Abstract
It has been reported that the ramification pattern of spinal motor nerves reflected the spatial orientation of motoneuron pools in the ventral horn of spinal cord and this topography of spinal motor nuclei was very similar in different vertebrates. Therefore, the ramification pattern of spinal nerves was an important criterion for discussing the phylogenetic homology of muscles. It has been reported that the human subscapularis muscle was innervated by several branches, the proximal branch of them was from the ventral layer of the dorsal cord and the distal one from the dorsal layer of the dorsal cord of the brachial plexus. This fact suggested the human subscapularis had different phylogenetic origins. In this study, I unveil the phylogenetic origin of the mammalian subscapularis. The animals observed were a chimpanzee, a lar gibbon, a cat, a fetal pig, a koala, a possum (mammals), a lizard, an iguana (reptiles) and salamanders (amphibians). The branches to the mammalian subscapularis were divided into proximal and distal groups based on the origin from the brachial plexus, just like the human subscapularis. In salamanders and lizards, the homologous branch with the mammalian proximal branch to the subscapularis was observed and the segmentally higher branch innervating the latissimus dorsi was homologous with the distal branch to the mammalian subscapularis. Conclusively, I suppose that the dorsal-most portion of the reptilian latissimus dorsi muscle differentiates to the mammalian teres major, and the segmentally higher portion of the reptilian latissimus dorsi contributes to the formation of the mammalian subscapularis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the comparative anatomy of anterior limb muscles, Fürbringer (1873, 1874, 1876, 1900) clarified the homology of the shoulder girdle muscles from amphibians to mammals based on their innervation. In his study, Fürbringer identified and discussed each nerve branches from the brachial plexus with considering their ramification pattern in the plexus. The previous studies have reported that the ramification pattern of spinal nerves reflected the spatial orientation of motoneuron pools in the ventral horn of spinal cord (Tani et al. 1994) and the motoneuron clusters in the ventral horn appeared to be arranged very similarly in different vertebrates (Romanes 1964). Furthermore, this topography of spinal motor nuclei was reported to be independent of both the position of the target muscles and the functions exerted by the given muscles (Hörner and Kümmel 1993). Smith and Hollyday (1983) observed the neighboring of motoneurons innervated the muscles derived from common precursor muscle masses. These studies showed that the ramification pattern of the spinal nerves was an important criterion for discussing the homology of muscles.
In the previous study, Koizumi (1989) clarified the human coracobrachialis muscle received different nerve branches occupying the different layers in the brachial plexus and concluded that the human coracobrachialis muscle was formed by the fusion of two different muscles in prosimian, coracobrachialis superficialis and profundus muscles. Like the coracobrachialis muscle, the human subscapularis muscle, arising widely from the costal surface of scapula and inserting to the lesser tubercle of humerus, is innervated by the several branches occupying the different layers of the dorsal cord of the brachial plexus. Especially, the caudal portion of the subscapularis muscle is supplied by the common twigs with the teres major muscle and frequently by the twig from the axillary nerve (Frohse and Fränkel 1908; Kerr 1918). Additionally, among branches to the subscapularis muscle, the proximal ones arise from the ventral layer of the dorsal cord of the brachial plexus, and distal ones from the dorsal layer of the dorsal cord near the origin of the twigs to the teres major and latissimus dorsi muscles (Kato 1989). This finding suggests that the human subscapularis muscle may have some different phylogenetic origins. On the other hand, according to the previous studies of comparative anatomy, the teres major developed from the same anlagen with the subscapularis in mammals (Diogo et al. 2009) and the subscapularis itself has been reported to exist as an independent muscle in amphibians (Romer and Parsons 1977; Dilkes 2000). In this study, I focus on the ramification pattern of the branches to the subscapularis, teres major and latissimus dorsi muscles from salamanders to mammals and unveil the phylogenetic relationship among those muscles.
Materials and methods
The animals observed in this study were summarized in Table 1.
The primate specimens were obtained from the Cooperative Research Program in 1984 of the Primate Research Institute of Kyoto University in Aichi, Japan. All these specimens were kept in 10% formalin after death in the Primate Research Institute of Kyoto University.
The fetal pig was obtained from the General Science Cooperation Japan, which was by-product of the pork industry in the USA and sold for the educational purpose.
