Abstract
Agriculture has the potential to improve dietary diversity through farm production diversity if farming households consume what they produce. However, the linkages between a household’s own agricultural production and dietary diversity are not well understood. This study uses a count of crop species, animal species, production diversity score, and the Simpson’s index as measures of farm production diversity to assess the effect of production diversity on the dietary diversity of households, women and children. A Poison model was employed on a sample of 779 farming households selected using a multistage sampling technique in a household survey representative at the County level in Kisii and Nyamira Counties, Kenya. The findings of the study indicate that farm production diversity is significantly associated with the dietary diversity of women and that of the entire household, but is not associated with the dietary diversity of children. The count of the animal species has the highest magnitude of association with dietery diversity in this study. Every additional animal species kept leads to a 0.33 and 0.13 increase in household dietery diversity and the dietery diversity of women respectively. Children’s dietary diversity is significantly associated with the education of the mother, household size and age of the child. The study highlights the need to consider individual dietary requirements when developing nutrition interventions and policy, as opposed to general dietary interventions targeting the entire household.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Close to one billion people in the world are undernourished (FAO 2018). According to FAO (2018), a majority of the people who are undernourished live in the rural areas of low-income countries in Africa and Asia. In Kenya, approximately 24.2% of the population is undernourished (FAO 2018). Undernutrition causes severe economic losses at the individual, household and national levels (Gödecke et al. 2018; World Health Organization [WHO] 2006). The annual cost of micronutrient deficiencies in low-income countries has been estimated at between 2.4 and 10% of Gross Domestic Product (GDP) (Freijer et al. 2018, 2013; Stein and Qaim 2007; Horton and Ross 2003). Women and children are more vulnerable to the effects of undernutrition because of their high nutritional requirements for growth and development, different physiological requirements, and contribution to the intergenerational cycle of undernutrition and ill health (Merchant and Kurz 2018; Kim et al. 2017; Blossner et al. 2005).
Besides enhanced access to sufficient quantities of food, better dietary diversity and quality of diets are central components of nutrition security. Dietary diversity is a qualitative measure of food consumption that reflects household access to a variety of foods, and is also a proxy for nutrient adequacy of the diet of individuals (Kennedy et al. 2011). Studies have shown that dietary diversity is correlated with nutrient adequacy of the diet, micronutrient adequacy and indicators of growth and nutritional status of women and children (Steyn et al. 2006; Kennedy et al. 2007; Arimond et al. 2010; Fongar et al. 2019). Nutrient adequacy results from consumption of energy and essential nutrients, including micronutrients, in sufficient amounts over time. Micronutrient adequacy plays an important role in preventing deficiency diseases, improving immune function, physical work capacity, cognitive development and learning capacity in children, as well as preventing diet-related chronic diseases (Black et al. 2008; WHO 2003). Overall, individual dietary indicators are good proxies for individual dietary quality and micronutrient adequacy. Promotion of dietary diversity is thus an effective approach to improving micronutrient and dietary quality for women and children. The household-level dietary diversity is an indicator of household economic access to diverse food and includes purchased foods such as sugars and sweets (Swindale and Bilinsky 2005; Kennedy et al. 2011; Verger et al. 2017).
Despite increased global food availability, undernutrition remains an increasingly difficult challenge in low-income countries (FAO 2018; Black et al. 2008). A majority of the people who are undernourished live in the rural areas of low-income countries and depend on agriculture as a source of food and livelihood (FAO et al. 2017). Thus, nutrition interventions would be expected to focus on enabling these households to meet their nutrition requirements from the food they produce. However, there has been little nexus between nutrition and agricultural interventions and policy. Most of the policy interventions in agriculture have focused on improving the quantity of food staples produced, resulting in commercialization and intensification of staple food production systems, rather than quality and diversity of diets (Fanzo et al. 2013; Larochelle and Alwang 2014; Pingali 2015; Magrini and Vigani 2016). Nonetheless, farm commercialization and market access can substitute for own production through selling surplus farm produce and purchasing food items not produced in the farm (Sibhatu and Qaim 2017; Koppmair et al. 2017; Hoddinott et al. 2015; Sibhatu et al. 2015). However, studies have shown little effect of commercialization and intensification of staple food production systems on the nutritional status of vulnerable groups such as the poor, women and children (Berti et al. 2004; Hawkes and Ruel 2008; Masset et al. 2012). This is because commercialization and intensification of staple food production systems crowd out traditional non-staple foods that are rich in essential micronutrients (Pingali 2015). For subsistence households that consume mainly what is produced from their own farms, this transition may deprive them of diverse diets and micronutrient-rich vegetables, fruits, and animal-source foods.
