Abstract
Over the years a number of investigators have analysed the morphology of wormian bones in different population groups across the world. There have been significant variations between findings reported in these studies, and this has prompted researchers to focus on the influence of genetic factors on the morphology of these bones. In the light of the above observation, we considered it justified to conduct anatomical studies on wormian bones in different population groups; hence, we undertook the present study to look into the morphological details of these bones among a population in the eastern part of India. We observed a total of 120 adult dry human skulls of unknown age and sex, and noted the anatomical details of wormian bones when present. It was observed that wormian bones were present in 45 % of skulls, and that 30 % of skulls had more than one wormian bone. We also found that 2.5 % of the skulls had ten or more wormian bones, which is considered as pathognomonic. Maximum incidence (53.33 %) was observed at the lambdoid suture and minimum incidence at the bregma and metopic suture (0.61 % in each case). We noted a high incidence (21.21 %) of Inca bone/lambdoid ossicle, and bilaterally symmetrical wormian bones were present in 12.5 % study skulls. There were statistically significant (P < 0.05) variations between the findings of the present study and values reported in previous studies conducted in other regions of India and different parts of the world. Our observations favour the view that genetic influence primarily determines the morphology of wormian bones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wormian bones, or ossa suturalia, are accessory ossicles located in or near sutures of the skull, and are irregular in size, shape and number. They are isolated bones that appear in addition to the usual centres of ossification of the human cranium and, although unusual, are not rare (Soames 1995). They frequently lie along the lambdoid suture, occasionally seen within the sagittal and coronal suture, but may be found anywhere in or between skull bones (Chambellan 1883). Sutural bones were first described by the Swiss physician Theophrastus Aureolus Bombastas von Hohenheim (1493–1541), who is popularly known as Paracelsus (Jeanty et al. 2000). He referred to a bony ossicle located in the posterior fontanelle as the ‘ossiculum antiepilepticum’ as sutural bones were believed to have antiepileptic virtues in those days. After him, this particular sutural bone is nowadays referred to as the ‘Paracelsian ossicle’ (Marti et al. 2013). However, it was the Danish anatomist, Ole Worm (1588–1654), who was first to make a detailed description of accessory or supernumerary bones present within the cranial sutures and fontanelle, in a letter addressed to the famous anatomist, Thomas Bartholin (1616–1680). Later, Bartholin himself christened these bones as ‘Ossa wormiana’ or ‘wormian bones’ (Romero-Reverón and Arráez-Aybar 2015). Descriptions of individual wormian bones have been documented by various anatomists over the years. The French physician, Jean Guinter d’Andernach (1487–1574) detailed the anatomy of the sutural bone present at the level of the obelion (posterior interparietal region in front of the lambda) and it came to be known as ‘Andernach’s ossicle’ (Olry 1994). The interparietal portion of the squamous part of occipital bone when separated from the supraoccipital portion by the sutura mendosa (transverse occipital suture) is known as ‘Goethe’s ossicle’ after the German anatomist Johann Wolfgang von Goethe (1749–1832), who first described it. These sutural bones are also known as Os Incae, or Inca bone, as they resemble the triangular architectural monument design of the Inca tribe (Yucel et al. 1998; Udupi and Srinivasan 2011).
Wormian bones are often considered to be present as simple anatomical variants; nevertheless, they can be associated with certain bony dysplasias like cleidocranial dysostosis, pycnodysostosis, congenital hypothyroidism, rickets and most commonly osteogenesis imperfecta (Marti et al. 2013). However, when wormian bones occur as normal variants, they tend to be smaller in size and less in number than when they are associated with skeletal dysplasias (Kaplan et al. 1991). In fact, an abnormally high number of wormian bones is a strong pointer towards a diagnosis of osteogenesis imperfecta (Semler et al. 2010). Moreover, the presence of wormian bones is also associated with a number of congenital disorders, particularly those involving the central nervous system (Pryles and Khan 1979). Andernach and Andre Vesalius (1514–1564) were the first to associate their presence with cerebral disorders (Parker 2009). Some recent studies have even reported that the presence of wormian bones may serve as a marker for identification of anomalies involving the central nervous system (Jeanty et al. 2000). Anatomical details of wormian bones are also valuable from medico-legal point of view in the forensic investigation of non-accidental skull injuries in children as well as adults (Govsa et al. 2014).
