Abstract
Many studies have been conducted to determine the composition of the glycoconjugates of the mucus-secreting cells of the fundic glands of the stomach. However, the chief cells of these glands have been largely ignored because they secrete mainly zymogens with a lower glycosylation. The aim of this work was to analyze the glycoconjugates of the gastric chief cells by a battery of 17 different lectins, recognizing Fucose, N-acetylgalactosamine, Galactose, N-acetylneuraminic acid, N-acetylglucosamine and Mannose containing oligosaccharides. Histochemical techniques were performed with several lectins and also combined with two pre-treatments; β-elimination, which removes O-linked oligosaccharides, and incubation with Peptide-N-Gycosidase F, which removes N-linked oligosaccharides. In addition, acid hydrolysis was performed before WGA histochemistry, and incubation with glucose oxidase before Con A labeling. Many lectins did not stain the chief cells. In addition, the presence of O-glycans in the apical cell membrane was demonstrated with the lectins AAL, HPA, MPA/MPL, PNA, RCA-I, and WGA. Some of these O-glycans were resistant to short-term β-elimination pre-treatments. Mannose-binding lectins stained the basal cytoplasm of the chief cells. The level of glycosylation of the chief cells was lower than that of the mucous cells. The presence of O-glycans in the apical cell membrane is consistent with the presence of mucins such as MUC1 in the apical membrane of chief cells. Moreover, Mannose-binding lectins revealed N-glycosylation in the basal cytoplasm. The knowledge of gastric chief cell glycoconjugates is relevant because of their potential involvement not only in in physiological but also in pathological processes, such as cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of glycoconjugates has been relatively uncommon for a long time, probably due to their great variety and structural complexity. However, in recent years, the analysis of glycans present in glycoproteins and glycolipids—called glycobiology—has become a rapidly growing field in the life sciences, with a great relevance in many areas of basic research, biomedicine and biotechnology (Varki and Kornfeld 2017). In addition, glycans have been shown to play a large variety of biological roles, from the very subtle to those that are essential for development, growth, or organism survival. Moreover, glycoconjugates have important roles in pathological processes, including cancer and infection by microorganisms (Varki and Gagneux 2017; Varki and Kornfeld 2017; Varki et al. 2017). Glycans often have a complex structure that greatly hinders their study. Combinations of various techniques (physical, chemical and enzymatic) are frequently used to analyze them, but very interesting and basic information on the composition of glycans can be obtained in situ by means of lectins or other glycan-binding proteins and antibodies (Cummings et al. 2017; Mulloy et al. 2017).
The cells of the gastric mucosa produce a glycoconjugate-rich secretion that has attracted the attention of researchers (Holmen Larsson et al. 2013; Rodríguez-Piñeiro et al. 2013; Jin et al. 2017). The rat gastric mucosa is divided into several regions. The corpus is the medial region and contains the fundic tubular glands with several types of secretory cells that arise from stem cells. Some of them are mucus-secreting cells. Others, the zymogenic or chief cells, located at the bottom of the gland, are serous cells that secrete digestive enzymes (Hoffmann 2013). Several lectin histochemical studies have been performed to analyze the glycoprotein composition of these cells and their secretion, mainly for the mucous cells (Madrid et al. 1990, 1998; Gómez-Santos et al. 2018). The combination of lectin histochemistry with various deglycosylation pre-treatments allows not only to find out the carbohydrate residues present in the glycoproteins, but also the type of oligosaccharide they belong to, i.e., N- or O-glycan (Brooks and Hall 2012). Mucous cells have been studied more extensively as they secrete highly glycosylated mucins, a very diverse family of O-glycosylated proteins. However, the chief cells produce mainly a zymogenic secretion, formed by proteins with a very low or absent glycosylation level, although they also express some mucins.
The characterization of the fundic gland glycoconjugates is interesting because of their important role in normal physiological processes, in development and in pathological processes, such as gastric cancer (Su et al. 2004; Duarte et al. 2016). Notably, mucins, O-glycosylated proteins produced in the gastric glands, are involved in the development of gastric cancer. The MUC1 protein, located in the apical membrane of chief cells, is known to be in the binding of Helicobacter pylori to the gastric mucosa, which can produce chronic gastritis and result in gastric cancer. The most common types of gastric cancer are also known to arise from chief cells (Duarte et al. 2016; Mills and Goldenring 2017). MUC1 is also known to be an oncogene with an anti-apoptotic function in cancer cells, but its MUC1 protein product has also been suggested to play a protective role against inflammation and carcinogenesis in the normal gastric mucosa (Saeki et al. 2014). However, there is no report analyzing the glycan composition of chief cells using lectins combined with deglycosylation pre-treatments. This would allow a better characterization of the presence of glycoconjugates even at a subcellular level, showing precisely where they are located.