The cat was provided after euthanized in other experimental use for the brain research in Kumamoto University, Japan. That brain research using cats was approved for by the institutional animal use committee. Koala (No. Q-UNI 1091) and Common brushtail possum (no specimen number) were provided from the Department of Zoology, University of Queensland, Australia. All were found dead and then kept frozen. The green iguana and western tiger salamander were provided after death from Dr. Kawashima (Toho University, Japan). The monitor lizard and Chinese giant salamanders were from Kumamoto University, where they were kept in 10% formalin after death in Kanazawa Sunny Land Zoo, Japan. They had no specimen numbers. No animals were killed for this study.
All specimens were fixed in 10% formalin and preserved in 60% alcohol solution. After dissecting to show the brachial plexuses and shoulder muscles, each spinal root participating the brachial plexus and the shoulder girdle muscles were cut off from the body. Next, the origins of the nerves innervating the subscapularis, teres major and latissimus dorsi muscles were observed carefully macroscopically and under a stereomicroscope if necessary. In a chimpanzee, a lar gibbon and a fetal pig, the intramuscular distribution of nerves into the subscapularis and teres major muscles was also observed.
The protocol for the present study did not include any specific issue that required approval from the Ethics Committee of my university. The present study conformed to the AAA's Guiding Principles in the Care and Use of Animals.
Results
Spinal segments of the brachial plexus and the number of cervical vertebrae
The spinal segments contributing the brachial plexuses and the number of cervical vertebrae were summarized in Table 2. The number of cervical vertebrae is important when identifying the segments of the cervical and thoracic nerves, especially in reptiles.
Attachments of each muscle
The attachments (origins and insertions) of the subscapularis, teres major and latissimus dorsi muscles were summarized in Table 3. The origin of the latissimus dorsi in primates could not be observed because the muscle was already removed from the body.
Origins of the branches innervating each muscle
The origins (layers and spinal segments) of the muscular branches to each muscle from the brachial plexus were shown in Table 4 (mammals), Table 5 (reptiles) and Table 6 (amphibians). In tables, the origins from the dorsal cords of the brachial plexuses were mentioned. The ventral cords, ramifying the median, ulnar and musculocutaneous nerves, never branch off the muscular twigs to those muscles in all species and did not considered in this study.
Mammals
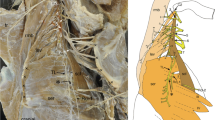
Chimpanzee (Figs. 1a-c) and lar gibbon (Fig. 2)
a Photograph of the subscapularis muscle and its innervation from the dorsal cords of the right brachial plexus in a chimpanzee. Scale bar: 10 mm. b Diagram of the branches innervating the subscapularis, teres major and latissimus dorsi muscles from the dorsal cords (yellow) of the brachial plexus. The ventral cords (shaded) are reflected ventrally. c Intramuscular distribution of each branch supplying the subscapularis muscle. Ax axillary nerve; C4–8 ventral rami of the fourth to eighth cervical nerves; ld latissimus dorsi muscle; M median nerve; Mc musculocutaneous nerve; p pectoralis muscle; R radial nerve; sbs subscapularis muscle; Ss suprascapular nerve; T1 ventral ramus of the first thoracic nerve; tma teres major muscle; U ulnar nerve; 1–7 branches to the subscapularis muscle. Red numbers show the branches from the ventral layer of the dorsal cord, and the branches indicated by pale orange include nerve fibers from the dorsal layer of the dorsal cord. Black supplies the shoulder joint
Diagram showing the origin of branches to the subscapularis muscle from the right brachial plexus in a lar gibbon. The ventral cords (shaded) of the brachial plexus is reflected ventrally. Ax axillary nerve; cbr coracobrachialis muscle; C4–8 ventral rami of the fourth to eighth cervical nerves; ld latissimus dorsi muscle; p pectoralis muscle; Phr phrenic nerve; R radial nerve; sbs subscapularis muscle; Ss suprascapular nerve; T1 ventral ramus of the first thoracic nerve; tma teres major muscle; 1–5 branches to the subscapularis muscle. The numbers are colored in the same manner as in Fig. 1b; + , cutaneous branch
The proximal branches innervating the subscapularis arose from the ventral layer of the dorsal cord of the ventral rami of the fifth and sixth spinal nerves (C5d and 6d) (chimpanzee: sbs-1–4, lar gibbon: sbs-1,2) or of C5d-7d (lar gibbon: sbs-3). A fine branch in a chimpanzee (sbs-5) got into the terminal part of the muscle to supply the shoulder joint. The distal branches (chimpanzee: sbs-6,7, lar gibbon: sbs-4,5) were formed by the fibers from the ventral layer of C5d + 6d in a chimpanzee or C5d-7d in a lar gibbon joined with the fibers from the dorsal layer of C5d + 6d in both species. Some of these nerve fibers from the dorsal layer contributed to the branch innervating the teres major muscle (tma). In both species, the branches from the ventral layer supplied most of the muscle and the branch including the fibers from the dorsal layer distributed in the small caudal part of the muscle (chimpanzee: Fig. 1c). The branch innervating the latissimus dorsi (ld) was composed of the fibers from the dorsal layer of C6d + 7d (chimpanzee) or C5d-T1d (lar gibbon).