At national levels, a positive correlation between income growth and nutrition has been documented in the literature (Haddad et al. 2003; Webb and Block 2012). At the household level, income can be used to purchase diverse foods (Koppmair et al. 2017). However, income is a viable pathway only if households have access to markets to sell farm produce and purchase food, a persistent challenge for many households in rural areas of low-income countries (Binswanger-Mkhize and McCalla 2010; Jayne et al. 2010; Sibhatu et al. 2015; Koppmair et al. 2017). Consequently, the nutrition of the vulnerable categories of the population in developing countries benefits least from interventions that target economic growth in the short term (Dubé et al. 2012). Haddad et al. (2003) found that although increases in income at the household and national levels are correlated with a decrease in overall undernutrition, this growth alone does not reduce the prevalence of undernutrition in children.
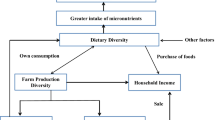
According to Hawkes and Ruel (2008), the subsistence production pathway is one of the most fundamental solutions to food insecurity for the poor and pre-transition countries. Some recent interventions are promoting “nutrition-sensitive” agriculture which aims to make agriculture more responsive to nutrition needs of small scale households. Farm production diversification is one of the components of “nutrition-sensitive” agriculture (FAO 2014; Ruel et al. 2018). Since most of the households facing undernutrition depend on subsistence farming as a source of food, having a variety of crops or animals on the farm could translate to a diverse diet. Production of staples should result in greater access to and consumption of energy, while production of fruit, vegetables, and animal source foods (dairy, eggs, fish, and meat) should result to greater access to energy, protein, and fat, as well as improved quality and micronutrient content of diets.
However, the current empirical evidence on the association of farm production diversity with dietary diversity is not conclusive (Sibhatu and Qaim 2018a). A review of recent studies by Sibhatu and Qaim (2018a) using meta-analysis reveals mixed evidence. Only a small proportion of studies find a positive and significant association between production diversity and dietary diversity and/or nutrition across various indicators and sub-samples, while a majority of studies report significant associations only for certain indicators or subsamples, or no association at all (Sibhatu and Qaim 2018a). However, most of the studies analyze the associations only for specific indicators or sub-samples. Consequently, the findings are context specific and not generalizable across sub samples within the population (Sibhatu and Qaim 2018a). This study contributes to the existing literature by using the same data set to compute dietary diversity scores for children, women and household and analyzing the scores against different indicators of farm production diversity to evaluate the linkages thereof. The study aims to provide evidence on the suitability of farm production diversification as a strategy of improving diet diversity of households and individuals.
Diet diversity of individuals is measured differently from household dietary diversity to capture specific individual nutritional needs. For example, women of reproductive age are more vulnerable due to physiological demands of pregnancy and lactation and have a higher requirement for iron than men (FAO and FHI 360 2016). For children, vitamin A is essential to support rapid growth, prevent blindness and prevent mortality and morbidity caused by infections such as diarrhea, measles, and respiratory infections (Villamor and Fawzi 2000; West and Keith 2002; Mayo-Wilson et al. 2011). Dietary diversity measurements for individuals are designed to include foods that meet these nutrition-specific requirements.
Using both individual and household level measures of dietary diversity in the study provides an opportunity to analyze, compare and report on the effects at both levels. Analyzing this association at both the individual and household level is important in illustrating the effect of farm production diversity on the nutrition of vulnerable groups in the household, such as women and children. We tested the hypotheses that: (i) an increase in the farm production diversity does not have an effect on household dietary diversity; (ii) an increase in the farm production diversity does not have an effect on dietary diversity of women; and, (iii) an increase in the farm production diversity does not have an effect on dietary diversity of children.
2 Study methods
2.1 Data sources
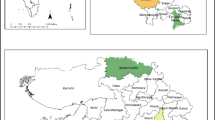
This study uses primary data collected between October and December in 2015 from a survey of 779 farming households in Kisii and Nyamira Counties of Kenya. The two Counties are located in a highland equatorial climate region that receives rainfall in all months of the year. There are two main rainfall seasons, the long rain season occurring between February and June and the short rain season occurring between September and early December. The two Counties are suitable for agricultural production, farmers grow a wide range of food crops such as maize, beans, indigenous vegetables, sorghum, millet, and cash crops such as tea. In spite of the favourable agricultural potential, the two counties are characterized by high levels of undernutrition. More than one-quarter of children below 5 years of age are stunted, while close to 10% are underweight (Kenya National Bureau of Statistics 2014). Paradoxically, a vibrant agriculture sector in the two Counties seems to co-exist with high levels of undernutrition, suggesting a failure to match the agricultural potential of the region with its nutrition needs.
