deposition. These amyloid deposits show as ‘granular sparkling’, because of increased ‘granular’ myocardial echogenicity (Fig. 1). The granular sparkling appearance is characteristic but not specific for the diagnosis of CA. Nevertheless, this finding, in association with increased LV wall thickness, has a sensitivity of 87 % and a specificity of 81 % [13]. The increased LV wall thickness is associated with low voltage on the ECG in the precordial and/or limb leads <5 mm in height, often associated with extreme left or right axis deviation and patterns of pseudoinfarction that may be misinterpreted as ischemia [14]. Amyloid deposition in the conductive tissue may cause atrioventricular conduction abnormalities, mainly second- and third-degree atrioventricular block. These features are specific electrocardiographic aspects of infiltrative disease, such as amyloidosis. Some authors showed that septal thickness >1.98 cm combined with low ECG voltages, in the absence of systemic hypertension, has a sensitivity of 72 % and a specificity of 91 % for the diagnosis of CA.
The ‘mismatch’ between the electrocardiographic (low QRS voltage) and the echocardiographic pictures (increased LV wall thickness) is a distinctive aspect of cardiac involvement (Fig. 2). If atrial enlargement is present in association with ECG/ECHO findings, the noninvasive diagnosis of CA can reach a sensitivity of 100 %. The increase in left atrial size is mainly induced by the severity and chronicity of left atrial pressure elevation resulting from diastolic dysfunction. In CA, it was found that the LA diameter is almost normal in TTR-related CA, slightly increased in AL amyloid and significantly enlarged in senile amyloid [15].
Among conventional 2-DE, integrated backscatter (IB) is a useful non-invasive method that can provide information about the structure of myocardial tissue by its acoustic properties. It provides an approach for defining the composition of cardiac muscle tissue. A software package incorporated into the echocardiographic machine must be used. For the analysis, magnitudes of cyclic variation of IB were calculated as the difference between maximal values recorded at end-diastole and minimal values obtained at end-systole [16]. Two-dimensional standard transthoracic echocardiography in short-axis view of the LV (at chordae level) must be performed. Two regions of interest subsequently must be placed in the ventricular septum and in the LV posterior wall, avoiding endocardial and epicardial echoes. IB tissue ultrasound reflectivity must be measured in dB. Acoustic quantification is not only performed in CA but in a variety of pathological conditions, such as aging heart [17], hypertrophic cardiomyopathy [18], myocardial ischemia [19], and diabetes mellitus [20]. In a study by Koyama et al., the cyclic variation of IB in CA was recorded at the level of the interventricular septum and in the LV posterior wall both in controls and in patients with amyloidosis. The results obtained demonstrated that the magnitude of cyclic variation progressively decreased from amyloidosis without heart failure to amyloidosis with heart failure. In addition, the reduction recorded in the LV posterior wall was higher than that recorded in the interventricular septum [21].
Other information about the changes in myocardial function that occur in patients with CA may be obtained by calculating the myocardial performance index (MPI). This index, also called the Tei index, is obtained by combining systolic and diastolic time intervals. The index appears to be a more useful predictor of clinical outcome in amyloidosis patients [22].
In summary, echocardiographic findings that characterize patients with CA are the following:
-
1.
Increased LV wall thickness in the absence of causes such as hypertension.
-
2.
‘Mismatch’ between echocardiographic and electrocardiographic findings.
-
3.
‘Granular sparkling’ appearance of myocardial walls.
-
4.
Biatrial dilatation in contraposition to the normal or reduced LV cavity dimensions.
Other echocardiographic findings found in CA but not specific are:
-
5.
Early E/A ratio <1 and late E/A ratio >2 with tissue Doppler echocardiography reduction.
-
6.
Reduced magnitude of cyclic variation of myocardium at IB.
-
7.
Increased Tei index (MPI) value indicative of systolic/diastolic LV dysfunction.
New echocardiographic techniques
Tissue Doppler imaging
Tissue Doppler imaging (TDI) is a novel echocardiographic technique based on principles of pulsed wave and color Doppler echocardiography. A high-pass wall filter is used to measure blood flow velocities, and a low-pass filter is used to estimate tissue velocities. The Doppler-based technique is able to provide real-time quantification of longitudinal myocardial function. The measure of longitudinal contraction and relaxation is obtained by annular displacement toward the ventricular apex in systole and away from the same in diastole.
