Abstract
The tongue of an adult degu was examined by light and scanning electron microscopy. It consists of an apex, corpus, and radix and contains a lingual prominence. The aim of this study was to describe the course of muscle fascicles of the proper lingual muscle, the presence and nature of the lingual salivary glands, and particularly the appearance and distribution of the lingual papillae. Three major types of papillae have been observed: filiform, conical, and vallate. The dorsal surface of the lingual apex extends in caudally bent filiform papillae with two spines. The lingual corpus bears long filiform papillae with a single tip. The lingual radix contains crown-like papillae in the region of the prominence and conical papillae in the remaining areas. Two oval vallate papillae were discovered caudally on the lingual radix. This first description of the lingual structures in a degu could be used for comparative studies or as basic data for differentiation of lingual morphology in this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The degu (Octodon degus) is a caviomorph rodent, a member of the family Octodontidae and is commonly kept as a pet or laboratory animal (Jekl et al. 2011).
Degus are social diurnal rodents native to Mediterranean-type semi-arid shrub land ecosystem called “matorral”, which can be found on the western slopes of the Andes in north-central Chile between 28° and 35° south latitude (Nowak 1991). Degus typically weigh between 170 and 300 g, and measure between 325 and 440 mm in length, including the tail (Lee 2004).
Degus are generalist herbivores and are foliovores, granivores and lignivores. They feed on the leaves, bark, stems and seeds of shrubs and forbs. Among their favourite foods are the bark of Cestrum palqui and Mimosa cavenia, leaves and bark of Proustia cuneifolia, Atriplex repunda and Acacia caven, annuals such as Erodium cicutarum when in season, green grasses and thistle seeds. Degus are selective feeders and choose food items with reduced fiber and increased nitrogen and moisture content and thus prefer young leaves and avoid woodier shrubs (Gutierrez and Bozinovic 1998). In nature, they prefer to eat shrubs such as Adesmia bedwellii, Baccharis paniculata and Chenopodium petioare, which are less fibrous and less thorny than other shrubs (Gutierrez and Bozinovic, 1998). In captivity, pelleted diet and meadow/timothy hay should be the main component of their diet.
Degus are true herbivores, with incisors and all premolar and molars continually growing throughout their life as in rabbits, guinea pigs and chinchillas.
The tongue is a muscular organ located in the oral cavity. It is used for food processing. The surface is covered by stratified squamous epithelium. Dorsum linguae extends in the form of mechanical and gustatory papillae. The gustatory papillae contain taste buds.
Previous studies of the microscopic structure of the tongue in rodents have described all five standard types of lingual papillae (filiform, conical, fungiform, vallate and foliate) in the house mouse, Mus musculus (Kobayashi et al. 1989), Patagonian cavy, Dolichotis patagonum (Emura et al. 2011), hazel dormouse, Muscardinus avellanarius (Wolczuk 2014), Manchurian chipmunk, Tamias sibiricus (Kobayashi et al. 1992) and bank vole, Clethrionomys glareolus (Jackowiak and Godynicki 2005). Filiform, fungiform, vallate and foliate papillae have been found in the agouti, Dasyprocta aguti (Ciena et al. 2013), capybara, Hydrochaeris hydrochaeris (Watanabe et al. 2013), American beaver, Castor canadensis (Shindo et al. 2006), nutria, Myocastor coypus (Emura et al. 2001), large vesper mouse, Calomys callosus (Watanabe et al. 1997), porcupine, Hystrix cristata (Kubota et al. 1966) and Japanese dormouse, Glirus japonicus (Kubota and Togawa 1966). Foliate papillae were absent in the Middle East blind mole rat, Spalax ehrenbergi (Kilinc et al. 2010), Japanese grass vole, Microtus montebelli (Emura et al. 1999) and squirrel, Sciurus vulgaris (Unsaldi 2010). Vallate papillae have not been revealed in the tongue of the guinea pig, Cavia porcellus (Sakr et al. 2013). The taste buds have been described in various rodents (Watanabe et al. 2013; Ciena et al. 2013; Kubota and Togawa 1966; Atalar and Karan 2011; Unsaldi 2010). There have also been studies that have described the lingual glands (Nagato et al. 1997; Shindo et al. 2006; Ciena et al. 2013).