A multistage sampling technique was used to select the respondents in the field survey. The first stage involved the development of a sampling frame by listing all existing Common Interest Groups (CIGs) in Kisii and Nyamira Counties with assistance from Africa Harvest Biotech Foundation International, a non-governmental organization working in the region. In this study, CIGs are defined as groups of farmers from the same area who voluntarily agree to cooperate on agricultural activities and are registered with the State Department of Social Services. In the second stage, a simple random sampling technique with a probability proportionate to the total number of CIGs was used to select 48 groups (32 and 16 groups were selected from Kisii and Nyamira Counties respectively) from the sampling frame. In the third stage, a sample of 20 households was selected from each group through a simple random sampling technique. In groups that had 20 or fewer members, all members were selected. Interviews were conducted in local languages by trained enumerators who were supervised by the researchers. After dropping 29 questionnaires that had incomplete information, a sample of 779 households was used for the analysis. Within the 779 households, data from 570 women within the childbearing age of 18–45 years and 263 children who were between 6 and 59 months of age was also analyzed.
2.2 Analytical model
This study employs the Poisson regression model to estimate the effect of farm production diversity on dietary diversity. A total of 15 bivariate Poisson regression equations were estimated with the dietary diversity scores as the dependent variable while the five measures of farm production diversity constituted the independent variables. The Poisson regression model is suitable for analyzing data in cases where the dependent variable takes the form of a non-negative integer 0, 1, 2, which is in form of count data (Cameron and Trivedi 2005; Greene 2003). The model is suitable for this study since the dependent variables are measured as a count of food groups to be analyzed against a set of covariates (x). Count data can be analyzed using Ordinary Least Squares (OLS) as found in Jones et al. (2014), but can yield biased results due to among other reasons; the inability of OLS to account for the zero truncated data, heteroscedasticity, and non-normality (Sturman 1999; Greene 2003).
The Poisson model assumes equidispersion, that is the variance of yi is equal to its mean. The likelihood ratio test for equidispersion is conducted by running the negative binomial regression and interpreting the overdispersion parameter, alpha, which becomes zero when the data does not suffer from overdispersion (StataCorp 2009). Although the Poisson model is nonlinear, it can be estimated by the maximum likelihood method as the observations (yi| xi) are independent. The coefficient estimates of a Poisson model are interpreted as semi-elasticities while the marginal effects, which are presented in this study, are interpreted as the change in the dietary diversity score when the explanatory variable changes by one unit. The model has been applied in similar studies on diet diversity recently (Sibhatu et al. 2015; Koppmair et al. 2017). All the models were estimated with standard errors corrected for group clusters to eliminate possible error term correlation within common interest groups (Cameron and Miller 2015).
2.3 Empirical model
The Poisson regression model in this study was estimated in two forms. First, the effect of Farm production Diversity (FD) on the Dietary Diversity Scores (DDS) is analyzed. The models are specified in Eq. 1 as follows:
Where the DDS of household i or individual j is a function of farm production diversity of the farm cultivated by household i. The terms α and ℓ denotes the coefficients to be estimated and random error term respectively. Different forms of this model are estimated using various measures of farm production diversity (Simpson’s Index, animal species count, crop species count and production diversity score) against household and individual dietary diversity scores as the dependent variables in each model.
Since dietary diversity depends not just on the sources of the food, but also on the ability of the household to produce the food or obtain it from other sources, the models are estimated again, but this time adjusted for market access and market participation (farm commercialization) factors and socioeconomic factors. The adjusted models are specified in Eq. 2 as follows;
In the adjusted models, the DDS of household i or individual j is a function of farm production diversity (FD), market access (MA) and market participation or farm commercialization (MP) factors, and individual and household socio-economic factors (SE).
2.4 Definition and measurement of variables
The dependent variables in this study are the dietary diversities of the household and individuals. The explanatory variables include farm production diversity, market access, market participation, and socioeconomic factors.
2.5 Dietary diversity
Dietary diversity is defined as “a qualitative measure of food consumption that reflects household access to a variety of foods and is also a proxy for nutrient adequacy of the diet of individuals” (Kennedy et al. 2011). This study uses three measures of dietary diversity namely; Household Diet Diversity Score (HDDS), Child Dietary Diversity Score (CDDS) and Women Dietary Diversity Score (WDDS) (Table 1). The HDDS and WDDS are computed following FAO guidelines of 2011 (Kennedy et al. 2011) while the CDDS is computed following WHO (2010) guidelines for children aged 6–23 months. The age of children in this study was extended to 6–59 months to cover the first five years of life when children are most susceptible to negative impacts of undernutrition (Grantham-McGregor et al. 2007). The scores are a count of the number of different food groups consumed over a certain period of time, usually 7 days or 24 h, for either the household or for individual members. Studies have shown that the individual dietary diversity scores are significantly correlated with more comprehensive measures of diets and nutrition (Fongar et al. 2019; Headey and Ecker 2013) and are thus appropriate for research as they are easy to measure.
The HDDS was computed using data on 12 food groups from a 7-day food consumption recall. Individual dietary diversity scores were computed for women (WDDS) and children (CDDS) using a 24-h dietary recall data. Recall data for WDDS was collected twice on two non-consecutive days. Special days such as celebrations and holidays were excluded. The WDDS was calculated using 9 food groups, while the CDDS was calculated for children aged 6–59 months using 7 food groups. The food group classes for all three scores are shown in Table 1.