Longitudinal TDI recorded in CA is able to differentiate between cardiac ‘pseudohypertrophy’ due to the infiltration of amyloid and true cardiac hypertrophy (Fig. 3). The myocardial profiles of the systolic/diastolic curves of velocity recorded from septal or lateral mitral annulus in amyloidosis patients are significantly reduced with regard to the velocity curves recorded in patients with true hypertrophy, despite LVEF being preserved in patients affected by CA. This occurs when there is a reduced amount of contractile tissue contained in the LV walls of patients with CA and for diastolic heart failure following amyloid intracardiac deposition [23].
Speckle tracking echocardiography (STE)
Velocities measured by TDI in one myocardial segment are determined by function in other segments as well as due to the tethering between adjacent segments and cardiac translational motion. Different to TDI, STE strain permits the calculation of myocardial deformation with high spatial and temporal resolution. This parameter is calculated on frame-to-frame analysis of speckle displacement. Thus, STE is a new method for the angle-independent and objective quantification of myocardial deformation.
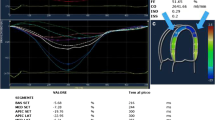
The incremental value of STE strain over tissue Doppler velocities in the diagnosis of CA was first demonstrated by Koyama et al. They showed that this echocardiographic method can identify patients with subclinical CA earlier, at a time when routine conventional 2-DE was unable to diagnose CA [24]. Other authors have confirmed the higher value of STE strain in comparison to TDI to differentiate biopsy-proven CA patients with normal 2-DE and Doppler echocardiograms from healthy controls [25]. 2-D STE is able to differentiate CA from other causes of LV wall thickening. Global myocardial deformation is lower in patients with CA compared to those with LV hypertrophy [26]. The impaired LV function characteristic of CA, results in altered LV function evidenced by the heterogeneity of basoapical contractility. In the normal heart, LV contractility progressively decreases from the basal region to the apex. On the contrary, an impairment of LV contractility is evident in CA, with hypokinesia at the base and/or at mid-ventricular level preserved function at the apex. The non-uniform contractility of LV walls and the reduction of basoapical gradient were also observed on longitudinal speckle tracking (Fig. 4); this was also confirmed by Belkin et al. in a study performed using 2-DE [27]. Using 2-D STE, another recent study showed the importance of this method in differentiating the increased thickness of LV walls from other causes of LV hypertrophy. In particular, it was found that the amyloid heart is characterized by reduced longitudinal strain from the base to the apex [28]. The heterogenicity of LV contractility was further confirmed by cardiac magnetic resonance that examined the regional distribution of gadolinium uptake. In some patients with CA, high enhancement scores were more frequent at the mid-ventricular level compared with apical and basal levels. Segments with extensive transmural involvement were more likely to be hypokinetic or akinetic. Probably, the reduced segment deformation is due to preferential deposition of amyloid accumulation [29]. It is uncertain if the pattern of preserved motion at the LV apex with hypokinesis in the basal segments (with the inversion of the normal gradient from base to apex) is characteristic of patients with CA. It is possible that the altered segmental wall motion is due to the preferential deposition of amyloid proteins in the basal segments in comparison to the apical zones [27].
3-D echocardiography
3-D echocardiography (3-DE) is a useful method to assess the geometry and mechanics of the LV. The potential role of 3-DE to detect some peculiar aspects of CA was evaluated by Migrino et al. They demonstrated that 3-DE can be a novel method able to show regional LV dyssynchrony at the same time as characteristic temporal pattern dispersion of regional volume systolic change (Fig. 5) [30]. Although this echocardiographic method has not been extensively evaluated, it represents a potential technique for specific and early diagnosis of CA in the future.
In conclusion, the ultrasound method is an effective and non-invasive tool to recognize the cardiac infiltration of amyloid. In addition, unlike conventional echocardiography, new echocardiographic techniques (such as tissue Doppler, speckle tracking and 3-DE) were unable to specifically characterize CA but have been shown to allow the detection of CA at a subclinical stage, when conventional techniques were not able to demonstrate cardiac involvement.
References
Falk RH, Comenzo RI, Skinner M. The systemic amyloidoses. New Engl J Med. 1997;337:898–909.
Khan MF, Falk RH. Amyloidosis. Postgrad Med. 2001;77:686–93.
Gertz MA, Lacy MQ, Dispenzieri A. Amyloidosis. Hematol Oncol Clin North Am. 1999;13:1211–33.
Shah K, Inoue Y, Mehra M. Amyloidosis of the heart: a comprehensive review. Arch Intern Med. 2006;166:1805–13.
Falk RH. Diagnosis and management of the cardiac amylodoses. Circulation. 2005;112:2047–60.
Klein AL, Hatle LK, Burstow DJ, et al. Doppler characterization of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1989;13:1017–26.
Dubrey SW, Cha K, Anderson J, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998;91:141–57.