Scanning electron microscopic studies of the tongue have already been performed on agouti, Dasyprocta aguti (Ciena et al. 2013), capybara, Hydrochaeris hydrochaeris (Watanabe et al. 2013), Patagonian cavy, Dolichotis patagonum (Emura et al. 2011), nutria, Myocastor coypus (Emura et al. 2001), large vesper mouse, Calomys callosus (Watanabe et al. 1997), porcupine, Hystrix cristata (Atalar and Karan 2011), Middle East blind mole rat, Spalax ehrenbergi (Kilinc et al. 2010), house-mouse, Mus musculus (Kobayashi et al. 1989), Manchurian chipmunk, Tamias sibiricus (Kobayashi et al. 1992), Japanese grass vole, Microtus montebelli (Emura et al. 1999), guinea pig, Cavia porcellus (Sakr et al. 2013), American beaver, Castor canadensis (Shindo et al. 2006), hazel dormouse, Muscardinus avellanarius (Wolczuk 2014).
However, the tongue morphology of a degu has not yet been published.
The aim of this study was to investigate the microscopic structure of the tongue of a degu with a special focus on the distribution and the structure of the lingual papillae by light and scanning electron microscopy and to compare the results with other rodents.
Materials and methods
Animals
Six adult male degus (Octodon degus) used for this study were kept as pet animals in plastic and wire-mesh cages. The degus were fed twice to three times a day with a balanced pelleted diet and had free access to water and meadow hay. The animals were euthanized using T61 euthanasia solution for veterinary use (containing Embutramide, Mebezonium iodide, Tetracaine hydrochloride) at the Avian and Exotic Animal Clinic, FVL VFU Brno due to various reasons (polytrauma, neoplasia, etc.). Prior to the T61 administration, Isoflurane was used. All the tongues came from animals with healthy jaws and oral cavities.
Light microscopy
The tongues were immediately fixed in 10 % neutral buffered formaldehyde. After fixation had been completed, samples for light microscopy were dehydrated in a graded alcohol series (the ethanol concentration in each subsequent bath was increased by 10 %), acetone, and three baths of xylene. At the end of dehydration process, samples were infiltrated with hot paraffin and embedded in paraffin wax. Then, 3–4 µm thin sections were cut in a routine manner. The sections were dried, stained with haematoxylin and eosin, mounted and examined, and finally photographed under an Olympus BX51 light microscope using an Olympus DP70 digital camera.
Scanning electron microscopy
The samples for scanning electron microscopy were dehydrated in a graded alcohol series (60, 70, 96 and 100 %, 20 min for each concentration), transferred to absolute acetone, dried at the critical point (Bal-tec CPD 030 Critical Point Dryer, Bal-Tec, UK), coated with gold (Balzers SCD 040 by current 30 mA for 4 min.) and finally examined and photographed under a Tescan VEGA TS 5136 XM scanning electron microscope in a high vacuum and accelerated voltage 20 kV by using an SE detector.
Results
The tongue of an adult degu is wedge-shaped and measures 18.5 mm in length and 6.2 mm in width on average. Three distinct areas are easy to distinguish: lingual apex, corpus and radix (Fig. 1). In our findings, the radix linguae bulged dorsally, thus forming a torus-like lingual prominence. The lingual septum divided the tongue into two halves in the area of the apex and corpus. The septum was incomplete because it was interrupted by a dome-shaped prominence in the middle of the lingual corpus.
The underlying skeletal muscular tissue was densely packed with a small amount of endomysial and perimysial connective tissue that generally lacked adipocytes.
Dorsally, in the area of lingual apex, bundles of muscle fibers with longitudinal and transverse orientation predominated. Ventrally, the apex was formed from muscle fibers, the absolute majority of which were of the transverse orientation.