Seasonality of agricultural production can cause variation in diet diversity across the year due to associated effects on food security (Hirvonen et al. 2016). The data used in this study was collected in the month of November and early December, just before harvesting of maize which is a major staple food. Although this would be considered a lean season (Hirvonen et al. 2016), Kisii and Nyamira Counties receive rainfall in all months of the year, thus would be expected to be comparatively less vulnerable to effects of seasonality.
2.6 Farm production diversity
Farm production diversity is defined in this study as the variety of crop species grown and animal species kept on the farm in the previous 12 months. Farm production diversity is measured using four methods: Simpson’s Index; crop species count; animal species count and production diversity score. The use of multiple measurements allowed for the comparison of results and to test for consistency of the association between farm production diversity and dietary diversity. The Simpson’s Index is a weighted index that takes into account the number of species (richness), as well as the relative abundance of each species (evenness) (Jones et al. 2014; Simpson 1949). The crop species count, animal species count, and production diversity score is an unweighted count of the different animal species and/or plant species that are kept or grown within the farm. The production diversity score adopts a dietary approach in measuring farm production diversity by mapping crop and animal species produced on the farm on the number of food groups used to compute dietary diversity (Berti 2015; Koppmair et al. 2017; Sibhatu and Qaim 2018b). According to Sibhatu and Qaim (2018b), a major disadvantage of the production diversity score method is that it counts species that deliver products in two different food groups twice (for example, chicken is a source of eggs and meat). This study overcomes the double-counting problem by adopting the 16 broad food groups from which dietary diversity scores are derived as found in FAO guidelines of 2011 (Table 3). This ensured that species were not counted in two food groups (for example, eggs are only counted if they were actually produced, while chicken was only counted as a source of meat). Industrial cash crops were excluded from the production diversity score as household do not consume them from the farm but were included in the crop species count.
2.7 Market access and market participation (farm commercialization)
This study uses a commercialization index as a proxy for market participation and farm commercialization. Following Carletto et al. (2017) and Ogutu and Qaim (2019), the index was calculated as the value of farm produce sold divided by the value of total farm produce. Commercialized households were expected to consume more diversified diets. Similarly, households that are able to access employment off-farm could also access diversified diets from the market (Sibhatu et al. 2015). Such farmers may, however, be constrained by physical access to the market. This study defines market access as the geographic distance in kilometres from the home to the nearest market where households purchase or sell food. Households located close to these markets were expected to have more diversified diets.
The models were also adjusted for socioeconomic and demographic factors that were expected to influence dietary diversity such as household size, education of household head and female spouse, as well as age and gender of the household head.
3 Results and discussions
3.1 Descriptive results
Table 2 presents the descriptive statistics for the key variables in the study. The average counts of crop species and animal species per household were nine and two respectively (Table 2). Farm sizes in Kisii and Nyamira Counties were 1.6 acres on the average (Table 2). The most common plant species grown were maize, beans, bananas, millet, amaranths and local vegetables (black nightshade, spider plant and jute mallow). Other crops included tea, coffee, avocado, kale, cowpeas, sugarcane, pumpkins, and onions. The most common livestock species kept were cattle, goats, chicken, sheep and rabbits (Table 3). The dietary diversity scores on the average were 9, 4 and 5 for HDDS, WDDS and CDDS respectively (Table 2).
Annual farm revenues on the average were Kenya Shillings 96,020 (1 dollar is equivalent to Kshs.100 as at 22nd December 2019; source, Central Bank of Kenya). Households are relatively large in Kisii and Nyamira Counties, with 7 members being the average size (Table 2).
3.2 Econometric results
Table 4 presents the results of 15 bivariate Poisson regression models between the measures of dietary diversity as dependent variables and farm diversity as independent variables. The chi-squared statistic for the likelihood ratio test in all models was not significant, thus we fail to reject the hypothesis that alpha = 0 and conclude that the negative binomial regression results are equal to the Poisson regression results. The data, therefore, do not suffer from overdispersion.
Results presented in Table 4 show that for every additional food group that the household produced, HDDS and WDDS increased by 0.19 and 0.13 respectively. Animal species count has the highest association with diet diversity in this study. Every additional animal species kept leads to a 0.33 and 0.13 increase in HDDS and WDDS respectively (Table 4). When farm production diversity is measured only as a crop species count, every additional crop grown leads to an increase of 0.07 and 0.05 in the number of food groups consumed by the household and women respectively (Table 4). The WDDS is significantly associated with all indicators of farm production diversity. In contrast, CDDS is not associated with any of the indicators of farm production diversity.
