Abstract
Coronary vessel development has been investigated in avian and mouse embryonic hearts. Quail embryos are a useful tool to examine vascular development, particularly because the QH1 antibody and transgenic quail line, Tg (tie1:H2B-eYFP), are useful to trace endothelial cells. However, there are only a few descriptions of the quail coronary vessels. Using ink injection coronary angiography, we examined the course of coronary vessels in the fetal quail heart. The major coronary arteries were the right and left septal arteries, which, respectively, branched off from the right and left coronary stems. The right septal artery ran posteriorly (dorsally) and penetrated the ventricular free wall to distribute to the posterior surface of the ventricles. The left septal artery ran anteriorly (ventrally) and penetrated the ventricular free wall to distribute to the anterior surface of the ventricles. The right and left circumflex arteries were directed posteriorly along the atrioventricular sulci. The cardiac veins consisted of three major tributaries: the middle, great, and anterior cardiac veins. The middle cardiac vein ascended along the posterior interventricular sulcus and emptied into the right atrium. The great cardiac vein ran along the anterior interventricular sulcus, entered the space between the left atrium and conus arteriosus and emptied into the right atrium behind the aortic bulb. The anterior cardiac vein drained the anterior surface of the right ventricle and connected to the anterior base of the right atrium. The course of coronary vessels in the quail heart was basically the same as that observed in chick but was different from those of mouse and human.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coronary circulatory system is essential to maintain nutrients and oxygen provision for the cardiac muscle to function throughout life. Coronary circulation starts immediately after metamorphosis, during which embryos change their anatomy and physiology to form those of fetuses. At this time, the way oxygen is supplied to the cardiac muscle changes dramatically, i.e., diffusion from the endocardium is replaced by the adult coronary circulatory system consisting of coronary arteries and veins. Impaired development of the coronary vessels leads to life-threatening defects, including aberrant origins of the coronary artery and coronary arteriovenous fistula (Perez-Pomares et al. 2016). Coronary arteriosclerosis causes ischemic heart disease in adult life. Revascularization of the ischemic or infarcted lesions is a potential therapeutic strategy to restore cardiac contractility. Therefore, it is important to understand the mechanisms regulating coronary vessel formation in order to develop the revascularization therapies (Sedmera and Watanabe 2006).
The development of the coronary system has been examined extensively in avian and mouse models (Reese et al. 2002; Tian et al. 2015). In avian models, the proepicardial organ, which consists of surface mesothelium and a sinus venosus-derived inner core mesenchyme, generates the coronary vascular system of the ventricular free wall (Kamimura et al. 2018). Genetic tracing experiments demonstrated that the endothelial cells lining the sinus venosus and ventricles are the major sources of coronary vascular endothelium. Although the origin of coronary vessels has been concluded, the mechanisms leading to coronary vessel patterning as well as arteriovenous segregation processes are largely unknown (Nakajima and Imanaka-Yoshida 2013; Tian et al. 2015).
Mammalian and avian hearts have two coronary artery systems, in which the right and left coronary arteries originate from the right and left aortic sinuses, respectively. However, the patterns and courses of coronary vessels are very different between species. In human hearts, coronary arteries course along the interventricular and coronary sulci subjacent to the epicardium, whereas mouse coronary arteries course intramurally. In birds, the major branches of coronary artery are the septal arteries that branch off from the coronary stem, but there exist some differences in coronary patterning between birds (Myczkowski, 1960; Lindsay and Smith 1965; Bezuidenhout 1984). Quail embryonic hearts are a useful tool to examine vascular development, because the quail endothelium-specific antibody QH1 and transgenic quail model, Tg (tie1:H2B-eYFP), are available to trace endothelial cells (Pardanaud et al. 1987; Sato and Lansford 2013). However, there have been few published studies presenting the actual macroscopic anatomy of the quail coronary vessels (Fitzgerald 1969). In the present report, using ink injection coronary angiography, we observed the origin and course of coronary arteries and veins in fetal quail heart. The major coronary arteries were the right and left septal arteries, which respectively branched off from the right and left coronary stems. Cardiac veins were classified into three major veins including the middle, great, and anterior cardiac veins according to their opening into the right atrium.