In the area of the lingual corpus, vertically oriented fascicles of muscle fibers appeared in the dorsal half of the tongue. Caudally, muscle fibers with vertical orientation became more numerous. Ventrally, mixed orientation of transverse and longitudinally running bundles of muscle fibers underlaid the tongue.
The lingual radix was underlaid by fascicles of muscle fibers, which regularly changed their transverse and vertical orientation in the entire height of the tongue.
Lingual salivary glands were located within the lingual musculature of the radix (Fig. 2). They consisted of several lobules ventrally at the beginning of the lingual radix. Dorsally and caudally, glands were more developed. These glands were purely serous under the lingual surface medially and purely mucous deeper, scattered among lingual musculature laterally (Fig. 3). Their ducts were rather inconspicuous and opened onto the lingual surface of this area.
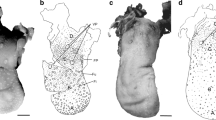
The dorsal surface of the lingual apex bore numerous long, slender filiform papillae, which tended to bend caudally (Fig. 4). These papillae had two spines of uneven height and extended above the lingual surface from a small opening (pore). Papillae grew in series, between which there were tortuous elevations. Each papilla was covered by rounded, flattened cells with a stippled surface on which droplets of secretion were found.
Papillae on the lingual corpus were more numerous and had a similar layout (Fig. 5). Their shape was different—the papillae extended in the form of caudally bended single spines. The surface cells of the papillae were dome-shaped and smooth. Tortuous elevations that coursed between the papillae split into pieces. These formations were extremely corrugated. Two separate taller conical papillae extended from the lingual surface rostrally, close to the lingual sulcus. These papillae were also thicker compared to the surrounding papillae of this area.
Papillae on the lingual prominence were less frequent and they were of a different shape (Fig. 6). They had a wider base, which formed several spines on the periphery of the papilla. Usually, there were three or four spines formed. Papillae were sometimes even rounded with more spines, thus resembling a crown. Flattened surface cells desquamated more intensely compared to the other lingual regions. The spaces between papillae were filled with densely packed cells that had a mosaic arrangement in some areas. Tortuous elevations, described on the lingual corpus were not that conspicuous.
Laterocaudally from the lingual prominence, the dorsal surface of the tongue was covered with the conical papillae (Fig. 7a). These papillae resembled the papillae on the lingual corpus, compared to which they were much shorter, had a wide base (thus conical), and their tips were also caudally bended (Fig. 7b). Interpapillary regions were similar to those of the lingual prominence.
Two oval vallate papillae were found caudally on the lingual radix (Fig. 8). These papillae were incompletely surrounded by the circumpapillary sulcus in the form of rostromedially and caudolaterally oriented arches. The papillae were covered by a distinct keratinized layer and contained numerous taste buds in the epithelium covering lateral surfaces of the papillae (Fig. 9). These taste buds were facing the circumpapillary sulcus. No other taste buds were found in the epithelium of the lingual mucosa. In the vicinity of the vallate papillae, a small amount of lymphatic tissue was located within the lamina propria mucosae of the dorsal lingual surface.
Discussion
The tongue of the degu was wedge-shaped as in the agouti (Ciena et al. 2013) and porcupine (Atalar and Karan 2011). The lingual prominence was formed similarly as in the agouti (Ciena et al. 2013), Middle East blind mole rat (Kilinc et al. 2010), Patagonian cavy (Emura et al. 2011), American beaver (Shindo et al. 2006), bank vole (Jackowiak ans Godynicki 2005) and porcupine (Atalar and Karan 2011). Compared to these species, an inconspicuous lingual prominence was described in the Manchurian chipmunk (Kobayashi et al. 1992). This supports a theory by Sonntag (1924), who considered the rodents of the Sciuridae family as a primitive group of rodents. In these animals, the lingual prominence was not formed at all or was only poorly developed. On the contrary, this does not correspond with Shindo et al. (2006), who considered lingual prominence as a characteristic structure in herbivorous animals in general.