Tables 5, 6, 7 and 8 present the results of the Poisson regression estimates adjusted for market access, market participation (farm commercialization) and socioeconomic factors. The full model results show that farm diversity has an independent association with HDDS and WDDS. The magnitude does not change considerably from the findings in Table 4 and the results are consistent across all the models. When the model is adjusted for market access, farm commercialization and socioeconomic factors, we find that WDDS is not significantly associated with the Simpson’s Index.
Other factors that are positively associated with HDDS include off-farm income, market access, farm commercialization, gender and education level of household head (Tables 5, 6, 7 and 8). Off-farm income is not significantly associated with increased dietary diversity for women and children. Similarly, farm commercialization was significantly associated with increased HDDS, but not WDDS and CDDS. Contrary to expectations, larger households were associated with increased WDDS. The association, however, turned negative for the squared term, indicating a non-linear relationship. Using the formula -βt/2βq, we determine the turning point to be 10 household members. Thus, as household size increased beyond 10 members, the diet diversity of women begun to decline.
The education level of the woman was positively associated with CDDS. Maternal education was expected to have a positive association with diet diversity because women are the primary family caregivers in the majority of households, this was confirmed herein as women were the majority respondents of the dietary recall questionnaires. As expected, the age of the child was significantly associated with diet diversity of children (Tables 5, 6, 7 and 8).
3.3 Discussion of key findings
One of the key findings of this study is that the magnitude of the association between farm production diversity and dietary diversity first depends on the method used to measure farm production diversity. When measured as an unweighted crop species count, animal species account and production diversity score, farm production diversity is significantly associated with HDDS and WDDS but not with CDDS. The magnitude of the effect is however small but higher for HDDS than WDDS. When measured as a Simpson’s Index, farm production diversity is only significantly associated with WDDS. The magnitude of the association is smallest for the crop species count and highest for the animal species count. Similar to Koppmair et al. (2017), the results show a larger effect of farm production diversity on dietary diversity scores when measured as production diversity score, relative to crop species count. Most previous studies report a magnitude of association in the range of 0.05 to 0.20 (Bellon et al. 2016; Koppmair et al. 2017; Sibhatu and Qaim 2018a, b), which compares favourably with our findings. Jones et al. (2014) reported higher effects while using the OLS method instead of a Poisson model that is applied in this study. We find a higher magnitude effect for the animal count, which has not been analyzed separately by any of the previous studies.
The hypotheses that an increase in farm production diversity has no effect on household dietary diversity is rejected. Similarly, the hypothesis that an increase in farm production diversity has no effect on women dietary diversity is rejected. Our findings are consistent with findings from previous studies by Sibhatu et al. (2015) and Jones et al. (2014) that farm production diversity is independently associated with household dietary diversity. Sibhatu et al. (2015) however argue that this association is only true in specific conditions, and can turn negative as farm production diversity exceeds a certain threshold because households may lose the economic benefits of specialization. Overall, the Poisson model results show that as households diversify in the farm, their economic access to diverse diets follows a similar pattern.
As would be expected, other factors that contribute to the household economic status, such as farm commercialization, off-farm income and education level of household head are significantly associated with HDDS. Households that practiced commercialized farming relative to subsistence had more diversified diets. This is however not the case for diets of women and children. It is plausible that off-farm income and farm commercialization is only significantly associated with HDDS, since unlike individual diet diversity; HDDS is an indication of the household’s economic access to diverse diets. HDDS includes food groups that are obtained only from the market to measure the economic ability of the household to acquire diverse diets. Nevertheless, the finding is in agreement with previous studies that report little effect of commercialization and intensification of staple food production systems on the nutritional status of vulnerable groups such as the poor, women and children (Berti et al. 2004; Hawkes and Ruel 2008; Masset et al. 2012; Pingali 2015). Thus, even when farm commercialization and incomes are increasing, some degree of farm production diversity is still vital for diet diversity of women and of households that are constrained by income.
Farm production diversity is not associated with dietary diversity of children in this study. Based on these findings, we fail to reject the hypothesis that increase in farm production diversity has no effect on children dietary diversity. Though positive, the association between farm production diversity and dietary diversity of children is not significant. The results here do not agree with results from some other recent studies undertaken in various regions using other measures of farm diversity. For instance, Hirvonen and Hoddinott (2017), found that CDDS among children in Ethiopia is strongly correlated with farm production diversity computed by mapping farm diversity over the 7 food groups used to compute CDDS. However, findings from previous studies have shown little change in children nutrition as a result of interventions in agriculture (Berti et al. 2004; Masset et al. 2012).
Across all models, our findings show that children’s dietary diversity is associated with maternal education, household size and age of the child. These findings agree with previous studies, that maternal education leads to improved children nutrition outcomes (Kabubo-Mariara et al. 2008; Ruel et al. 2013). Schooling improves nutrition through nutrition knowledge, empowerment, optimization of time and physical and mental health which is observed through diet choices and attitude (Ruel et al. 2013). We also find that as children grow older, their diets become more diversified. This confirms similar results reported from the region by Harvey et al. (2017), that older children were more likely to attain minimum acceptable diet and dietary diversity. Obviously, new types of foods are introduced as the child grows older. However, this is an indication that the dietary requirements of younger children, especially those who are not breastfeeding, are possibly not met.