The aim of this work was to analyze the presence of glycoconjugates in the chief cells of rat fundic glands by means of histochemistry with a battery of 17 lectins combined with several deglycosylation pre-treatments.
Materials and methods
Reagents
We purchased several reagents from EY (San Mateo, CA): biotinylated Aleuria aurantia lectin (AAL), Galanthus nivalis agglutinin (GNA), Glycine max agglutinin (SBA), Ulex europaeus agglutinin-I (UEA-I), Canavalia ensiformis agglutinin (Concanavalin A, Con A), and peroxidase-labeled agglutinin from Limax flavus (LFA).
We used some other reagents from Sigma Aldrich (Spain): type III glucose oxidase from Aspergillus niger, Bovine serum albumin (BSA), 3,3′-diaminobenzidine (DAB), peroxidase-labeled agglutinins from Helix pomatia (HPA) and Maclura pomifera (MPA/MPL), and biotinylated Arachis hypogaea (PNA) and Lotus tetragonolobus (LTA) agglutinins.
Roche (Spain) supplied the following reagents: the enzyme Peptide-N-glycosidase F (PNGase F) from Flavobacterium meningosepticum and expressed in Escherichia coli, peroxidase-labeled anti-digoxigenin antibody (HRP-anti-DIG), digoxigenin-labeled Datura stramonium (DSA) and Sambucus nigra (SNA) agglutinins.
In addition, we purchased other reagents from Atom (Barcelona, Spain): avidin–biotin–peroxidase complex (Vectastain ABC kit peroxidase standard), Avidin–biotin blocking kit, biotinylated Maackia amurensis haemagglutinin (MAH), Bandeiraea simplicifolia lectin I-B4 (BSI-B4), Ricinus communis agglutinin-I (RCA-I), Dolichos biflorus (DBA) and Triticum vulgaris (WGA) agglutinins.
Samples
We used some paraffin-embedded samples obtained during the years 2002–2003 from six adult male Sprague–Dawley rats weighing between 250 and 300 g that were previously employed in other studies (Gómez-Santos et al. 2007, 2018). We also used samples of gall bladder, testis and intestine from our archives for the controls of deglycosylation techniques (Madrid et al. 1994). At the time we obtained the samples, we followed all the applicable international, national and institutional guidelines for the care and use of experimental animals. Samples were stored at room temperature in the dark until use.
Histochemical techniques
The lectins used are listed in Table 1. Their specificities were indicated in one of our previous works (Gómez-Santos et al. 2018) and are summarized in Table 1. We obtained 4 µm-thick sections from the paraffin-embedded samples. After removal of paraffin and hydration, we blocked the endogenous peroxidase and carried out the histochemical techniques. In addition, when digoxigenin- or biotin-labeled lectins were used, the nonspecific binding of antibodies was blocked by incubating the samples with BSA. All the procedures were performed as previously described (Gómez-Santos et al. 2018). The lectin dilutions were as follows: 6 µg/ml HPA, 20 µg/ml MPA/MPL, 25 µg/ml LFA, 10 µg/ml DSA, 30 µg/ml SNA, 5 µg/ml RCA-I, WGA and MAH, 3 µg/ml BSI-B4, 50 µg/ml DBA, PNA, LTA and SBA, 10 µg/ml AAL, UEA-I and Con A, and 60 µg/ml GNA.
Peroxidase-labeled lectins were developed with DAB. When digoxigenin-labeled lectins were used, samples were incubated with 0.6U/ml HRP-anti-DIG antibody and then the peroxidase was developed. The samples treated with biotin-labeled lectins were incubated with ABC kit before peroxidase development. Finally, we counterstained the sections with hematoxylin.
Deglycosylation pretreatments
In addition to performing histochemical techniques with each lectin, in other tissue sections the same techniques were performed after different deglycosylation procedures. The deglycosylation procedures used were chemical deglycosylation by β-elimination, which removes O-linked oligosaccharides (Ono et al. 1983), and enzymatic deglycosylation with PNGase F, which removes N-linked oligosaccharides. We performed the β-elimination procedure for every lectin in different sections for 1 and 5 days, to discriminate both labile and resistant O-glycans (Gómez-Santos et al. 2007). In these procedures, unpublished observations have shown an increase of endogenous biotin labeling by ABC kit in gastric gland cells, so in these cases it is also necessary to block endogenous biotin. The detailed procedure for β-elimination and PNGase F incubation, including blocking of endogenous biotin, has been previously described (Martínez-Menárguez et al. 1993; Gómez-Santos et al. 2018).
To determine specifically which carbohydrate was labeled by WGA, a lectin that recognizes both N-Acetylglucosamine (GlcNAc) and sialic acid (NeuAc), we also tested the lectin after removal of terminal NeuAc by acid hydrolysis. This was carried out by immersing the sections in 0.1 N HCl at 82–84 °C for 3 h before the lectin histochemical procedure (Madrid et al. 1994).