Materials and methods
Fertilized quail eggs (Quail COSMOS, Toyohashi, Japan) were incubated at 37.5°C and 60% humidity. Embryos were staged by days of incubation (embryonic day, E) using the Hamburger and Hamilton system (1951) adapted for quail (Ainsworth et al. 2010), and E6–14 (stage 29–43) hearts were examined. The number of embryos examined is tabulated in Table 1. Eggs were placed on ice for 30 min to stop the heart-beat, the embryo was extirpated and placed in ice-cooled phosphate buffered saline (PBS). The thoracic cavity was opened, then a pulled glass needle was inserted into the ascending aorta, and 4% paraformaldehyde (PFA)/PBS was gently injected. To observe the coronary artery, 20% carbon ink (PLATINUM, Tokyo, Japan) in PBS was injected into the ascending aorta. The resulting hearts were extirpated and re-fixed in 4% PFA/PBS at 4°C for more than 12 h. The fixed hearts were immersed in a graded series of glycerin/0.5% potassium hydroxide (1:3, 15 min; 1:1, 30 min; 3:1, 2 h) for transparency. To observe the cardiac vein, 20% carbon ink/2% gelatin in PBS was injected into the ascending aorta; after the cardiac veins connecting to right atrium were visualized, the hearts were removed and re-fixed in ice-cooled 4% PFA/PBS for more than 48 h. Gelatin was used to keep the carbon ink from diffusing out of the cardiac vein. Samples were observed using a stereoscopic microscope and photographed. Animal handling and procedures were approved by the Osaka City University Animal Care and Use Committee, as set forth in the NIH Guide for the Care and Use of Laboratory Animals (eighth edition).
Results
Coronary arteries
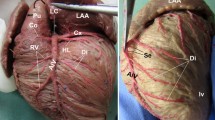
The right coronary stem, which originated from the right aortic sinus, gave off surface and deep branches. The surface arteries included the conus branch (cb in Fig. 1a), subepicardial branches of the right ventricular free wall (rvb in Fig. 1a), and the right circumflex branch (cx in Fig. 1a). The right circumflex branch ran along the right coronary sulcus. It gave off right atrial branches along the pectinate muscles as well as terminal branches on the right lateral surface of the right ventricle. The deep artery—the right septal artery—was thicker than the right circumflex artery (sa in Fig. 1b). The right septal artery pierced the supraventricular crista to enter into the interventricular septum (right side of the yellow dotted line in Fig. 1b and gray area in Fig. 1b′) and gave off branches to the posterior (dorsal) aspect of the interventricular septum and apex. The peripheries of the right septal artery penetrated the ventricular free wall transmurally at the posterior interventricular sulcus and distributed on the posterior surface of both ventricles and the apex (small arrows in Fig. 1c).
Right and left coronary arteries. a Surface branches of the right coronary artery include the conus branch, branches on the right ventricle, and right circumflex branch. b Right septal artery. The right ventricular free wall was removed to show the surface of the ventricular septum (right side of the dotted line in b and gray color in b′). c Peripheral branches of the right septal artery penetrate the ventricular wall at the posterior interventricular sulcus (arrows) and distribute onto the posterior surface of the ventricle. d Surface branches of the left coronary artery including the interatrial branch, conus branch, and left circumflex branch. e Left septal artery. The right ventricular free wall was removed to show the surface of the ventricular septum (left side of the dotted line in e and gray color in e′). Peripheries of the left septal artery penetrated the ventricular free wall to distribute onto the left ventricular surface (arrows). a, d E9.0 (stage 36–37) heart; b, c, e E12.0 (stage 42). Ao Aorta, cb conus branch, cx circumflex branch, iab interatrial branch, LV left ventricle, Pt pulmonary trunk, RA right ventricle, rvb branches on the right ventricle, sa septal artery. Bar 500 μm
The left coronary stem, which originated from the left aortic sinus, gave off the interatrial branch, left septal artery, left conus branch, and left circumflex branch. The left circumflex branch ran into the left coronary sulcus (cx in Fig. 1d) and terminated at the left posterolateral wall of the ventricle. The deep thick artery, the left septal artery, pierced the infundibulum to enter the interventricular septum (sa in Fig. 1e) and gave off branches toward the anterior (ventral) aspect of the interventricular septum and apex (left side of the yellow dotted line in Fig. 1e and gray area in Fig. 1e′). The peripheries of the left septal artery penetrated the ventricular free wall and distributed onto the surface of the left ventricular free wall and apex (small arrows in Fig. 1d, e).