Over the years a number of researchers have reported the incidence and distribution of wormian bones in the skulls of different geographical population groups, and there are significant differences in the observations documented (Saxena et al. 1986; Murlimanju et al. 2011; Khan et al. 2011; Marti et al. 2013; Cirpan et al. 2015). This prevalence of racial variations in the morphology of wormian bones can be explained in accordance with the theory put forward by some authors that occurrence of wormian bones is determined by genetic factors (El-Najjar et al. 1985; Opperman 2000). It may be opined that the influence of genetic factors justifies the study of wormian bones individually in different human population groups. Hence, we undertook the present study to look into the morphological details of wormian bones with regards to their incidence, number and topography in skulls from the population of an eastern part of India.
Materials and methods
The study was conducted at the Department of Anatomy, ESI-PGIMSR and ESIC Medical College, Joka, Kolkata, India. Prior to the onset of the study, we obtained ethical approval from the Ethics Committee of the above-mentioned institution. We examined the wormian bones, when present, in 120 adult dry skulls of unknown age and sex. We looked for wormian bones in all the skulls from anterior aspect to the posterior aspect at seven bilateral (right and left) sites, i.e. orbit, coronal suture, pterion, squamosal and parieto-mastoid suture, lambdoid suture, asterion, occipito-mastoid suture, and four unilateral sites, i.e. metopic suture (when present), bregma, sagittal suture, lambda. In the present study, the ossicle at lambda, i.e. interparietal portion of the squamous part of the occipital bone divided by the transverse occipital suture, was defined as the Inca bone. The skulls included in the present study were procured from the bone bank of the Department of Anatomy. We ensured that all selected skulls were without any evident sign of ante-mortem or post-mortem injuries. The following parameters were evaluated in the present study:
-
1.
Percentage of skulls where wormian bones were present.
-
2.
Incidence of wormian bones per skull when present.
-
3.
Topographic distribution of wormian bones in the skull.
-
4.
Incidence of wormian bones with respect to sutures in the skull.
-
5.
Symmetry of wormian bones with respect to sutures in the skull.
In order to assess the symmetry of wormian bone, we first identified those skulls where wormian bone was present on both sides of the bilateral sites as mentioned before. These bones were then divided into those present in front of the external occipital protuberance and those present behind. Finally, with the help of a measuring tape, we measured the distance of the wormian bones on either side of the skull from the bregma in the case of the first group, and from lambda in the second group. The difference in distance was analysed with the help of the paired student’s t test. When the difference was not found to be statistically significant (P ≥ 0.05), wormian bones were considered to be present in symmetrical orientation for that particular bilateral suture in that particular skull.
Statistical analysis
Pearson’s Chi square test was used to assess the differences between incidences of wormian bones observed at each of the sites in the skull mentioned earlier. Fisher’s exact test was employed when the incidence of wormian bones at any of the sites was less than five. Likewise, the differences in incidence of wormian bones between right and left sides were analysed in case of bilateral sites in the skull. Differences between an observation made in the present study and related observations from previous studies on wormian bones were analysed with the help of Student’s t test. All statistical analyses were performed with the help of SPSS (Statistical Package for Social Sciences) version 18.0 (SPSS, Chicago, IL). A P value <0.05 was considered as statistically significant.
Results
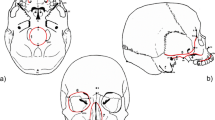
In the present study, single or multiple wormian bones were observed in 54 (45 %) out of the 120 skulls examined (Table 1). We noted that 18 (15 %) skulls had a single wormian bone, 18 (15 %) skulls had more than one but less than five, 15 (12.5 %) skulls had five or more than five but less than ten, and 3 (2.5 %) skulls had ten or more wormian bones. In other words, 36 (30 %) skulls had multiple wormian bones, i.e. more than one wormian bone per skull. A total of 165 wormian bones were observed in the skulls, and the topographical distribution of these bones has been detailed in a schematic manner in Fig. 1a, b. We found that 46 (27.88 %) wormian bones were present along the midline of the skull, whereas 61 (36.97 %) and 58 (35.15 %) wormian bones were present at the left side and right side of the skull, respectively (Fig. 1b). The difference in incidence of wormian bones between right and left side of the skull was statistically not significant (P ≥ 0.05).