Cat (Fig. 3)
Diagram of the right brachial plexus of a cat. The dorsal cords and the branches to the subscapularis (sbs), teres major (tma) and latissimus dorsi (ld) muscles are in yellow. The ventral cords (shaded) are reflected ventrally. The ramification of the dorsal cord in the root of C7 is shown in detail on the upper left. Ax axillary nerve; Axa axillary artery; C6–8 ventral rami of the sixth to eighth cervical nerves; cbr-br coracobrachialis and biceps brachii muscles; ld latissimus dorsi muscle; M median nerve; Mc musculocutaneous nerve; p pectoralis muscle; Phr phrenic nerve; R radial nerve; sbs subscapularis muscle; Ss suprascapular nerve; T1 ventral ramus of the first thoracic nerve; tma teres major muscle; U ulnar nerve; 1–4 branches to the subscapularis muscle. The numbers are colored in the same manner as in Fig. 1b; + , cutaneous branch
The dorsal cord of the brachial plexus was composed of C7, C8 and T1 (C7d, C8d and T1d). The proximal three branches to the subscapularis (sbs-1–3) originated from the ventral layer of C7d. The distal branch (sbs-4) was formed by the union of the dorsal and ventral branches of C7d. A twig from sbs-4 joined with the dorsal branch from the root of the axillary nerve to make the branch innervating the teres major (tma). The branch to the latissimus dorsi (ld) arose from the dorsal layer of C8d.
Fetal pig (Fig. 4)
Diagram of the right brachial plexus of a fetal pig. The ventral cords (shaded) are reflected ventrally. The ventral cords (shaded) are reflected ventrally. Ax axillary nerve; C5–8 ventral rami of the fifth to eighth cervical nerves; ld latissimus dorsi muscle; R radial nerve; sbs subscapularis muscle; T1 ventral ramus of the first thoracic nerve; tma teres major muscle; 1–4 branches to the subscapularis muscle. The numbers are colored in the same manner as in Fig. 1b; + , cutaneous branch
Two proximal branches to the subscapularis (sbs-1,2) arose from the ventral layers of C6d + 7d. Two distal branches (sbs-3,4) were formed by the nerve fibers from the ventral surface of C6d + 7d and from the twig innervating the teres major (tma). The sbs-4 received additionally the fibers from the dorsal layer of C6d + 7d. The branches to the teres major (tma-1,2) arose from the dorsal layer of C6d + 7d. The nerve fibers from the dorsal layers of C7d and C8d joined to form the branch to the latissimus dorsi (ld).