ConA can label Glucose (Glc) and Mannose (Man), thus in some sections we made a pre-incubation with 50 U/ml type III Glucose oxidase at 37 °C overnight to convert Glc into gluconic acid, which is not recognized by Con A (Alonso et al. 2006).
Controls
We used controls for all the procedures as previously reported (Gómez-Santos et al. 2018). Briefly, they were the following: (1) substitution of the lectins or the enzyme by buffer alone, (2) preincubation of the lectins with the corresponding hapten sugar inhibitor at a concentration of 0.2 M, and (3) staining of sections of other tissues of known altered binding pattern for each of the chemical and enzymatic pretreatments.
As specific control of the β-elimination procedure, we combined the deglycosylation treatment with HPA staining of rat testis sections, because this tissue loses the HPA-labeling when β-elimination works correctly (Martínez-Menárguez et al. 1993).
For deglycosylation with PNGase F, the control consisted of verifying the absence of labeling in sections of rat testicle with AAL, since incubation with PNGase F prevents the binding of this lectin (Martínez-Menárguez et al. 1993).
Semi-quantitative evaluation of the staining
For each lectin and each deglycosylation procedure, three samples were evaluated by three independent observers. They classified the staining intensity in the chief gastric cells into six categories: no labeling (0), very weak (1), weak (2), moderate (3), strong (4), and very strong (5). Sometimes a different staining intensity was observed in different sections or in different fields of the same section. When this happened, the staining intensity was classified in a range indicated with the minimum and maximum values.
Results
We analyzed the expression of glycoconjugates in the chief cells of gastric glands in the rat stomach by means of lectin histochemistry. For each lectin, in addition to the lectin histochemical staining in some histological sections, the same staining was performed in other sections after different deglycosylation methods: the β-elimination technique, which removes O-linked oligosaccharides, and incubation with the enzyme PNGase F, which removes N-linked oligosaccharides. For the lectin WGA, removal of NeuAc was performed by acid hydrolysis with HCl. Moreover, for the lectin Con A some sections were preincubated with the enzyme glucose oxidase, which converts Glc into gluconic acid.
We used 17 lectins that recognize the most abundant sugar moieties in glycoconjugates, i.e., Fucose (Fuc), N-acetylgalactosamine (GalNAc), Galactose (Gal), NeuAc, GlcNAc and Mannose (Man). The results are shown in Table 2. Below, we highlight the most important by grouping the lectins according to the sugar that they recognize.
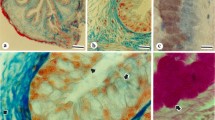
In contrast to other neighboring cell types, such as parietal cells, none of the Fuc-recognizing lectins labeled the cytoplasm of the chief cells. However, AAL clearly labeled the apical surface of these cells. This labeling remained after incubation with PNGase F, but pretreatment of the sections with a β-elimination protocol completely removed it (Fig. 1).
Histochemical staining of the rat fundic glands for Fuc-labeling lectins. AAL labeled the apical surface of the chief cells (a, arrow); the staining remained with the pretreatment with PNGAse F (b, arrow) and was absent after ß-elimination for 1 day pretreatment (c). The other two Fuc- binding lectins, LTA (d) and UEA-I (e) were negative. AAL Aleuria aurantia lectin, LTA Asparagus pea agglutinin, UEA-I Ulex europaeus agglutinin-I, pF pre-incubation with PNGase F, β1d β-elimination for 1 day. Asterisk: parietal cell. Scale bar: 20 µm
The only GalNAc-labeling lectin which labeled the cytoplasm of the chief cells was MPA/MPL. This lectin strongly labeled the supranuclear cytoplasm, corresponding to the area occupied by the secretory vesicles, in a lattice-like pattern. In addition, the apical surface of the cells was strongly labeled. The labeling was unaltered by pretreatment with PNGAse F and disappeared after β-elimination for 5 days. Chief cells were negative for the remaining GalNAc-binding lectins, except HPA, which labeled the apical membrane of the cells. This labeling remained after PNGase F pretreatment but was no longer observed after ß-elimination for 1 day procedure (Fig. 2).