Hattori T, Takei Y, Koyama J, et al. Clinical and pathological studies of cardiac amyloidosis in transthyretin type familial amyloid polyneuropathy. Amyloid. 2003;10:229–39.
Merlini G, Westermark P. The systemic amyloidoses: clearer understanding of the molecular mechanisms offers hope for more effective therapies. J Intern Med. 2004;225:159–78.
Dubrey SW, Cha K, Simms RW, et al. Electrocardiography and Doppler echocardiography in secondary (AA) amyloidosis. Am J Cardiol. 1996;77:313–5.
Takayama F, Miyazaki S, Morita T, et al. Dialysis-related amyloidosis of the heart in long-term hemodialysis patients. Kidney Int Suppl. 2001;78:S172–6.
Berk J, Keane J, Seldin D, et al. Persistent pleural effusions in primary systemic amyloidosis: aetiology and prognosis. Chest. 2003;124:969–77.
Ardehali H, Qasim A, Cappola T, et al. Endomyocardial biopsy plays a role in diagnosing patients with unexplained cardiomyopathy. Am Heart J. 2004;147:919–23.
Weymann AE. Principles and Pratice of Echocardiography. 2nd ed. New York: Lippincott Williams and Wilkins; 1994. p. 809.
Mutagh B, Hammill SC, Gertz MA, et al. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95:535–7.
Rapezzi C, Merlini G, Quarta CC, et al. Systemic amyloidoses: disease profiles and clinical course of the main types. Circulation. 2009;120:1203–12.
Miller JG, Perez JE, Sobel BE. Ultrasonic characterization of myocardium. Prog Cardiovasc Dis. 1985;28:85–110.
Masuyama T, Nellesen U, Schnitter I, et al. Ultrasonic tissue characterization with a real time integrated backscatter imaging system in normal and aging heart. J Am Coll Cardiol. 1989;14:702–8.
Masuyama T, Goar F, Tye TL, et al. Ultrasonic tissue characterization in of human hypertrophied hearts in vivo with a real-time integrated backscatter imaging system. Circulation. 1989;80:925–34.
Vitale DF, Bonow RO, Gerundo G, et al. Alterations in ultrasonic backscatter during exercise-induced myocardial ischemia in humans. Circulation. 1995;92:1452–7.
Di Bello V, Talarico L, Picano E, et al. Increased echogenicity of myocardial wall in the diabetic heart: an ultrasound tissue characterization study. J Am Coll Cardiol. 1995;25:1408–15.
Koyama J, Ray-Sequin PA, Falk RH. Prognostic significance of ultrasound myocardial tissue characterization in patients with cardiac amyloidosis. Circulation. 2002;106:556–61.
Tei C, Dujardin KS, Hodge DO, et al. Doppler index combining systolic and diastolic myocardial performance: clinical value in cardiac amyloidosis. J Am Coll Cardiol. 1996;28:658–64.
Oki T, Tanaka H, Yamada H, et al. Diagnosis of cardiac amyloidosis based on the myocardial velocity profile in the hypertrophied left ventricular wall. Am J Cardiol. 2004;93:864–9.
Porciani MC, Lilli A, Perfetto F, et al. Tissue Doppler and strain imaging: a new tool for early detection of cardiac amyloidosis. Amyloid. 2009;16(2):63–70.
Bellavia D, Abraham TP, Pellikka PA, et al. Detection of left ventricular systolic dysfunction in cardiac amyloidosis with strain rate echocardiography. J Am Soc Echocardiogr. 2007;20:1194–202.
Sun JP, Stewart WJ, Yang XS, et al. Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am J Cardiol. 2009;103:411–5.
Belkin RN, Kupersmith AC, Kalique O, et al. A novel two-dimensional echocardiographic finding in cardiac amyloidosis. Echocardiography. 2010;27:1171–6.
Phelan D, Collier P, Thavediranathan P, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle tracking is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98:1442–8.
Perugini E, Rapezzi C, Piva T, et al. Non-invasive evaluation of the myocardial substrate of cardiac amyloidosis by gadolinium cardiac magnetic resonance. Heart. 2006;9:343–9.
Migrino RQ, Harmann L, Woods T, et al. Intraventricular dyssynchrony in light chain amyloidosis: a new mechanism of systolic dysfunction assessed by 3-dimensional echocardiography. Cardiovasc. Ultrasound. 2008;6:40.
Conflict of interest
The author declares that there are no conflicts of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cacciapuoti, F. The role of echocardiography in the non-invasive diagnosis of cardiac amyloidosis . J Echocardiogr 13, 84–89 (2015). https://doi.org/10.1007/s12574-015-0249-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12574-015-0249-1