Koala (Fig. 5) and common brushtail possum (Fig. 6)
Diagram of the left brachial plexus of a koala. The dorsal cords and the branches to the subscapularis (sbs), teres major (tma) and latissimus dorsi (ld) are in yellow. The ventral cords (shaded) are reflected ventrally. Ax axillary nerve; C5–8 ventral rami of the fifth to eighth cervical nerves; ld latissimus dorsi muscle; R radial nerve; sbs subscapularis muscle; T1 ventral ramus of the first thoracic nerve; tma teres major muscle; 1–4 branches to the subscapularis muscle. The numbers are colored in the same manner as in Fig. 1b; + , cutaneous branch
Diagram of the right brachial plexus of a brushtail possum. The dorsal cords and the branches to the subscapularis (sbs), teres major (tma) and latissimus dorsi (ld) muscles are in yellow. The ventral cords are shaded. Ax axillary nerve; C4–8 ventral rami of the fourth to eighth cervical nerves; ld latissimus dorsi muscle; Phr phrenic nerve; Ss suprascapular nerve; R radial nerve; sbs subscapularis muscle; T1 ventral ramus of the first thoracic nerve; tma teres major muscle; 1–5 branches to the subscapularis muscle. The numbers are colored in the same manner as in Fig. 1b
Among the branches to the subscapularis, the proximal ones (koala: sbs-1, possum: sbs-1–3) originated from the ventral surface of C5d + 6d. The distal ones (koala: sbs-2–4, possum: sbs 4,5) were formed by the fibers from the dorsal surface of C5d-7d (koala) or C5d + 6d (possum) and from the twig to the teres major. In both species, the nerve innervating the teres major arose from the dorsal layer of C5d-7d (koala) or C5d + 6d (possum). The latissimus dorsi in both species was innervated by the branch from the dorsal layer of C7d-T1d (koala) or C7d + 8d (possum).
Reptiles
Monitor lizard (Figs. 7a,b, 8)
Photograph (a) and drawing (b) showing the subscapularis (sbs), scapulohumeralis posterior (shp) and latissimus dorsi (ld) muscles with their innervations in a monitor lizard. Dorsal view of the right shoulder girdle. The subcoracoscapularis (sbc) and sbs arise from the inner surface of the shoulder girdle and shp from the dorsal (outer) surface. Scale bar: 10 mm. del, origin of the deltoideus muscle; sha, scapulohumeralis anterior muscle
Diagram of the left brachial plexus in a monitor lizard. Ax axillary nerve; C7–9 ventral rami of the seventh to ninth cervical nerves; bi biceps brachii muscle; bra brachialis muscle; cbr coracobrachialis muscle; Fl flexor nerve; ld latissimus dorsi muscle; p pectoralis muscle; Ri inferior radial nerve; Rs superior radial nerve; sbc subcoracoideus muscle; sbs subscapularis muscle; sha scapulohumeralis anterior muscle; shp scapulohumeralis posterior muscle; spc supracoracoideus muscle; tr tractor radii muscle (named following to Haines 1939); tri triceps brachii muscle; + , cutaneous branch
The monitor lizard had the well-developed coracoid bone, from inner (costal) surface of which the subcoracoideus muscle arose (sbc in Fig. 7a, b). The subcoracoideus muscle had the common insertion with the subscapularis, arising from the inner (costal) surface of the scapular bone (sbs in Fig. 7a, b). Both muscles have been sometimes put together as a subcoracoscapularis muscle. Further, in a monitor lizard, there was a muscle that arose from the outer caudal surface of the scapular blade and inserted into the proximal tubercle of the humerus, joining with the subcoracoscapularis muscle (shp in Fig. 7a, b). In this study, this muscle was defined as a scapulohumeralis posterior muscle.
The nerve to the subcoracoscapularis (sbs + sbc) arose from the ventral layers of the roots of C7d + 8d (Fig. 8). Distally, from the ventral surface of C7d + C8d, the branch supplying the subscapularis and scapulohumeralis posterior muscles arose (shp + sbs). The branch to the latissimus dorsi (ld) was from the dorsal layer of C8d. In a monitor lizard, the teres major muscle was not observed.
Green iguana (Fig. 9)
Diagram of the right brachial plexus in a green iguana. Ax axillary nerve; C6-9 ventral rami of the sixth to ninth cervical nerves; bi biceps brachii muscle; cbr coracobrachialis muscle; ld latissimus dorsi muscle; M median nerve; p pectoralis muscle; Ri inferior radial nerve; Rs superior radial nerve; sbc subcoracoideus muscle; sbs subscapularis muscle; sha scapulohumeralis anterior muscle; spc supracoracoideus muscle; tri triceps brachii muscle; U ulnar nerve; + , cutaneous branch
In a green iguana, the scapulohumeralis posterior muscle, observed in a monitor lizard, was not found. The nerve to the subcoracoscapularis (sbs + sbc) originated from the ventral surfaces of C6d + C7d. The branches to the latissimus dorsi (ld) were from C6d + 7d and C8d + 9d.