Cardiac veins
Cardiac veins were classified into three major veins according to their openings into the right atrium, i.e., middle cardiac vein, great cardiac vein, and anterior cardiac vein. The middle cardiac vein was the largest vein and was observed in all samples examined (20/20) (mcv in Fig. 2a). The middle cardiac vein, which drained from the posterior surface of both ventricles including the apex, ran along the posterior interventricular sulcus and emptied into the base of the left precaval vein (left superior vena cava) before E9 (stage 36–37) and emptied into the sinus venosus of the right atrium after E10 (stage 38–39). The left circumflex vein (cx in Fig. 2a), which originated from the left lateral wall of the left ventricle and passed into the left coronary sulcus, was observed in 14 hearts (14/20) and terminated at the left precaval vein (5/14), right atrium (5/14) or middle cardiac vein (4/14). Small cardiac veins were observed in 5 (5/20) hearts and joined into the middle cardiac vein (scv in Fig. 2a).
Cardiac veins. a Middle cardiac vein. In this sample, the middle cardiac vein receives the left circumflex vein and small cardiac vein. b The great cardiac vein consists of the anterior interventricular segment and its downstream basal segment. The basal segment runs between the left atrium and great arteries to empty the right atrium behind the aortic bulb (open arrowhead). c Anterior cardiac vein drains from the anterior right ventricular wall and empties into the base of the right auricle (open arrowhead). a E7.25 (stage 32) heart; b E8.0 (stage 35); c E7.0 (stage 32); acv anterior cardiac vein, aivs anterior interventricular segment of great cardiac vein, Ao aorta, bs basal segment of great cardiac vein, cx left circumflex vein, LV left ventricle, Pt pulmonary trunk, RV right ventricle, scv small cardiac vein, mcv middle cardiac vein. Bar 500 μm
The great cardiac vein consisted of two major segments, the anterior interventricular segment and a downstream basal segment. The anterior interventricular segment ascended along the left lateral side of the anterior interventricular sulcus (aivs in Fig. 2b) and entered the space between the pulmonary trunk and left auricle to continue the basal segment (bs in Fig. 2b). The basal segment ran along the anterior basal rim of the left atrium (accompanying the left coronary artery stem) and finally emptied into the right atrium behind the base of the ascending aorta (open arrowhead in Fig. 2b). In 2 of 18 hearts, no apparent interventricular segment was observed. The left conus vein was observed in all samples with a visible great cardiac vein (18/18). A direct anastomosis between the great cardiac vein and left circumflex vein was detected in six samples (6/18).
The anterior cardiac vein, which drained from the anterior wall of the right ventricle via several tributaries and the right conus vein, emptied into the base of the right auricle (acv and open arrowhead in Fig. 2c). The opening of the anterior cardiac vein had a single orifice (13/16; three openings were found in one sample and undetectable in two samples).
Discussion
Coronary artery
In quail hearts, the main coronary arteries were the right and left septal arteries, which branched off from the right and left coronary stems, respectively, and penetrated the supraventricular crista and infundibulum to enter the interventricular septum. These deep arteries distributed into the interventricular septum, subsequently penetrated the ventricular free wall to exit into the subepicardial space, and distributed into the ventricular surface and apex (Fig. 3a). The anatomy of quail coronary arteries resembled chick and phalanger (a marsupial, Dowd 1974) but were different from monotremes (Dowd 1969), rodents and human hearts (Halpern 1957; Fernandez et al. 2008; Loukas et al. 2009a).