a Schematic representation of the sites of the skull, both bilateral and unilateral, where wormian bones were observed in the present study shown as shaded areas. O-M Occipito-mastoid, P-M parieto-mastoid. b Schematic representation of the topographic distribution of the wormian bones (n = 165) in the skulls observed in the present study
Maximum incidence of wormian bones were observed at the lambdoid suture (53.33 %) followed by the lambda (21.21 %) and squamosal and parieto-mastoid sutures (10.9 %) (Figs. 2, 3). The difference in incidence of wormian bones at the lambdoid suture and other observed sites in the skull was found to be statistically significant (P < 0.05). Similarly, the incidence of wormian bones at the lambda suture had a statistically significant difference (P < 0.05) from that of the other observed sites in the skull. The incidence of wormian bones at other sites of the skull as observed in the present study has been documented in Figs. 2 and 4. The difference between the incidence of wormian bones at the right and left sides in the case of bilateral sites in the skull was not statistically significant (P ≥ 0.05) for any of the sutures of the skull.
In the present study, we did not find any skull where wormian bones were symmetrically present in all seven bilateral sites observed. There were two (1.67 %) skulls with symmetrically arranged wormian bones in at least three bilateral sites, and five (4.17 %) skulls with symmetrical wormian bones in at least two bilateral sites. In eight (6.67 %) skulls, at least one of the bilateral sites had symmetrically arranged wormian bones (Fig. 5). Overall, bilaterally symmetrical wormian bones were observed in 15 (12.5 %) skulls (Table 1).
Discussion
Wormian bones usually occur in the sutures separating the flat bones that constitute the neurocranium of the skull (Schoenwolf et al. 2009). Over the years, numerous theories have been suggested in an attempt to explain the development of wormian bones, but none of these has been universally accepted (Bellary et al. 2013). Earlier, environmental factors were the most cited cause in the literature regarding the development of wormian bones, and it was suggested that wormian bones developed in response to mechanical deformities of the cranium that were either pathological or induced artificially (Riveiro and Von Tschudi 1853; Bennett 1965). In 1977, El-Najjar and Dawson refuted this theory by comparing the incidence of wormian bones between skulls with deformities and those without (El-Najjar and Dawson 1977). Moreover, based on their study of skulls from South-western Pueblo Indian population, El-Najjar et al. (1985) supported the existence of both environmental as well as genetic factors in the formation of wormian bones. Their hypothesis was based on the observation that the stress generated by the induced deformation of infant skulls among the Pueblo Indians led to an increased number of wormian bones in the affected skulls without affecting the overall incidence in the population group (El-Najjar et al. 1985). Bergman et al. (1988) suggested that development of wormian bones could possibly be related to the rapid cranial expansion that spreads sutures apart and develops dural strain within sutures and fontanelles (Bergman et al. 1988). Recent studies have reported an increased frequency of wormian bones associated with craniosynostosis (premature fusion of cranial sutures), which results in the abnormal dural strain (mechanical stress) that initiates formation of islands of wormian bones in the membranous portion of the fontanelle (Opperman 2000). In summary, to date there is disagreement among researchers regarding the extent to which morphology of wormian bones can be attributed to environmental or genetic influences (Sanchez-Lara et al. 2007; Barberini et al. 2008).
The percentage of skulls with wormian bones as reported by different authors has been highly variable. In the present study, we report an incidence of 45 % of wormian bones in dry skulls from the population of the eastern part of India. Our observation was significantly lower (P < 0.05) than the findings of Murlimanju et al. (73.1 %) and Patil and Sheelavant (52.2 %), with both the studies conducted on skull samples from South India (Murlimanju et al. 2011; Patil and Sheelavant 2012). However, the incidence we observed was significantly higher (P < 0.05) than that seen by Walulkar et al. (34.22 %) in skull samples from the Western part of India (Walulkar et al. 2012) (Table 1). Considerable genetic variations have been documented among population groups residing in different regions of India (Balgir 1992). Accordingly, we are inclined to suggest that there may be an influence of genetic factors on the difference in incidence of wormian bones among regional population groups in India. We noted considerable variations between our findings and observations made by researchers elsewhere in the world (Table 1), which highlights the influence of racial variations, and thereby genetic factors, on the incidence of wormian bones. The archaeologist Brothwell studied the incidence of wormian bones in different population groups across the world, and observed remarkable variations, reporting the highest incidence among the Chinese (80 %) (Brothwell 1959). Such an observation implies that we have to consider the co-existence of genetic influence as well as mechanical factors regarding the incidence of wormian bones, as researchers have suggested that the high incidence of wormian bones in crania of Chinese populations as compared to other population groups could be due to the traditional supine infant sleep position leading to brachycephalic deformations among the Chinese (Graham et al. 2005).