GalNAc-binding lectins. HPA stained the apical surface of the chief cells when it was used alone (a, arrow) or in combination with PNGase F pretreatment (b, arrows); however this staining was not visible when ß-elimination for 1 day was used (c). DBA and SBA were negative in all cases (d, e), although DBA stained the parietal cells (d, asterisk). MPA/MPL (f) was positive in the cytoplasm and also at the apical surface (arrows) and negative after ß-elimination for 5 days (g). HPA Helix pomatia agglutinin, DBA Dolichos biflorus agglutinin, SBA soybean agglutinin, MPA/MPL Maclura pomifera lectin, pF incubation with PNGase F, β1d β-elimination for 1 day, β5d β-elimination for 5 day. Scale bar: 20 µm
The cytoplasm of the chief cells was negative for most Gal-labeling lectins (PNA, BSI-B4 and RCA-I). A weak cytoplasmic labeling was seen with PNA, but this was not detectable when β-elimination was previously carried out. Furthermore, both PNA and RCA-I labeled the apical surface of the chief cells, but this staining disappeared after β-elimination in both cases. Moreover, the chief cells showed a very weak lattice-like cytoplasmic labeling when RCA-I was performed after β-elimination for 1 day, but not after 5 days (Fig. 3).
Gal-binding lectins. PNA showed a very weak labeling in the cytoplasm (a) and a stronger staining at the apical surface (a, arrows), neither labeling was observed when the sections were pre-treated with β-elimination procedure for 1 day (b). BSI-B4 was negative (c). The apical surface of the chief cells was moderately positive to RCA-I (d, arrow) and this staining was absent after β-elimination (e, f). The cytoplasm turned from negative to RCA-I (d) to slightly positive in a lattice-like manner after β-elimination for 1 day (e) and again negative if the β-elimination pretreatment was extended to 5 days (f). PNA peanut agglutinin, BSI-B4 Bandeiraea simplicifolia-I-B4 lectin, RCA-I Ricinus communis agglutinin, β1d β-elimination for 1 day, β5d β-elimination for 5 day. Asterisks: parietal cells. Scale bar: 20 µm
LFA was the only NeuAc-binding lectin showing positive labeling in the chief cells. Labeling was detected at the supranuclear cytoplasm and it was resistant to incubation with PNGase F or β-elimination. The chief cells were negative for MAH and SNA, except when MAH staining was performed after β-elimination for 5 days, when a very weak lattice-like labeling could be seen (Fig. 4).
NeuAc-binding lectins. MAH was initially negative (a), but the cytoplasm was very slightly stained after ß-elimination for 5 days pre-treatment (b). SNA was negative (c, d), while LFA was positive in the cytoplasm in a lattice-like way (e, f). MAH Maackia amurensis hemagglutinin, SNA Sambucus nigra agglutinin, RCA-I Limax flavus agglutinin, β1d β-elimination for 1 day, β5d β-elimination for 5 day. Asterisk: parietal cell. Scale bar: 20 µm
WGA labeled the cytoplasm and the apical surface of the chief cells. This labeling was also resistant to most of the deglycosylation pre-treatments employed (including acid hydrolysis), except for β-elimination for 5 days, which reduced cytoplasmic staining and abolished apical surface staining. However, DSA, the other lectin that recognizes GlcNAc, very weakly labeled the chief cells, and this labeling was also resistant to β-elimination. After PNGase-F procedure the apical surface became very slightly positive (Fig. 5).
GlcNAc-binding lectins. WGA was positive in the cytoplasm (a) and in the apical surface (a, arrow). After PNGase-F the WGA-staining increased in the apical surface (b, arrow), and the labeling was absent in the apical surface after β-elimination for 5 days (c). DSA showed a lattice-like pattern in the cytoplasm (d) and after PNGase-F (e) and this staining increased after β-elimination for 5 days (f). WGA wheat germ agglutinin, DSA Datura stramonium agglutinin, pF incubation with PNGase F, β5d β-elimination for 5 day. Scale bar: 20 µm
Finally, both Man-binding lectins, GNA and Con A, moderately labeled the cytoplasm of the chief cells. The staining was more intense in the basal than in the supranuclear cytoplasm. When lectin histochemistry was performed after incubation with PNGase F, no labeling was seen in either case. When samples were pre-treated with Glucose-oxidase, the basal cytoplasm Con A-labeling was absent or very weak, but the supranuclear cytoplasm and the apical surface of the chief cells appeared labeled instead (Fig. 6).
Man-binding lectins. GNA and Con A were positive, mainly in the basal cytoplasm (a, d) and this staining was absent after PNGase-F (b, e); however β-elimination for 5 days did not modify it (c). Pretreatment with Glucose oxidase removed the staining to Con A in the basal cytoplasm (f) while the apical surface was slightly positive (f, arrows). GNA Galanthus nivalis agglutinin, Con A Concanavalin A agglutinin, pF incubation with PNGase F, β5d β-elimination for 5 day, GO incubation with Glucose oxidase. Scale bar: 20 µm
Discussion
In this work we examined the expression of glycoconjugates in the rat gastric chief cells by means of lectin histochemistry alone and in combination with deglycosylation pre-treatments: β-elimination to remove O-linked oligosaccharides (Ono et al. 1983) and incubation with PNGase F to remove N-linked glycans (Martínez-Menarguez et al. 1993). Beta-elimination was carried out for each lectin in different sections for 1 and 5 days, to discriminate between labile and resistant O-linked oligosaccharides (Gómez-Santos et al. 2007). In the case of WGA histochemistry, it was also carried out after acid hydrolysis with HCl, which removes NeuAc, to distinguish whether this lectin recognized GlcNAc or NeuAc (Madrid et al. 1994). Finally, Con A, which can bind to Man or Glc, was also tested after incubation with glucose oxidase to convert Glc into gluconic acid, which is not labeled by the lectin (Alonso et al. 2006).