Schematic showing the coronary arteries and cardiac veins of the fetal quail heart. a The major coronary arteries are the right and left septal arteries (rsa and lsa), which originate from the right and left coronary stems, respectively. The right septal artery pierces the supraventricular crista and left septal artery infundibulum to enter the ventricular septum (gray color). Peripheries of the right and left septal arteries penetrate the ventricular free wall at the posterior and anterior interventricular sulci, respectively, and distribute over the ventricular surface. b The cardiac veins consist of three major veins according to their openings into the right atrium, the middle cardiac vein, great cardiac vein, and anterior cardiac vein. The most characteristic feature of the quail cardiac veins is the course of the great cardiac vein, which ascends along the anterior interventricular sulcus (anterior interventricular segment) and then courses between the left atrium and great arteries (basal segment) to open into the right atrium. Note that a shows the ventricular septum seen form the right ventricle after removing the right ventricular free wall and b shows the cardiac base after removing the atria. acv Anterior cardiac vein, aivs anterior interventricular segment of the great cardiac vein, bsbasal segment of the great cardiac vein, Ao aorta, lcx left circumflex branch, lsa left septal artery, mcv middle cardiac vein, MV mitral valve, Pt pulmonary trunk, rcx right circumflex branch, rsa right septal artery, TV tricuspid valve
In mice, the right and left coronary arteries originate from the right and left aortic sinuses, respectively, and course intramurally immediately after originating. The right coronary artery runs into the right coronary sulcus, giving off an acute marginal branch and turns obliquely toward the apex as a dorsal interventricular branch in C57BL/6 strains (Fernandez et al. 2008) or right ventricular distal branch in Swiss albino mouse (Yoldas et al. 2010). The septal artery predominantly branches off from the right coronary stem in several mice, including Balb/c, Swiss albino, and wild mice, whereas the septal artery originates evenly from the right, left or both coronary stems in strain C57BL/6 (Lopez-Garcia et al. 2016). The left coronary artery gave off two major branches, the left circumflex artery in the left coronary sulcus terminating at the crux and the obtuse branch along the obtuse margin to the apex in strain C56BL/6 (Fernandez et al. 2008), whereas it was the paraconal interventricular branch in Swiss albino mouse (Yoldas et al. 2010). These observations indicated that the fundamental patterns/courses of coronary arteries are distinct between not only species but also strains. Unusual anatomical variations/anomalies including a single ostium, high take-off, and aortic intramural course occur with relatively high incidence in certain strains (Lopez-Garcia et al. 2016). Therefore, knowledge of the species/strain-specific topographical coronary anatomy is necessary to investigate coronary biology.
Cardiac vein
The quail cardiac vein system consisted of three cardiac veins according to their openings into the right atrium: the middle cardiac vein, great cardiac vein, and anterior cardiac vein (Fig. 3). The patterns of quail cardiac veins were similar to those observed in chick hearts (Lindsay 1967). The most characteristic feature of the quail cardiac veins was the course of the great cardiac vein. In this vein, the anterior interventricular segment ascended the lateral aspect of the interventricular sulcus followed by the basal segment. The basal segment ran between the left atrium and conus arteriosus and opened solely into the right atrium behind the aortic bulbs. This type of great cardiac vein is commonly observed in chicks [78% (60/78) Lindsay 1967], monotremes (Dowd 1969), and phalangers (marsupials) (Dowd 1974), but rarely in pigs (Alejandro Gomez et al. 2015) and humans [0.3% (1/337), Kawashima et al. 2003]. The middle cardiac vein is the largest cardiac vein in birds, and it drains from the apex and posterior ventricular surface, ascends along the interventricular sulcus, and opens into the right atrium. The middle cardiac vein is commonly observed in birds as well as in mammals, suggesting that the middle cardiac vein is conserved across species. The anterior cardiac vein drained the anterior free wall of the right ventricle via the right conal vein and several tributaries of the anterior ventricular wall. These tributaries united into a single canal to open the anterior basal rim of the right auricle. The course and opening of the quail anterior cardiac vein resembled with those in chicks and humans, in which one (most common) to three anterior cardiac veins receive tributaries from the right ventricular free wall, empty the right atrium via the luminal vein (Lindsay 1967; von Lüdinghausen 1987; Loukas et al. 2009b).