Amphibians
Tiger salamander (Figs. 10, 11) and giant salamander (Fig. 12)
Photograph of the left shoulder girdle and related muscles in a tiger salamander. Inner view. Scale bar: 5 mm. bi biceps brachii muscle; cuc cucullaris muscle; cor coracoid region of the shoulder girdle; ld latissimus dorsi muscle; p pectoralis muscle; procor procoracoid region of the shoulder girdle; sbs subscapularis muscle; scap scapular region of the shoulder girdle; spc supracoracoideus muscle; tri triceps brachii muscle
Diagram of the right brachial plexus in a western tiger salamander. Ax axillary nerve; Fl flexor nerve; ld latissimus dorsi muscle; p pectoralis muscle; Ri inferior radial nerve; Rs superior radial nerve; sh scapulohumeralis muscle; spc supracoracoideus muscle; Sp3–5 ventral rami of the third to fifth spinal nerves; tri triceps brachii muscle; + , cutaneous branch
Diagram of the left brachial plexus in a giant salamander. bi biceps brachii muscle; bra brachialis muscle; del (cor) coracoid part of the deltoideus muscle; del (scap) scapular part of the deltoideus muscle; Fl flexor nerve; ld latissimus dorsi muscle; p pectoralis muscle; Ri inferior radial nerve; Rm middle radial nerve; Rs superior radial nerve; sh scapulohumeralis muscle; spc supracoracoideus muscle; Sp3–5 ventral rami of the third to fifth spinal nerves; tri triceps brachii muscle; + , cutaneous branch
The shoulder girdle in both species remained mostly cartilaginous and was incompletely divided into three regions; scapular, procoracoid and coracoid regions. Unlike in mammals and reptiles, in both species, the muscles arising from the inner surface of the shoulder girdle were less developed and a small muscle arising from the caudal portion of the scapular region (giant salamander) or of the boundary among the three regions of the girdle (tiger salamander, sh in Fig. 10). This muscle was innervated by the branch arising from the ventral layer of the dorsal cord of the 3rd and 4th spinal nerves (Sp3d and 4d) (sh in Figs. 11, 12). The nerve to the latissimus dorsi (ld) was from the caudal margin of Sp4d (tiger salamander) or Sp3d + 4d (giant salamander).
Discussion
Identification of muscles
When discussing the homology of muscles among different species from amphibians to mammals, the criterion on which the identification is based is most important. Howell (1935) mentioned that topography was, of course, often of considerable value in the identification of some individual units, but it was often extremely misleading. Further he continued that the only trustworthy criterion was the innervation. Although Diogo et al. (2009) mentioned that there were cases in which the same muscles had different innervations in different taxa, there have been a wealth of evidence that the innervation was the important key to clarify the phylogenetic significance of muscles (Arakawa et al. 2006; Honma et al. 1998; Kato 1989; Koizumi and Sakai 1995; Koizumi 2019; Sakamoto et al. 1996). In this study, the homology of muscles was discussed based on their innervations.
The origins of the nerves to the subscapularis, teres major and latissimus dorsi muscles in mammals
The origins of the branches innervating the subscapularis, teres major and latissimus dorsi muscles from the brachial plexus in mammals are summarized in Fig. 13. The nerves to the subscapularis are grouped into the proximal (red line) and distal groups (light-blue line). The branches in the proximal group arose from the ventral layer of the dorsal cord formed by the higher spinal nerve segments, and the distal ones from the dorsal layer of the dorsal cords by the lower spinal nerve segments. In chimpanzee, cat and pig, the distal branch of the proximal group and the proximal branch of the distal group joined together to supply the subscapularis muscle. This branching pattern was commonly observed in mammals. Consequently, the present study clarifies that the subscapularis muscle in mammals is innervated by two different nerve groups, originating from the different layers and different spinal nerve segments. This fact suggests that the subscapularis in mammals is composed of two phylogenetically different muscular portions. Further, the fact that the nerve in the distal group arose from the same layer and the spinal segments as the branch to the teres major indicates that a part of the subscapularis innervated by the nerve in the distal group has the close relationship with the teres major muscle.