We can simplify the results obtained with lectins into three labeling patterns: (1) lectin did not label any cell structure, (2) lectin stained relatively large areas of the internal cytoplasm, and (3) the apical cell surface was positive for lectin. The last two staining patterns could be observed with some of the 17 tested lectins.
The first observed pattern, i.e. the absence of labeling, was shown by several lectins that recognize Fuc, GalNAc, Gal or NeuAc. Other lectins that also recognize these sugars showed one or both of the other two labeling patterns before mentioned. This discrepancy between lectins recognizing the same carbohydrate can be explained by the different affinities displayed by each of the lectins towards different carbohydrate sequences summarized in Table 1.
The second staining pattern, the labeling of the internal cytoplasm, with stronger or weaker intensity, was observed with only a few of the lectins that bind to Fuc, GalNac, Gal or NeuAC and with all those that recognize GlcNAc and Man. Among the lectins that recognize Fuc, GalNAc, Gal or NeuAc, only MPA/MPL and LFA stained the internal cellular cytoplasm with some intensity. Labeling with MPA/MPL disappeared after β-elimination for 5 days, indicating the presence of GalNAc in resistant O-linked oligosaccharides (Gómez-Santos et al. 2007). The LFA staining did not disappear with any of the deglycosylation pre-treatments, which indicates that NeuAc may be present in both N- and O-linked oligosaccharides. Both MPA/MPL and LFA labeling showed a lattice-like pattern in the supranuclear cytoplasm, which corresponds to the cell region typically occupied by the secretory granules.
WGA can label to GlcNAc or NeuAC. Removal of NeuAc by acid hydrolysis did not alter the WGA-labeling suggesting that WGA is recognizing GlcNAc. This labeling decreased but did not completely disappear after β-elimination. On the contrary, the weak labeling seen after histochemistry with DSA slightly increased after β-elimination. All these results suggest that lectins are recognizing different GlcNAc moieties, some in N- and other in O-linked oligosaccharides.
We used two lectins, GNA and Con A, that recognize Man. Con A can also bind to Glc. Both lectins stained the basal and supranuclear cytoplasm of the chief cells. When histochemistry was performed with Con A after incubation with glucose oxidase, the cells remained positive, with minimal differences in the pattern of labeling, suggesting that Con A may be identifying mostly Man residues. Although most Man residues are usually located in N-linked oligosaccharides, Man has also been described in O-linked oligosaccharides (Varki and Kornfeld 2017). Our results showed that removal of the N-linked oligosaccharides by PNGase F incubation turned the gastric chief cells negative for the two Man-labeling lectins, indicating that most of the Man in chief cells was located in N-linked oligosaccharides.
Most lectins that labeled the cytoplasm stained the supranuclear region, occupied by the Golgi apparatus and secretory granules. Usually, this labeling showed a lattice-like staining pattern, which was apparently due to the fact that the cytoplasm surrounding the secretion granules was positive but the granules themselves were negative. Comparing the results obtained in the chief cells with those obtained in a previous work on the mucus-producing cells of the gastric glands, it can be pointed out that the chief cells show a lower content of oligosaccharides (Gómez-Santos et al. 2018). In addition, lectins usually label the secretion granules in mucous cells, whereas in the chief cells the granules were rarely stained. Surely, this is related to the type of secretion produced by each cell type. Whereas mucous cells have a mucin-rich secretion, which are highly glycosylated proteins, the chief cells secrete mostly zymogens, which are proteins with few or no glycosylation and once secreted they are converted into digestive enzymes (Baudyš and Kostka 1983; Dekker et al. 1989; Kageyama 2002; Holmén Larsson et al. 2013). The degree of glycosylation of zymogenic proteins from the gastric chief cells is diverse. Some of them are somewhat glycosylated, whereas others lack glycans. Even the same protein can be glycosylated in some species and not glycosylated in others (Kageyama and Takahashi 1978; Green and Roberts 2004; Hassam et al. 2010).