Researchers have opined that wormian bones can be found in healthy individuals as a normal variant; however, they tend to be less numerous than when associated with pathological conditions like skeletal dysplasias (Marti et al. 2013). Notably, most authors have opined that the presence of multiple wormian bones is not always pathognomonic (Vishali et al. 2014). In the present study, we observed that multiple wormian bones (more than one per skull) were present in 30 % skulls, which, although similar to the finding of an earlier study (Patil and Sheelavant 2012), is notably different from previous reports (Table 1). Hence, it may be suggested that the influence of racial variations and genetic factors could have a bearing on the number of these bones per skull, which is in accordance with the argument presented by El-Najjar et al. (1985). We further observed that 2.5 % of the skulls in the present study had ten or more wormian bones, an entity that has been defined as significant number of wormian bones (SNWB) and is possibly associated with some pathological conditions (Cremin et al. 1982; Semler et al. 2010). The presence of SNWB can be a clinically useful radiographic sign for successful diagnosis of osteogenesis imperfecta, a potentially fatal hereditary disease, at a young age (Semler et al. 2010).
We observed a total of 165 wormian bones in the present study, of which an overwhelming majority, i.e. 53.33 %, were present at the lambdoid suture (Figs. 2, 3). This is in accordance with findings from studies undertaken on Indian populations in recent times (Table 1). In fact, in one of those studies, it was noted that the wormian bones were present only in the lambdoid suture (Vedula et al. 2015). Researchers elsewhere in the world have also reported a maximum incidence of wormian bones at the lambdoid suture (Table 1). We noted a very high incidence (21.21 %) of wormian bones at the lambda; this entity is also known as Os Incae or Inca bone (Figs. 2, 3). The available literature suggests a highly variable incidence of the Inca bone, e.g. 0.4 % has been reported in North Indian population, 1.32 % in Central India, and 14 % in a South Indian population (Singh et al. 1979; Marathe et al. 2010; Murlimanju et al. 2011). Elsewhere in the world, an incidence of 2.9–4.6 % has been reported in an American population of the south west coast, whereas an incidence of 57 % was observed in an Italian population of North Sardinia (Berry and Berry 1967; Brasili et al. 1999). Hanihara and Ishida studied the frequency of Inca bones among major human populations in the world and reported significant regional variations. Maximum frequency was observed in North America, followed by Tibet/Nepal/Northeast India, Central/South America and sub-Saharan Africa. They opined that such variations could possibly be due to genetic factors regulating the development of the Inca bone (Hanihara and Ishida 2001).
In the present study, we report an incidence of 10.9 % of wormian bones at the squamosal and parieto-mastoid suture (Figs. 2, 3), 5.45 % at the sagittal suture, 3.03 % at the occipito-mastoid suture, and 2.42 % at the coronal suture of the skulls (Fig. 2). We noted significant variations (P < 0.05) between our findings and those from South Indian (2.7, 5.56, 7.22 and 0.56 % respectively) and North Sardinian (9.5, 4.25, 6.58 and 11.24 % respectively) populations (Patil and Sheelavant 2012; Brasili et al. 1999). In the present study, we observed an incidence of 2.42 % for wormian bones in the orbit or Os orbitale (Figs. 2, 4), which, although lower than previous reports by Patil and Sheelavant (5 %) and Manjunath (4.29 %), is considerably higher than the findings of Malhotra (0.8 %) (Patil and Sheelavant 2012; Manjunath 2013; Malhotra et al. 1980). An incidence of 0.61 % was noted for wormian bones at the bregma (Os Krukenberg), which is a rare occurrence as reported by previous studies by Brasilli and colleagues (1.05 %) and Patil and Sheelavant (0.56 %) (Brasili et al. 1999; Patil and Sheelavant 2012). We noted the presence of a single wormian bone at the metopic suture that was present as a complete/incomplete suture in 7.5 % of the skulls, and thereby report an incidence of 0.61 % in the present study (Figs. 2, 4). To the best of our knowledge, there are no previous reports on the incidence of wormian bones at the metopic suture. In contrast to previous reports, we did not find any wormian bones at the pterion and asterion (Saxena et al. 1986; Brasili et al. 1999; Patil and Sheelavant 2012).