Schematic diagram showing the origins of the branches to the subscapularis (sbs), teres major (tma) and latissimus dorsi (ld) muscles in mammals. Yellow bundles are the dorsal cords of the right brachial plexus. Ventral cords are not shown. Ventral view. The nerves to the subscapularis muscle are grouped into proximal (red line) and distal groups (light-blue line) as explained in the text. Ax axillary nerve; C4–8 ventral rami of the fourth to eighth cervical nerves; T1 ventral ramus of the first thoracic nerve; R radial nerve
The subscapularis muscle has been previously reported to be innervated by several branches from the dorsal cord of the brachial plexus (Frohse and Fränkel 1908; Kerr 1918; Kato 1989; Williams 1995). Especially, Kato (1989) described that the distal branch to the subscapularis formed the common bundle with the branch to the teres major. He suggested that the caudal portion of the subscapularis had the same muscular origin with the teres major. All these studies observed the branching pattern with focusing on the proximal–distal relationship, not on the ramification of each branch. Considering the ramification patterns of the innervating branches, this study has firstly clarified the morphological difference between the distal and proximal branches to the subscapularis and the close relationship among the subscapularis, teres major and latissimus dorsi muscles.
The comparative anatomy of the subscapularis, teres major and latissimus dorsi muscles
The origins of the branches from the dorsal cords of the brachial plexus to the subscapularis (or its homologous muscle) and latissimus dorsi muscles in reptiles and amphibians observed in this study are summarized in Fig. 14.
Schematic diagram showing the origins of the branches to the subscapularis (sbs), scapulohumeralis posterior (shp) and latissimus dorsi (ld) muscles in amphibians and reptiles. Yellow bundles are the dorsal cords of the right brachial plexus. Ventral view. Ax axillary nerve; IT inferior trunk of the brachial plexus; MT middle trunk of the brachial plexus; R radial nerve; ST superior trunk of the brachial plexus
In urodeles including salamanders, the muscles arising from the inner surface of the shoulder girdle and inserting to the humerus are poorly developed (Howell 1935). In this study, a muscle originated from the inner surface (tiger salamander) or from the inner caudal margin (giant salamander) of the shoulder girdle and inserting into the proximal end of the humerus was observed. This muscle has been identified as the scapulohumeralis brevis (Howell 1935) or subcoraco-scapularis (Fürbringer 1873; Romer 1944) and was considered to be homologous with the subscapularis muscle in mammals, although the innervation of the muscle was not observed in both studies. The muscle observed in this study was supplied by the branch arising from the ventral layer of the dorsal cords formed by the higher spinal segments that constituted the brachial plexus (red line in Fig. 14). Considering the origin of this branch from the brachial plexus, this branch is homologous with the proximal branch innervating the subscapularis muscle in mammals and this muscle was identified as the subscapularis muscle.
Further in reptiles, the subcoracoscapularis muscle is well developed to have the wide origin of the inner surface of the shoulder girdle from coracoid to scapular regions (Fürbringer 1900; Howell 1936; Romer and Parsons 1977). In this study, the scapular part of the subcoracoscapularis was identified as the subscapularis muscle. The subscapularis in reptiles observed in this study was innervated by the branch arising from the ventral layer of the dorsal cords formed by the higher spinal segments of the brachial plexus, just like the subscapularis in salamanders (red line in Fig. 14). This branch was also homologous with the proximal branch to the subscapularis in mammals. In a monitor lizard, distal more subscapularis branch which arose from the ventral layer was observed. This branch additionally supplied the small muscle, which arose from the outer caudal surface of the scapular blade and inserted into the proximal tubercle of the humerus and had the common insertion with the subcoracoscapularis (Fig. 7a,b). In this study, this small muscle was identified as the scapulohumeralis posterior muscle according to Fürbringer (1900), who observed this muscle in crocodiles. He considered the scapulohumeralis posterior as an independent muscle of the subscapularis. However, the fact that the scapulohumeralis posterior had the same innervation as the subscapularis and that both muscles were continuous near their insertions supported the idea that the scapulohumeralis posterior was an overhang formed by the development of the caudal part of the subscapularis onto the outer surface of scapula.