One of the highly glycosylated zymogen proteins secreted by gastric chief cells is gastric lipase (Barrowman and Darton 1970; Moreau et al. 1989). In particular, it has been suggested that 10–15% of the human gastric lipase mass is due to oligosaccharides, probably N-glycans (Wicker-Planquart et al. 1999). Since lectins recognizing mannose stained the cytoplasm surrounding the granules, but not the granules themselves, we cannot conclude that the staining is due to recognition of gastric lipase. Zymogenic protein expression by chief cells is known to be clinically relevant. For example, the expression of progastriscin, one of the main proteolytic enzymes present in the gastric fluid, has been shown to gradually decrease from the healthy stomach to benign lesions to precancerous lesions, and finally to gastric cancer (Hassam et al. 2010).
Only the Man-recognizing lectins labeled with some intensity the basal cytoplasm, where the rough endoplasmic reticulum is typically located in chief cells. These results are consistent with the fact that mannosylation occurs mostly in the rough endoplasmic reticulum, whereas other sugar moieties are added in the Golgi apparatus, (Stanley et al. 2017).
Finally, some lectins (AAL, HPA, MPA/MPL, PNA, RCA-I and WGA) labeled the apical surface of the chief cells, probably because they were bound to glycoconjugates of the apical cell membrane. This labeling was absent when O-linked oligosaccharides were removed before lectin staining. This is consistent with what was previously observed in immunohistochemical studies to identify mucin 1 (MUC1). These studies showed a diffuse staining in the cytoplasm, which was more distinguishable in the apical membrane of the cells (Saeki et al. 2014). MUC1 is a transmembrane protein that has a VNTR (variable number of tandem repeat) region rich in serine, threonine and proline that is highly O-glycosylated. O-linked oligosaccharides of mucins can be diverse. The simplest mucin O-glycan is a single N-acetylgalactosamine linked to serine or threonine, which can be recognized by MPA/MPL. The most common O-linked oligosaccharide in mucins is Galβ(1–3)GalNAc, which can be labeled by HPA, MPA/MPL and PNA. Other common O-glycans in mucins can be recognized by lectins that labeled the apical cell membrane of chief cells (Brockhausen and Stanley 2017). MUC1 has a prominent role as a barrier against external insults to the gastric cells, but its expression changes during pathological events: the MUC1 gene is silenced in intestinal metaplasia, a pre-neoplastic lesion, but is reactivated in gastric cancer. Furthermore, MUC1 is regarded as an oncoprotein because it suppresses the transcription of the p53 gene, a major tumor suppressor gene (Wei et al. 2007; Saeki et al. 2014). Our lectin-labeling results are consistent with the presence of MUC1 in the apical membrane of chief gastric cells. Moreover, this lectin histochemistry method could be clinically used as a prognostic tool to monitor early changes in the expression of mucins in the gastric epithelial surface, and correlate these changes with the development of cellular events contributing to gastric cancer. Experiments with gastric cancer patient samples have already pointed out an aberrant expression of glycoconjugates and the altered binding to some lectins as potential prognostic factors for this disease (Li et al. 2016).
In conclusion, we have demonstrated the presence of different glycoconjugates in the chief cells of the fundic glands of the rat stomach by means of a battery of 17 lectins, coupled to different deglycosylation treatments. Many of the lectins did not stain chief cells, and only a few of them stained some regions of the cytoplasm, suggesting a low level of glycosylation, especially when comparing to gastric mucous cells. Most of the labeling obtained with the lectins was observed in the apical membrane of the chief cells and could be unequivocally attributed to O-glycans. This labeling can be explained by the presence of MUC1 and possibly other mucins expressed by chief cells. Labeling of Man-binding lectins at the cytoplasmic region where rough endoplasmic reticulum is located would reflect the N-glycosylation of some secretory proteins. It could be very interesting to compare these lectin binding patterns of the chief cells in the healthy gastric mucosa with those of precancerous and cancerous lesions, to test this methodology as a possible tool for gastric cancer prognosis.