The course of cardiac veins in the mouse heart resembles that in rats, but is different from that in humans or quails (Ciszek et al. 2007; Kresakova et al. 2015). In mouse cardiac veins, three major cardiac veins emptying to the coronary sinus have been identified. These include the left cardiac vein (the largest cardiac vein and comparable to the human left marginal vein), caudal vein (middle cardiac vein), and right cardiac vein (right marginal vein). There is no great cardiac vein running in the anterior interventricular sulcus. The remarkable features of the mouse/rat cardiac vein are the conal veins, in which the right conal vein courses on the ventral aspect of the left ventricular outflow tract and opens into the right atrium, and the left conal vein runs behind the pulmonary trunk and connects with the right conal vein, right atrium, or right cranial caval vein. Therefore, the conal veins surrounding the great arteries are similar to the peritruncal endothelial plexus (peritruncal ring) observed in embryonic hearts (Ando et al. 2004). It may be suggested that the left conal vein passing behind the great arteries (pulmonary trunk and aortic bulb) is equivalent to the basal segment of the great cardiac vein in avian species.
Coronary arteriovenous fistula
In our observations, carbon ink injected into the aorta in E7–9 (stage 32–37) hearts delineated directly not only the coronary arteries but also the major cardiac veins without apparent staining of the ventricular free wall. This observation suggested direct connections between the coronary arteries and veins at the onset of and immediately after coronary circulation starts. The direct connection between the major coronary arteries and veins was consistent with the observations that the peritruncal endothelial plexus surrounding the outflow tract remodels into the main coronary arteries and cardiac veins (Vrancken Peeters et al. 1997; Tomanek et al. 2006). The direct connection was not evident from E10 (stage 38–39) onward. Therefore, the capillary beds interposing the arteries and veins may rapidly develop after the coronary circulation starts. Coronary arteriovenous fistula (a type of congenital heart defect), in which the right coronary artery connect the right atrium without a capillary bed (Perez-Pomares et al. 2016), may occur due to the persistence of the direct connection of the coronary artery and cardiac vein.
We described the topographical anatomy of the quail coronary arteries and cardiac veins. The major coronary arteries of the quail heart are the right and left septal arteries, which originate from the right ant and left coronary stems, respectively. The cardiac veins are classified into the middle, great, and anterior cardiac veins according to their openings into the right atrium. The course of the quail coronary vessels was basically the same as that in chick heart but was different from mouse and human hearts.
References
Ainsworth SJ, Stanley RL, Evans DJ (2010) Developmental stages of the Japanese quail. J Anat 216:3–15
Alejandro Gomez F, Ballesteros LE, Stella Cortes L (2015) Morphological description of great cardiac vein in pigs compared to human hearts. Braz J Cardiovasc Surg 30:63–69
Ando K, Nakajima Y, Yamagishi T, Yamamoto S, Nakamura H (2004) Development of proximal coronary arteries in quail embryonic heart: multiple capillaries penetrating the aortic sinus fuse to form main coronary trunk. Circ Res 94:346–352
Bezuidenhout AJ (1984) The coronary circulation of the heart of the ostrich (Struthio camelus). J Anat 138:385–397
Ciszek B, Skubiszewska D, Ratajska A (2007) The anatomy of the cardiac veins in mice. J Anat 211:53–63
Dowd DA (1969) The coronary vessels and conducting system in the heart of monotremes. Acta Anat (Basel) 74:547–573
Dowd DA (1974) The coronary vessels in the heart of a marsupial, Trichosurus vulpecula. Am J Anat 140:47–56
Fernandez B, Duran AC, Fernandez MC, Fernandez-Gallego T, Icardo JM, Sans-Coma V (2008) The coronary arteries of the C57BL/6 mouse strains: implications for comparison with mutant models. J Anat 212:12–18