Unlike the agouti (Ciena et al. 2013), capybara (Watanabe et al. 2013), nutria (Emura et al. 2001), large vesper mouse (Watanabe et al. 1997), Japanese dormouse (Kubota and Togawa 1966) and American beaver (Shindo et al. 2006), where filiform, fungiform, foliate and vallate papillae were described, the dorsal lingual surface of the tongue in a degu bore filiform, conical and vallate papillae. Three types of the lingual papillae were reported also in the Middle East blind mole rat (Kilinc et al. 2010). These papillae included filiform, fungiform and vallate papillae. In the guinea pig (Sakr et al. 2013), three different types of lingual papillae were also found: filiform, fungiform and foliate. Five standard types of lingual papillae (filiform, conical, fungiform, vallate and foliate) were observed in the house mouse (Kobayashi et al. 1989) and in Patagonian cavy (Emura et al. 2011). Filiform, conical, fungiform and vallate papillae were scattered on the tongue of the Japanese grass vole (Emura et al. 1999). The presence of only three types of lingual papillae in degu and the lack of typical sensory papillae, such as fungiform and foliate papillae, can be explained by the uniform diet or by the expected occurrence of the taste buds in the epithelium of other structures within the oral cavity or oropharynx.
Filiform papillae of the agouti (Ciena et al. 2013) were short and multifilamentous. They were distributed throughout the tongue surface. The same distribution of the filiform papillae was described also in the capybara (Watanabe et al. 2013) and Middle East blind mole rat (Kilinc et al. 2010), where they were narrow with fingerlike processes and distinct tips that bent backward. Filiform papillae of the guinea pig (Sakr et al. 2013) were also scattered all over the dorsal surface of the tongue and they also bent backward, but they were of a conical shape. In the anterior area of the tongue of the guinea pig, the majority of the filiform papillae extended into two processes (one principle and one accessory)—as well as in the degu. Posteriorly, these papillae were non-branched. Filiform papillae in degu did not reveal only two (apex) spines but also one (corpus) or even several spines (lingual prominence), such that they resembled a crown. The similarity in the appearance of the filiform papillae in degu, guinea pig and capybara may be based on the diet—these steppe species are strictly herbivorous. The reason why the filiform papillae of agouti were different may be explained by the occasional insectivore habits and the plant food in its biotope. Three types of filiform papillae (conical, long and short) were described also by Emura et al. (2001) in nutria or Shindo et al. (2006) in American beaver (filiform, large filiform, and dome-like). Four types of filiform papillae were found in the Japanese grass vole by Emura et al (1999). A few filiform papillae were also found on the ventral surface of the free part of the tongue in the Middle East blind mole rat (Kilinc et al. 2010). Unlike the papillae on the dorsal surface, these were bent toward the lingual apex.
The tongue of the agouti (Ciena et al. 2013) contained four vallate papillae, compared to the tongue of a degu that has only two. Vallate papillae of these species were similar in appearance. In both species, the vallate papillae were surrounded by conical papillae. Two vallate papillae were also found in the capybara (Watanabe et al. 2013) and Middle East blind mole rat (Kilinc et al. 2010). In these species they were located on the dorsal-posterior surface of the tongue and surrounded by filiform papillae. Emura et al. (2001) also reported two vallate papillae on the tongue of the nutria, Emura et al. (2011) on the tongue of the Patagonian cavy and Atalar and Karan (2011) on the tongue of the porcupine. In comparison, tongues of the Japanese dormouse (Kubota and Togawa 1966), Manchurian chipmunk (Kobayashi et al. 1992) and American beaver (Shindo et al. 2006) contained three vallate papillae. Three vallate papillae surrounded by conical papillae were observed in the hazel dormouse (Wolczuk, 2014). One vallate papilla was described on the tongue of the Japanese grass vole (Emura et al. 1999), large vesper mouse (Watanabe et al., 1997) and bank vole (Jackowiak and Godynicki 2005). We do not see any correlation between the arrangement and number of vallate papillae on the one hand and the environment and feeding habits on the other, as discussed by Kilinc et al (2010).