In the present study, we analyzed the incidence of wormian bones at right and left side of the skull (Fig. 1b) and observed that the differences were not statistically significant for any of the bilateral skull sutures. Previously, Sanchez-Lara et al. (2007) and Jeanty et al. (2000) observed that wormian bones were more frequent on the right side of the skull; however, Cirpan et al. (2015) reported that wormian bones were more frequent on the left side of the skull, except for the coronal suture, where the frequency was equal on each side. Previous reports have documented an asymmetrical orientation of wormian bones; however, Cirpan and colleagues recently observed considerable symmetry of wormian bones in their skull samples, with 23.33 % of skulls showing symmetrical wormian bones in one pair of sutures and 6.67 % skulls with symmetrical wormian bones in two pairs of sutures (Cirpan et al. 2015). We also report the existence of symmetry in the orientation of wormian bones as 6.67 % of skulls in the present study showed symmetrical wormian bones in one pair of skull sutures (Fig. 5), 4.17 % of skulls in two pairs of sutures, and 1.67 % of skulls in three pairs of sutures. Overall, Cirpan et al. (2015) had reported bilaterally symmetrical wormian bones in 30 % of their skull samples, whereas we found the same in 12.5 % of the skulls (Table 1).
The eastern part of India happens to be very close to Northeast India, a region with evidence of having one of the highest incidence of Inca bone (lambdoid ossicle) among its population (Hanihara and Ishida 2001). Demographic studies have documented crossovers between these two geographically close population groups (Gazi et al. 2013), which could possibly be reflected in the findings of the present study, where we observed an incidence of 21.21 % of Inca bone in skulls of a population from Eastern India (Figs. 2, 3), which is significantly higher than the incidence reported from other regions of India, as well as most of the other population groups of the world (Singh et al. 1979; Marathe et al. 2010; Berry and Berry 1967). We were aware that, apart from the genetic factors, environmental/mechanical factors are also critical in determining the incidence of wormian bones (El-Najjar et al. 1985; Graham et al. 2005). Prevalent customs related to the manipulation of skull shape are referred to as “cultural cranial deformation”, and researchers such as Dorsey (1897) and El-Najjar et al. (1985) have documented their influence on the number of wormian bones. However, as “cultural cranial deformation” is usually associated with ‘plagiocephaly’, i.e. asymmetry due to pressure exerted on infant skull (Marti et al. 2013), and, as we observed, the existence of notable symmetry in terms of wormian bones in our skulls samples, this might be considered as evidence of the absence of practices related to cranial deformation in Eastern India. Regarding all the parameters evaluated in the present study in relation to morphology of the wormian bones, considerable variations (statistically significant in most cases) were observed, with values reported from studies conducted on skulls from other regions of India as well as different parts of the world. In the event of such findings, we are inclined to believe that genetic factors could be the primary determinant of the morphogenesis of wormian bones. However, we suggest cautious interpretation of the opinions expressed in the present study on the basis of comparison with previous studies, considering the variable number of skull populations used in different reports, and also due to the fact that we took into account a study that was conducted by CT scan analysis of subadult skull samples (Table 1). Further, we would like to mention that the skull populations were of unknown age and sex, which is a limitation of the present study. Nevertheless, the findings of the present study could possibly be useful for clinicians in general. A knowledge of the anatomy of wormian bones could be critical for radiological diagnosis of wormian bones, which is a useful primary screening measure for central nervous system disorders early in life (Pryles and Khan 1979; Jeanty et al. 2000). It has been conclusively established that the presence of SNWBs can act as a marker for early diagnosis of bony dysplasias, particularly osteogenesis imperfecta (Semler et al. 2010). Details of the topographical distribution of wormian bones could be useful to radiologists and forensic experts in successfully differentiating a skull fracture/injury and a normal suture, and thereby exclude possibilities of physical abuse and brittle bones (Govsa et al. 2014).