Fitzgerald TC (1969) The Coturnix quail anatomy and histology. The Iowa State University Press, Ames, IA
Halpern MH (1957) The dual blood supply of the rat heart. Am J Anat 101:1–16
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryos. J Morphol 88:49–92
Kamimura T, Yamagishi T, Nakajima Y (2018) Avian coronary endothelium is a mosaic of sinus venosus- and ventricle-derived endothelial cells in a region-specific manner. Dev Growth Differ 60:97–111
Kawashima T, Sato K, Sato F, Sasaki H (2003) An anatomical study of the human cardiac veins with special reference to the drainage of the great cardiac vein. Ann Anat 185:535–542
Kresakova L, Purzyc H, Schusterova I, Fulton B, Maloveska M, Vdoviakova K, Kravcova Z, Boldizar M (2015) Variability in the cardiac venous system of Wistar rats. J Am Assoc Lab Anim Sci 54:10–16
Lindsay FE (1967) The cardiac veins of Gallus domesticus. J Anat 101:555–568
Lindsay FE, Smith HJ (1965) Coronary arteries of Gallus domesticus. Am J Anat 116:301–314
Lopez-Garcia A, Soto-Navarrete MT, Fernandez MC, Moncayo-Arlandi J, Duran AC, Lopez-Unzu MA, Alonso-Briales JH, Fernandez B (2016) Unusual anatomical origins of the coronary arteries in C57BL/6 mice. Are they strain-specific? J Anat 229:703–709
Loukas M, Groat C, Khangura R, Owens DG, Anderson RH (2009a) The normal and abnormal anatomy of the coronary arteries. Clin Anat 22:114–128
Loukas M, Bilinsky S, Bilinsky E, El-Sedfy A, Anderson RH (2009b) Cardiac veins: a review of the literature. Clin Anat 22:129–145
Myczkowski K (1960) Morphology of the coronary arteries in fowl and in some wild birds. Folia Morphol 11:21–30
Nakajima Y, Imanaka-Yoshida K (2013) New insights into the developmental mechanisms of coronary vessels and epicardium. Int Rev Cell Mol Biol 303:263–317
Pardanaud L, Altmann C, Kitos P, Dieterlen-Lievre F, Buck CA (1987) Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development 100:339–349
Perez-Pomares JM, de la Pompa JL, Franco D, Henderson D, Ho SY, Houyel L, Kelly RG, Sedmera D, Sheppard M, Sperling S, Thiene G, van den Hoff M, Basso C (2016) Congenital coronary artery anomalies: a bridge from embryology to anatomy and pathophysiology—a position statement of the development, anatomy, and pathology ESC Working Group. Cardiovasc Res 109:204–216
Reese DE, Mikawa T, Bader DM (2002) Development of the coronary vessel system. Circ Res 91:761–768
Sato Y, Lansford R (2013) Transgenesis and imaging in birds, and available transgenic reporter lines. Dev Growth Differ 55:406–421
Sedmera D, Watanabe M (2006) Growing the coronary tree: the quail saga. Anat Rec A Discov Mol Cell Evol Biol 288:933–935
Tian X, Pu WT, Zhou B (2015) Cellular origin and developmental program of coronary angiogenesis. Circ Res 116:515–530
Tomanek RJ, Hansen HK, Dedkov EI (2006) Vascular patterning of the quail coronary system during development. Anat Rec A Discov Mol Cell Evol Biol 288:989–999
von Lüdinghausen M (1987) Clinical anatomy of cardiac veins, Vv. cardiacae. Surg Radiol Anat 9:159–168
Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, Hungerford JE, Little CD, Poelmann RE (1997) The development of the coronary vessels and their differentiation into arteries and veins in the embryonic quail heart. Dev Dyn 208:338–348
Yoldas A, Ozmen E, Ozdemir V (2010) Macroscopic description of the coronary arteries in Swiss albino mice (Mus musculus). J S Afr Vet Assoc 81:247–252
Acknowledgements
The authors thank S. Uoya for preparing the manuscript. This work was supported by JSPS Grant-in-Aid Scientific Research ©) 16K08450.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kato, M., Narematsu, M. & Nakajima, Y. Anatomy of the coronary artery and cardiac vein in the quail ventricle: patterns are distinct from those in mouse and human hearts. Anat Sci Int 93, 533–539 (2018). https://doi.org/10.1007/s12565-018-0446-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-018-0446-x