In all salamanders and lizards observed in this study, the teres major was absent and all mammals had. In the previous reports, the teres major was absent in Sphendon and Iguana (Dilkes 2000) and in Lacertilia (Fürbringer 1900). On the other hand, some studies mentioned that the teres major was observed in crocodylian species (Romer 1944; Meers 2003), and in turtle, crocodiles and many lizards (Dilkes 2000). The teres major is thought to appear in reptiles in phylogeny.
Diogo et al. (2009) mentioned that the mammalian teres major corresponded to a part of the subcoracoscapularis. However, while the nerve to the subcoracoscapularis in monitor lizard and iguana arose from the ventral layer of the dorsal cord of the brachial plexus, the branch to the teres major in mammals from the dorsal-most layer. This fact strongly indicated that the mammalian teres major was never homologous with the subcoracoscapularis. Further, Dilkes (2000) described that the teres major in mammals was a derivative of the latissimus dorsi. His idea was based on the fossil evidence and the previous reports, not on his own findings. The present study has clarified that the origin of the branch to the teres major in the brachial plexus had the close relationship with that of the branch to the latissimus dorsi and suggested that the mammalian teres major was a derivative of the latissimus dorsi, as proposed by Dilkes (2000).
As a result, the phylogenetic origins of the mammalian subscapularis and teres major muscles are summarized in Fig. 15. In salamanders (Fig. 15A), the subscapularis (sbs) and latissimus dorsi (ld) muscles existed. The teres major was not be found. The subscapularis, arising from the caudal inner surface of the shoulder girdle and inserting into the proximal end of the humerus, was innervated by the branch from the ventral layer of the dorsal cord formed by the higher spinal segments of the brachial plexus. The latissimus dorsi was supplied by the branch from the dorsal layer of the dorsal cord of the brachial plexus. The spinal segments of the nerve to the latissimus dorsi were lower than that to the subscapularis. In reptiles (monitor and iguana) (Fig. 15B), the subscapularis (sbs) developed as the subcoracoscapularis, which arose widely from the inner surface of the scapular to coracoid portions of the shoulder girdle. In a monitor lizard, a caudal part of the subscapularis spread out over the dorsal surface of the scapula to be identified as scapulohumeralis posterior muscle (shp) (Fürbringer 1900). The latissimus dorsi (ld) was developed like in salamanders. The teres major was not observed in these species. The transitive stage from reptiles to mammals is supposed in Fig. 15C. The dorsal-most and segmentally high portion (★) of the latissimus dorsi (ld) was differentiated to form the teres major in mammals (tma in Fig. 15D). Additionally, the segmentally higher portion (*) was separated from the latissimus dorsi and constituted the dorsal part of the subscapularis in mammals (light-blue part of sbs in Fig. 15D). Finally in mammals (Fig. 15D), the subscapularis muscle was formed by uniting two muscular elements. One was the derivative from the subscapularis (red part) and the other was from the segmentally high portion of the latissimus dorsi (light-blue part) in amphibians and reptiles. The former was innervated by the nerve arising from the ventral layer of the dorsal cord of the brachial plexus, and the latter was by the nerve from the dorsal layer of the dorsal cord.
Schematic diagram representing the phylogenetic origin of the mammalian subscapularis (sbs) and teres major (tma) muscles. (C) is the transitive stage from reptiles to mammals. Each muscular rectangle is settled according to the spinal segments and layers of the nerves innervating the muscles. The horizontal arrows are representing the layer and the vertical arrows representing the spinal segments. The rectangles indicated by asterisk (*) and star (★) are the muscular portions that may contribute to the formation of mammalian subscapularis and teres major muscles, respectively. del deltoideus muscle; ld latissimus dorsi muscle; shp scapulohumeralis posterior muscle
Conclusively, this study clarifies the phylogenetic significance of the different origin of the nerves to the mammalian subscapularis muscle, and that the latissimus dorsi in reptiles and amphibians contribute to the formation of the mammalian subscapularis and teres major muscles.