The circumpapillary sulcus of the vallate papilla in the capybara (Watanabe et al. 2013), Middle East blind mole rat (Kilinc et al. 2010), Manchurian chipmunk (Kobayashi et al. 1992), American beaver (Shindo et al. 2006), hazel dormouse (Wolczuk 2014) and porcupine (Atalar and Karan 2011) formed a complete ring, whereas in the degu, agouti (Ciena et al. 2013), Japanese grass vole (Emura et al. 1999) or Patagonian cavy (Emura et al. 2011) it was interrupted, thus forming two arches (grooves).
Lingual salivary glands in the degu were both serous and mucous. They were found in the area of the lingual radix. Ciena et al. (2013) described only acinar von Ebner glands (serous-rich lingual glands) in the agouti as well as Watanabe et al. (2013) in the capybara, and Kubota and Togawa (1966) in the Japanese dormouse. The lingual glands ducts were observed on the dorsal and lateral surfaces of the root in the Patagonian cavy (Emura et al. 2011). Nagato et al. (1997) focused on the Weber’s salivary glands of the root of the tongue in the rat. These glands were tubulo-acinar (mixed), consisting of mucous tubules with serous demilunes. The presence of serous and mucous glands in the lingual radix of the degu, as well as mixed glands in this area of the rat's tongue is undoubtedly related to the taste perception and swallowing of dry food, respectively. This was also suggested by Nagato et al. (1997).
Muscle fascicles in the tongue of a degu are similarly organized as the muscle bundles in the tongue of the guinea pig (Sakr et al. 2013). According to the feeding habits, we do not expect any significant difference between the appearance of the proper lingual muscle of these two species.
Different types of lingual papillae within the superfamily Cavioidea and Octodontoidea, despite the same infraorder (i.e., the Hystricognathi), had a parallel evolution (Honeycutt et al. 2003). For instance, the differentiation in diet and habitat, also associated with jaw and teeth evolution, occurred independently in these two monophyletic groups (Alvarez et al. 2011).
References
Alvarez A, Perez SI, Verzi DH (2011) Early evolutionary differentiation of morphological variation in the mandible of South American caviomorph rodents (Rodentia, Caviomorpha). J Evol Biol 24:2687–2695
Atalar O, Karan M (2011) The light and scanning electron microscopic structure of the papilla vallatae in the porcupine (Hystrix cristata). J Anim Vet Adv 10:3069–3073
Ciena AP, de Sousa Bolina C, de Almeida SR, Rici RE, de Oliveira MF, da Silva MC, Miglino MA, Watanabe IS (2013) Structural and ultrastructural features of the agouti tongue (Dasyprocta aguti Linnaeus, 1766). J Anat 223:152–158
Emura S, Tamada A, Hayakawa D, Chen H, Jamali M, Ozawa Y, Shoumura S (1999) SEM study on the dorsal lingual surface of Microtus montebelli. Okajimas Folia Anat Jpn 76:171–178
Emura S, Tamada A, Hayakawa D, Chen H, Shoumura S (2001) SEM study on the dorsal lingual surface of the nutria, Myocastor coypus. Kaibogaku zasshi 76:233–238
Emura S, Okumura T, Chen H (2011) Morphology of the lingual papillae in the Patagonian cavy. Okajimas Folia Anat Jpn 88:121–125
Gutierrez J, Bozinovic F (1998) Diet selection in captivity by a generalist herbivorous rodent (Octodon degus) from the Chilean coastal desert. J Arid Environ 39:601–607
Honeycutt RL, Rowe DL, Gallardo MH (2003) Molecular systematics of the South American caviomorph rodents: relationships among species and genera in the family Octodontidae. Mol Phylogenet Evol 26:476–489