References
Alonso E, Gómez L, Madrid JF, Sáez FJ (2006) Identification of mannose moieties in N- and O-linked oligosaccharides of the primordial germ cells of Xenopus embryos. Microsc Res Tech 69:595–599. https://doi.org/10.1002/jemt.20318
Barrowman JA, Darnton S (1970) The lipase of rat gastric mucosa. Gastroenterology 59:13–21. https://doi.org/10.1016/S0016-5085(19)33786-2
Baudyš M, Kostka V (1983) Covalent structure of chicken pepsinogen. Eur J Biochem 136:89–99. https://doi.org/10.1111/j.1432-1033.1983.tb07709.x
Brockhausen I, Stanley P (2017) O-GalNAc Glycans. In: Varki A, Cummings RD, Esko JD et al (eds) Essentials of glycobiology [Internet], 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor. Chapter 10. https://www.ncbi.nlm.nih.gov/books/NBK453030/. https://doi.org/10.1101/glycobiology.3e.010
Brooks SA, Hall DM (2012) Lectin histochemistry to detect altered glycosylation in cells and tissues. Methods Mol Biol 878:31–50. https://doi.org/10.1007/978-1-61779-854-2_2
Cummings RD, Darvill AG, Etzler ME, Hahn MG (2017) Glycan-recognizing probes as tools. In: Varki A, Cummings RD, Esko JD et al (eds) Essentials of glycobiology [Internet], 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2015–2017. Chapter 48. https://www.ncbi.nlm.nih.gov/books/NBK453096/. https://doi.org/10.1101/glycobiology.3e.048
Dekker J, Van Beurden-Lamers WMO, Strous GJ (1989) Biosynthesis of gastric mucus glycoprotein of the rat. J Biol Chem 264:10431–10437
Duarte HO, Freitas D, Gomes C, Gomes J, Magalhães A, Reis CA (2016) Mucin-type O-glycosylation in gastric carcinogenesis. Biomolecules 6:33. https://doi.org/10.3390/biom6030033
Green J, Roberts RM (2004) Pepsin F. In: Barrett AJ, Rawlings ND, Woessner JF (eds) Handbook of proteolytic enzymes, Aspartic and metallo peptidases, vol 1, 2nd edn. Elsevier Academic Press, London, pp 137–139
Gómez-Santos L, Alonso E, Ferrer C, Zuasti A, Sáez FJ, Madrid JF (2007) Histochemical demonstration of two subtypes of O-linked oligosaccharides in the rat gastric glands. Microsc Res Tech 70:809–815. https://doi.org/10.1002/jemt.20465
Gómez-Santos L, Alonso E, Díaz-Flores L, Madrid JF, Sáez FJ (2018) Different glycoconjugate content in mucus secreing cells of the rat fundic gastric glands. Anat Rec 301:2128–2144. https://doi.org/10.1002/ar.23892
Hassam I, Toor A, Ahmad F (2010) Progastriscin: Structure, function, and its role in tumor progression. J Mol Cell Biol 2:118–127. https://doi.org/10.1093/jmcb/mjq001
Hoffmann W (2013) Self-renewal of the gastric epithelium from stem and progenitor cells. Front Biosci Schol Ed 5:720–731. https://doi.org/10.2741/s402
Holmén Larsson JM, Thomsson KA, Rodríguez-Piñeiro AM, Karlsson H, Hansson GC (2013) Studies of mucus in mouse stomach, small intestine, and colon. III. Gastrointestinal Muc5ac and Muc2 mucin O-glycan patterns reveal a regiospecific distribution. Am J Physiol Gastrointest Liver Physiol 305:G357–G363. https://doi.org/10.1152/ajpgi.00048.2013
Jin C, Kenny DT, Skoog EC et al (2017) Structural diversity of human gastric mucins glycans. Mol Cell Proteom 16:743–758. https://doi.org/10.1074/mcp.M116.067983
Kageyama T (2002) Pepsinogens, prograstricsins, and prochymiosins: structure, function, evolution, and development. Cell Mol Life Sci 59:288–306. https://doi.org/10.1007/s00018-002-8423-9
Kageyama T, Takahashi K (1978) Monkey pepsinogens and pepsins. III. Carbohydrate moiety of Japanese Monkey pepsinogens and the amino acid sequence around the site of its attachment to protein. J Biochem 84:771–778
Li X, Guan F, Li D, Tan Z, Yang G, Wu Y, Huang Z (2016) Identification of aberrantly expressed glycans in gastric cancer by integrated lectin microarray and mass spectrometric analyses. Oncotarget 7:87284–87300. https://doi.org/10.18632/oncotarget.13539
Madrid JF, Ballesta J, Castells MT, Hernández F (1990) Glycoconjugate distribution in the human fundic mucosa revealed by lectin and glycoprotein-gold cytochemistry. Histochemistry 95:179–187. https://doi.org/10.1007/bf00266591
Madrid JF, Castells MT, Martínez-Menárguez JA, Avilés M, Hernández F, Ballesta J (1994) Subcellular characterization of glycoproteins in the principal cells of human gallbladder. A lectin cytochemical study Histochemistry 101:195–204. https://doi.org/10.1007/bf00269544
Madrid JF, Leis O, Díaz-Flores L, Hernández F (1998) Secretion of fucosylated oligosaccharides related to the H antigen by human gastric cells. Histochem Cell Biol 110:295–301. https://doi.org/10.1007/s004180050291