References
Arakawa T, Sekiya S, Kumaki K, Terashima T (2006) Intramuscular nerve distribution pattern of the oblique and transverse heads of the adductor hallucis muscles in the human foot. Anat Sci Int 81:187–196
Dilkes DW (2000) Appendicular myology of the hardsaurian dinosaur Maiasaura peeblesorum from the Late Cretaceous (Campanian) of Montana. Trans R Soc Edinb Earth Sci 90:87–125
Diogo R, Abdala V, Aziz A, Lonergan N, Wood A (2009) From fish to modern humans—comparative anatomy, homologies and evolution of the pectoral and forelimb musculature. J Anat 214:694–716
Frohse M, Fränkel M (1908) Die Muskeln des menschlichen Armes. In: Bardeleben K (ed) Handbuch der Anatomie des Menschen. Bd II, Abt II, Teil II. Gustav Fischer, Jena
Fürbringer M (1873) Zur vergleichenden Anatomie der Schultermuskeln. I Theil. Jena Z Naturwiss Bd VII:237–320
Fürbringer M (1874) Zur vergleichenden Anatomie der Schultermuskeln II Theil. Jena Z Naturwiss Bd VIII:175–280
Fürbringer M (1876) Zur vergleichenden Anatomie der Schultermuskeln III Theil. Morphol Jahrb Bd I:636–816
Fürbringer M (1900) Zur vergleichenden Anatomie der Schultermuskeln IV Theil. Jena Z Naturwiss Bd XXXIV:215–718
Haines RW (1939) A revision of the extensor muscles of the forearm in tetrapods. J Anat 73:211–233
Honma S, Jun Y, Horiguchi M (1998) The human gemelli muscles and their nerve supplies. Acta Anat Nippon 73:329–335
Hörner M, Kümmel H (1993) Topographical representation of shoulder motor nuclei in the cat spinal cord as revealed by retrograde fluorochrome tracers. J Comp Neurol 335:309–319
Howell AB (1935) Morphogenesis of the shoulder architecture. Part III. Amphibia Quart Rev Biol 10:397–431
Howell AB (1936) Morphogenesis of the shoulder architecture. Part IV. Reptilia Quart Rev Biol 11:183–208
Kato K (1989) Innervation of the scapular muscles and its morphological significance in man. Anat Anz 168:155–168
Kerr AT (1918) The brachial plexus of nerves in man, the variations in its formation and branches. Am J Anat 23:285–395
Koizumi M (1989) A morphological study on the coracobrachialis muscle (in Japanese with English summary). Acta Anat Nippon 64:18–35
Koizumi M (2019) Two mammalian species in which the intercostal nerves innervate the serratus anterior or scalenus muscles together with the cervical nerves: an important clue to clarify the homology of cervico-thoracic trunk muscles in mammals. Anat Sci Int 94:295–306
Koizumi M, Sakai T (1995) The nerve supply to coracobrachialis in apes. J Anat 186:395–403
Meers MB (2003) Crocodylian forelimb musculature and its relevance to archosauria. Anat Rec A 274A:891–916
Romanes GJ (1964) The motor pools of the spinal cord. Prog Brain Res 11:93–119
Romer AS (1944) The development of tetrapod limb musculature—the shoulder region of lacerta. J Morph 74:1–41
Romer AS, Parsons TS (1977) The vertebrate body, 5th edn. WB Saunders Company, Philadelphia
Sakamoto H, Akita K, Sato T (1996) An anatomical analysis of the relationships between the intercostal nerves and the thoracic and abdominal muscles in man. I. Ramification of the intercostal nerves. Acta Anat 156:132–142
Smith CL, Hollyday M (1983) The development and postnatal organization of motor nuclei in the rat thoracic spinal cord. J Comp Neurol 220:16–28
Tani M, Kida MY, Akita K (1994) Relationship between the arrangement of motoneuron pools in the ventral horn and ramification pattern of the spinal nerve innervating trunk muscles in the cat (Felis domestica). Exp Neurol 128:290–300
Williams PL (ed) (1995) Gray’s anatomy, 38th edn. Churchill Livingstone, New York
Acknowledgements
The author specially thanks Dr. T. Kawashima (Department of Anatomy, Toho University) and Dr. K. Kodama (Professor Emeritus, Kumamoto University) for providing specimens.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koizumi, M. Comparative anatomy of the subscapularis, teres major and latissimus dorsi muscles from salamanders to mammals with special reference to their innervations from the brachial plexus. Anat Sci Int 97, 124–137 (2022). https://doi.org/10.1007/s12565-021-00636-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-021-00636-5