Seasonality of agricultural production affects the diets of rural households (Bellon et al. 2016). One limitation of this study is the failure to capture the possible seasonal changes in the linkages between farm production diversity and dietary diversity as it would have required further rounds of data collection. However, Kisii and Nyamira Counties receive rainfall in all the months of the year, thus farm production happens all year round, accordingly the seasonal variation in food production and consumption is minimal.
4 Conclusion and policy implications
This study evaluates the linkages between farm production diversity and dietary diversity of the household, women and children and uses different measures of farm production diversity to compare the results. Overall, the study’s findings are consistent with previous studies, yet offer some new insights. It can, therefore, be concluded that the association between farm production diversity and dietary diversity in Kenya is diverse, context-specific and depends on factors such as the market participation (farm commercialization), income sources and proximity to markets. Although the magnitude of association varies, the direction of association between farm production diversity and dietary diversity is consistent across the different measures of farm diversity except for the Simpson’s Index. The positive association between farm production diversity and dietary diversity though small in magnitude has important nutrition policy implications for government and development partners than can stem the high levels of undernutrition in Kenya.
Given that farm production diversity is significantly associated with the dietary diversity of the household and women, but not for children, there is need to target nutrition policy interventions at individuals such as women and children rather than developing programs for the entire household. This study recommends the expansion of child nutrition education programs that target to educate mothers and/or caregivers on the nutrition requirements of children.
The finding that factors contributing to household economic status such as farm commercialization, off-farm income and education are significantly associated with HDDS has important policy implication for improving household’s nutrition. This study recommends the implementation of policies and programs that target improving market access for farm produce and providing opportunities for off-farm employment since they will be more effective in improving household’s access to diverse diets.
References
Arimond, M., Wiesmann, D., Becquey, E., Carriquiry, A., Daniels, M. C., Deitchler, M., Fanou-Fogny, N., Joseph, M. L., Kennedy, G., Martin-Prevel, Y., & Torheim, L. E. (2010). Simple food group diversity indicators predict micronutrient adequacy of Women's diets in 5 diverse, resource-poor settings. The Journal of Nutrition, 140(11), 2059S–2069S.
Bellon, M. R., Ntandou-Bouzitou, G. D., & Caracciolo, F. (2016). On-farm diversity and market participation are positively associated with dietary diversity of rural mothers in southern Benin, West Africa. PloS one, 11(9), e0162535.
Berti, P. R. (2015). Relationship between production diversity and dietary diversity depends on how number of foods is counted. Proceedings of the National Academy of Sciences, 112(42), E5656–E5656.
Berti, P. R., Krasevec, J., & FitzGerald, S. (2004). A review of the effectiveness of agriculture interventions in improving nutrition outcomes. Public Health Nutrition, 7(5), 599–609.
Binswanger-Mkhize, H., & McCalla, A. F. (2010). The changing context and prospects for agricultural and rural development in Africa. Handbook of Agricultural Economics, 4, 3571–3712.
Black, R. E., Allen, L. H., Bhutta, Z. A., Caulfield, L. E., De Onis, M., Ezzati, M., et al. (2008). Maternal and child undernutrition: Global and regional exposures and health consequences. The Lancet, 371(9608), 243–260.
Blossner, M., De Onis, M., & Prüss-Üstün, A. (2005). Malnutrition: Quantifying the health impact at national and local levels. Environmental burden of disease series no. 12. World Health Organization, Geneva.
Cameron, C., & Miller, D. L. (2015). A practitioner’s guide to cluster-robust inference. The Journal of Human Resources, 50(2), 317–372.
Cameron, A. C., & Trivedi, P. K. (2005). Micro econometrics: Methods and applications. Cambridge: Cambridge university Press.
Carletto, C., Corral, P., & Guelfi, A. (2017). Agricultural commercialization and nutrition revisited: Empirical evidence from three African countries. Food Policy, 67(1), 106–118.
Dubé, L., Pingali, P., & Webb, P. (2012). Paths of convergence for agriculture, health, and wealth. Proceedings of National Academy of Sciences USA, 109(31), 12294–12301.
Fanzo, J., Hunter, D., Borelli, T., & Mattei, F. (Eds.). (2013). Diversifying food and diets: Using agricultural biodiversity to improve nutrition and health. New York: Routledge.
FAO (2014). Nutrition Sensitive Agriculture. ICN2 Second International Conference on Nutrition 19–21 November 2014. Rome, Italy.
FAO. (2018). The state of food security and nutrition in the world. Rome: Food and Agriculture Organization of the United Nations.