Jackowiak H, Godynicki S (2005) The distribution and structure of the lingual papillae on the tongue of the bank vole (Clethrionomys glareolus). Folia Morphol 64:326–333
Jekl V, Hauptman K, Knotek Z (2011) Diseases in pet degus: a retrospective study in 300 animals. J Small Anim Pract 52:107–112
Kilinc M, Erdogan S, Ketani S, Ketani MA (2010) Morphological study by scanning electron microscopy of the lingual papillae in the Middle East Blind Mole Rat (Spalax ehrenbergi, Nehring, 1898). Anat Histol Embryol 39:509–515
Kobayashi K, Miyata K, Takahashi K, Iwasaki S (1989) Three-dimensional architecture of the connective tissue papillae of the mouse tongue as viewed by scanning electron microscopy. Kaibogaku zasshi 64:523–538
Kobayashi S, Toh H, Tomo S (1992) Scanning electron microscopic study on the lingual papillae in the Manchurian chipmunk, Tamias sibiricus asiaticus. Okajimas Folia Anat Jpn 69:139–143
Kubota K, Togawa S (1966) Comparative anatomical and neurohistological observations on the tongue of Japanese dormouse (Glirus japonicus). Anat Rec 154:545–552
Kubota K, Fukuda N, Asakura S (1966) Comparative anatomical and neurohistological observations on the tongue of the porcupine (Hystrix cristata). Anat Rec 155:261–268
Lee TM (2004) Octodon degus: a diurnal, social, and long-lived rodent. ILAR J 45:14–24
Nagato T, Ren XZ, Toh H, Tandler B (1997) Ultrastructure of Weber’s salivary glands of the root of the tongue in the rat. Anat Rec 249:435–440
Nowak RM (1991) Walker’s mammals of the world, 5th edn. Johns Hopkins University Press, Baltimore, pp 1681–1682
Sakr SMI, Taki-El-Deen FMA, Aboelwafa HR (2013) Comparative light and scanning electron microscopic study of the lingual papillae in three different mammalian animals; Hemiechinus auritus (Erinaceomorpha: Erinaceidae), Cavia porcellus (Rodentia: Caviidae) and Mustela nivalis vulgaris (Carnivora: Mustelidae). Life Sci J—Acta Zhengzhou Univ Overseas Ed 10:3082–3093
Shindo J, Yoshimura K, Kobayashi K (2006) Comparative morphological study on the stereo-structure of the lingual papillae and their connective tissue cores of the American beaver (Castor canadensis). Okajimas Folia Anat Jpn 82:127–138
Sonntag CF (1924) The comparative anatomy of the tongues of the Mammalia X. Rodentia. In: Proceedings of the Zoological Society of London. pp 725–741
Unsaldi E (2010) Macroscopic and light microscopic structure of fungiform papillae on the tongue of squirrels (Sciurus vulgaris). Kafkas Univ Vet Fak Derg 16:115–118
Watanabe I, Utiyama C, Koga LY, Motoyama AA, Kobayashi K, Lopes RA, Junior BK (1997) Scanning electron microscopy study of the interface epithelium-connective tissue surface of the lingual mucosa in Calomys callosus. Ann Anat 179:45–48
Watanabe I, Santos Haemmerle CA, Dias FJ, Cury DP, Silva MCP, Sosthines MCK, Santos TC, Guimaraes JP, Miglino MA (2013) Structural characterization of the capybara (Hydrochaeris hydrochaeris) tongue by light, scanning, and transmission electron microscopy. Microsc Res Tech 76:141–155
Wolczuk K (2014) Dorsal surface of the tongue of the hazel dormouse (Muscardinus avellanarius): scanning electron and light microscopic studies. Zool Pol 59:35–47
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Cizek, P., Hamouzova, P., Jekl, V. et al. Light and scanning electron microscopy of the tongue of a degu (Octodon degus). Anat Sci Int 92, 493–499 (2017). https://doi.org/10.1007/s12565-016-0346-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-016-0346-x