Martínez-Menárguez JA, Avilés M, Madrid JF, Castells MT, Ballesta J (1993) Glycosylation in Golgi apparatus of early spermatids of rat. A high resolution lectin cytochemical study. Eur J Cell Biol 61:21–33
Mills JC, Goldenring JR (2017) Metaplasia in the stomach arises from gastric chief cells. Cell Mol Gastroenterol Hepatol 4:85–88. https://doi.org/10.1016/j.jcmgh.2017.03.006)
Moreau H, Bernadac A, Gargouri Y, Benkouka F, Laugier R, Verger R (1989) Immunocytolocalization of human gastric lipase in chief cells of the fundic mucosa. Histochemistry 91:419–423. https://doi.org/10.1007/BF00493829
Mulloy B, Dell A, Stanley P, Prestegard JH (2017) Structural analysis of glycans. In: Varki A, Cummings RD, Esko JD et al (eds) Essentials of glycobiology [Internet], 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY), 2015–2017. Chapter 50. https://www.ncbi.nlm.nih.gov/books/NBK453059/. https://doi.org/10.1101/glycobiology.3e.050
Ono K, Katsuyama T, Hotxhi M (1983) Histochemical application of mild alkaline hydrolysis for selective elimination of O-glycosidically linked glycoproteins. Stain Technol 58:309–312. https://doi.org/10.3109/10520298309066803
Rodríguez-Piñeiro AM, Bergström JH, Ermund A et al (2013) Studies in mouse stomach, small intestine, and colon. II. Gastrointestinal mucus proteome reveals Muc2 and Muc5A accompanied by a set of core proteins. Am J Physiol Gastrointest Liver Physiol 305:G348–G356. https://doi.org/10.1152/ajpgi.00047.2013
Saeki N, Sakamoto H, Yoshida T (2014) Mucin 1 gene (MUC1) and gastric-cancer susceptibility. Int J Mol Sci 15:7958–7973. https://doi.org/10.3390/ijms15057958
Stanley P, Taniguchi N, Aebi M (2017) N-Glycans. In: Varki A, Cummings RD, Esko JD et al (eds) Essentials of glycobiology [Internet], 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2015–2017. Chapter 9. https://www.ncbi.nlm.nih.gov/books/NBK453020/. https://doi.org/10.1101/glycobiology.3e.009
Su L, Hasui K, Sueyoshi K et al (2004) Expression of mucins in the human fetal and neonatal stomach. Acta Histochem Cytochem 37:163–172. https://doi.org/10.1267/ahc.37.163
Varki A, Kornfeld S (2017). Historical background and overview. In: Varki A, Cummings RD, Esko JD et al (eds) Essentials of glycobiology [Internet], 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2015–2017. Chapter 1. https://www.ncbi.nlm.nih.gov/books/NBK316258/. https://doi.org/10.1101/glycobiology.3e.001
Varki A, Gagneux P (2017) Biological functions of glycans. In: Varki A, Cummings RD, Esko JD et al (eds) Essentials of glycobiology [Internet], 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2015–2017. Chapter 7. https://www.ncbi.nlm.nih.gov/books/NBK453034/. https://doi.org/10.1101/glycobiology.3e.007
Varki A, Kannagi R, Toole B, Stanley P (2017) Glycosylation changes in cancer. In: Varki A, Cummings RD, Esko JD et al (eds) Essentials of glycobiology [Internet], 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2015–2017. Chapter 47. https://www.ncbi.nlm.nih.gov/books/NBK453023/. https://doi.org/10.1101/glycobiology.3e.047
Wei X, Xu H, Kufe D (2007) Human mucin 1 oncoprotein represses transcription of the p53 tumor suppressor gene. Cancer Res 67:1853–1858. https://doi.org/10.1158/0008-5472.CAN-06-3063
Wicker-Planquart C, Canaan S, Riviere M, Dupuis L (1999) Site-directed removal of N-glycosylation sites in human gastric lipase. Eur J Biochem 262:644–651. https://doi.org/10.1046/j.1432-1327.1999.00427.x
Acknowledgements
Mrs M. J. Fernández and Mrs C. Tobillas contributed to sample preparation. Mrs M. J. Aldasoro helped us with administrative work. This work was supported by the University of the Basque Country UPV/EHU (Grant numbers: EHUA13/15, EHUA15/26); and Fundación Séneca, Comunidad Autónoma de la Región de Murcia (Grant number 04542/GERM/06).
Funding
The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
LGS: conceptualization, formal analysis, investigation, data curation, writing—review and editing, funding acquisition. EA: formal analysis, investigation, data curation, funding acquisition. OC: investigation, writing—review and editing. GI: investigation, writing—review and editing. JFM: conceptualization, methodology, data curation, writing—review and editing, supervision, funding acquisition. FJS: conceptualization, methodology, writing–original draft, visualization, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gómez-Santos, L., Alonso, E., Crende, O. et al. Identification of sugar moieties in chief cells of the rat fundic gastric glands. Anat Sci Int 96, 221–230 (2021). https://doi.org/10.1007/s12565-020-00578-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-020-00578-4