FAO, & FHI 360. (2016). Minimum dietary diversity for women: A guide for measurement. Rome: Food and Agriculture Organization of the United Nations.
FAO, IFAD, UNICEF, WFP, & WHO. (2017). The State of Food Security and Nutrition in the World 2017. Building resilience for peace and food security. Rome: Food and Agriculture Organization of the United Nations.
Fongar, A., Gödecke, T., Aseta, A., & Qaim, M. (2019). How well do different dietary and nutrition assessment tools match? Insights from rural Kenya. Public Health Nutrition, 22(3), 391–403.
Freijer, K., Tan, S. S., Koopmanschap, M. A., Meijers, J. M., Halfens, R. J., & Nuijten, M. J. (2013). The economic costs of disease related malnutrition. Clinical Nutrition, 32(1), 136–141.
Freijer, K., van Puffelen, E., Joosten, K. F., Hulst, J. M., & Koopmanschap, M. A. (2018). The costs of disease related malnutrition in hospitalized children. Clinical Nutrition ESPEN, 23, 228–233.
Gödecke, T., Stein, A. J., & Qaim, M. (2018). The global burden of chronic and hidden hunger: Trends and determinants. Global Food Security, 17, 21–29.
Grantham-McGregor, S., Cheung, Y. B., Cueto, S., Glewwe, P., Richter, L., Strupp, B., & International Child Development Steering Group. (2007). Developmental potential in the first 5 years for children in developing countries. The lancet, 369(9555), 60–70.
Greene, W. H. (2003). Econometric analysis (5th ed.). New York: Prentice Hall.
Haddad, L., Alderman, H., Appleton, S., Song, L., & Yohannes, Y. (2003). Reducing child malnutrition: How far does income growth take us? World Bank Economic Review, 17(1), 107–131.
Harvey, S., Callaby, J., & Roberts, L. (2017). An exploration of complementary feeding of infants and young children in the rural area of Muhoroni, Nyanza province, Kenya: A descriptive study. Paediatrics and international child health, 37(3), 172–180.
Hawkes, C., & Ruel, M. T. (2008). From agriculture to nutrition: Pathways, synergies and outcomes. Agricultural and rural development notes; no. 40. World Bank, Washington, DC.
Headey, D., & Ecker, O. (2013). Rethinking the measurement of food security: From first principles to best practice. Food Security, 5(3), 327–343.
Hirvonen, K., & Hoddinott, J. (2017). Agricultural production and children's diets: Evidence from rural Ethiopia. Agricultural Economics, 48(4), 469–480.
Hirvonen, K., Taffesse, A. S., & Hassen, I. W. (2016). Seasonality and household diets in Ethiopia. Public Health Nutrition, 19(10), 1723–1730.
Hoddinott, J., Headey, D., & Dereje, M. (2015). Cows, missing milk markets, and nutrition in rural Ethiopia. The Journal of Development Studies, 51(8), 958–975.
Horton, S., & Ross, J. (2003). The economics of iron deficiency. Food Policy, 28(1), 51–75.
Jayne, T. S., Mather, D., & Mghenyi, E. (2010). Principal challenges confronting smallholder agriculture in sub-Saharan Africa. World Development, 38(10), 1384–1398.
Jones, A. D., Shrinivas, A., & Bezner-Kerr, R. (2014). Farm production diversity is associated with greater household dietary diversity in Malawi: Findings from nationally representative data. Food Policy, 46, 1–12.
Kabubo-Mariara, J., Ndenge, G. K., & Mwabu, D. K. (2008). Determinants of children's nutritional status in Kenya: Evidence from demographic and health surveys. Journal of African Economies, 18(3), 363–387.
Kennedy, G., Pedro, M. R., Seghieri, C., Nantel, G., & Brouwer, I. (2007). Dietary diversity score is a useful indicator of micronutrient intake in non-breast-feeding Filipino children. Journal of Nutrition, 137, 1–6.
Kennedy, G., Ballard, T., & Dop, M. C. (2011). Guidelines for measuring household and individual dietary diversity. Rome: Food and Agriculture Organization of the United Nations.
Kenya National Bureau of Statistics (2014). Demographic and health survey: Key indicators 2014. Nairobi, Kenya.
Kim, S., Fleisher, B., & Sun, J. Y. (2017). The long-term health effects of fetal malnutrition: Evidence from the 1959–1961 China great leap forward famine. Health Economics, 26(10), 1264–1277.
Koppmair, S., Kassie, M., & Qaim, M. (2017). Farm production, market access and dietary diversity in Malawi. Public Health Nutrition, 20(2), 325–335.
Larochelle, C., & Alwang, J. (2014). Impacts of improved bean varieties on food security in Rwanda. Selected Paper prepared for presentation at the Agricultural & Applied Economics Association’s 2014 AAEA Annual Meeting, Minneapolis, MN, July 27-29, 2014.
Magrini, E., & Vigani, M. (2016). Technology adoption and the multiple dimensions of food security: The case of maize in Tanzania. Food Security, 8(4), 707–726.
Masset, E., Haddad, L., Cornelius, A., & Isaza-Castro, J. (2012). Effectiveness of agricultural interventions that aim to improve nutritional status of children: Systematic review. BMJ, 344, d8222.
Mayo-Wilson, E., Imdad, A., Herzer, K., Yakoob, M. Y., & Bhutta, Z. A. (2011). Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: Systematic review and meta-analysis. BMJ, 343.
Merchant, K. M., & Kurz, K. M. (2018). Women’s nutrition through the life cycle: social and biological vulnerabilities. In the health of women (pp. 63-90). New York: Routledge.
Ogutu, S. O., & Qaim, M. (2019). Commercialization of the small farm sector and multidimensional poverty. World Development, 114, 281–293.
Pingali, P. (2015). Agricultural policy and nutrition outcomes – Getting beyond the preoccupation with staple grains. Food Security, 7(3), 583–591.
Ruel, M. T., Alderman, H., & Maternal and Child Nutrition Study Group. (2013). Nutrition-sensitive interventions and programmes: How can they help to accelerate progress in improving maternal and child nutrition? The Lancet, 382(9891), 536–551.
Ruel, M. T., Quisumbing, A. R., & Balagamwala, M. (2018). Nutrition-sensitive agriculture: What have we learned so far? Global Food Security, 17, 128–153.
Sibhatu, K. T., & Qaim, M. (2017). Rural food security, subsistence agriculture, and seasonality. PLoS One, 12(10), e0186406.
Sibhatu, K. T., & Qaim, M. (2018a). Review: Meta-analysis of the association between production diversity, diets, and nutrition in smallholder farm households. Food Policy, 77, 1–18.
Sibhatu, K. T., & Qaim, M. (2018b). Farm production diversity and dietary quality: Linkages and measurement issues. Food Security, 10(1), 47–59.
Sibhatu, K. T., Krishna, V. V., & Qaim, M. (2015). Production diversity and dietary diversity in smallholder farm households. Proceedings of the National Academy of Sciences, 112(34), 10657–10662.
Simpson, E. H. (1949). Measurement of diversity. Nature, 163(4148), 688–688.
StataCorp. (2009). Stata Base reference manual: Release 11. College Station: StataCorp LP.
Stein, A. J., & Qaim, M. (2007). The human and economic cost of hidden hunger. Food and Nutrition Bulletin, 28(2), 125–134.
Steyn, N. P., Nel, J. H., Nantel, G., Kennedy, G., & Labadarios, D. (2006). Food variety and dietary diversity scores in children: Are they good indicators of dietary adequacy? Public Health Nutrition, 9(5), 644–650.
Sturman, M. C. (1999). Multiple approaches to analyzing count data in studies of individual differences: The propensity for type 1 errors, illustrated with the case of absenteeism prediction. School of Hospitality Administration: Cornell University.
Swindale, A. & Bilinsky, P. (2005). Household Dietary Diversity Score (HDDS) for measurement of household food access: Indicator guide. Food and nutrition technical assistance project, academy for educational development, 2005. Washington, D.C.
Verger, E. O., Dop, M. C., & Martin-Prével, Y. (2017). Not all dietary diversity scores can legitimately be interpreted as proxies of diet quality. Public Health Nutrition, 20(11), 2067–2068.
Villamor, E., & Fawzi, W. W. (2000). Vitamin A supplementation: Implications for morbidity and mortality in children. The Journal of Infectious Diseases, 182(Supplement_1), S122–S133.
Webb, P., & Block, S. (2012). Support for agriculture during economic transformation: Impacts on poverty and undernutrition. Proceedings of the National Academy of Sciences, 109(31), 12309–12314.
West, J., & Keith, P. (2002). Extent of vitamin a deficiency among preschool children and women of reproductive age. Journal of Nutrition., 132(9), 2857S–2866S.
WHO (2003). Diet, nutrition and the prevention of chronic diseases: report of a Joint WHO/FAO Expert Consultation. WHO Technical Report Series, No. 916. Geneva.
WHO. (2006). Guidelines on food fortification with micronutrients. Geneva: World Health Organization.
WHO. (2010). Indicators for assessing infant and young child feeding practices: Part 2: Measurement. Geneva: World Health Organization.
Funding
This research was financially supported by the German Federal Ministry of Food and Agriculture (BMEL) based on a decision of the Parliament of the Federal Republic of Germany (grant number 2813FSNu01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Muthini, D., Nzuma, J. & Nyikal, R. Farm production diversity and its association with dietary diversity in Kenya. Food Sec. 12, 1107–1120 (2020). https://doi.org/10.1007/s12571-020-01030-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12571-020-01030-1