Conclusion
In the present study, we observed an incidence of 45 % of wormian bones in adult skulls from a population in the eastern part of India. We noted that 30 % of the skulls had multiple wormian bones, and 2.5 % of the skulls had significant number of wormian bones (SNWB), i.e. ten/more wormian bones per skull. The topographical distribution of wormian bones was detailed, and maximum incidence of wormian bones was noted at the lambdoid suture (53.33 %) whereas minimum incidence was observed at the bregma and metopic sutures (0.61 % in each case). We found a very high incidence of the Inca bone/lambdoid ossicle (21.21 %) among the skull samples included in the present study. The incidence of wormian bones was almost equal at the right and left side of the skull and bilateral symmetry, in terms of orientation of wormian bones, was noted in 12.5 % of the skulls. There were statistically significant variations between the findings of the present study and the values reported in studies from other regions of India as well as different parts of the world. Such an observation strengthens the prevalent view that genetic factors primarily determine the morphology of wormian bones and justifies the study of anatomical details of these bones in different population groups, which could be relevant in medical practice. We are optimistic that our findings will be beneficial to clinicians, particularly in early diagnosis and timely management of disorders associated with the presence of wormian bones.
References
Balgir RS (1992) Regional and genetic variations among the Hindu Gujjars of Northwestern India. Int J Anthropol 7:35–41
Barberini F, Bruner E, Cartolari R, Franchitto G, Heyn R, Ricci F, Manzi G (2008) An unusually wide human bregmatic wormian bone: anatomy, tomographic description, and possible significance. Surg Radiol Anat 30:683–687
Bellary SS, Steinberg A, Mirzayan N et al (2013) Wormian bones: a review. Clin Anat 26:922–927
Bennett KA (1965) The etiology and genetics of wormian bones. Am J Phys Anthropol 23:255–260
Bergman RA, Afifi AK, Miyauchi R (1988) Skeletal systems: cranium. In: Compendium of human anatomical variations. Urban and Schwarzenberg, Baltimore, pp 197–205
Berry AC, Berry RJ (1967) Epigenetic variation in the human cranium. J Anat 101:361–379
Brasili P, Zaccagni L, Gualdi-Russo E (1999) Scoring of nonmetric cranial traits: a population study. J Anat 195:551–562
Brothwell DR (1959) The use of non-metrical characters of the skull in differentiating populations. Dtsch Ges Anthropol 6:103–109
Chambellan V (1883) Étude anatomique et anthropologique sur les os Wormiens. thèse de médecine, Paris, p 66
Cirpan S, Aksu F, Mas N (2015) The incidence and topographic distribution of sutures including wormian bones in human skulls. J Craniofac Surg 26:1687–1690
Cremin B, Goodman H, Spranger J, Beighton P (1982) Wormian bones in osteogenesis imperfect and other disorders. Skelet Radiol 8:35–38
Dorsey GA (1897) Wormian bones in artificially deformed Kwakiuti crania. Am Anthropol 10:169–173
El-Najjar MY, Dawson GL (1977) The effect of cranial deformations on the incidence of wormian bones in the lambdoid suture. Am J Phys Anthropol 46:155–160
El-Najjar MY, Aufderheide AC, Ortner DJ (1985) Preserved human remains from the southern region of the North American continent: report of autopsy findings. Hum Pathol 16:273–276
Gazi NN, Tamang R, Singh VK et al (2013) Genetic structure of Tibeto-Burman populations of Bangladesh: evaluating the gene flow along the sides of Bay-of-Bengal. PLoS One 8:e75064
Govsa F, Ozer MA, Bayraktaroglu S, Aktas EO (2014) Anatomoradiological identification of intrasutural bones for importance of cranial fracture. Turk Neurosurg 24:357–362
Graham JM Jr, Kreutzman J, Earl D, Halberg A, Samoya C, Guo X (2005) Deformational brachycephaly in supine-sleeping infants. J Pediatr 146:253–257
Hanihara T, Ishida H (2001) Os incae: variation in frequency in major human population groups. J Anat 198:137–152
Jeanty P, Silva SR, Turner C (2000) Prenatal diagnosis of wormian bones. J Ultrasound Med 19:863–869
Kaplan SB, Kemp SS, Oh KS (1991) Radiographic manifestations of congenital anomalies of the skull. Radiol Clin N Am 29:195
Khan AA, Asari MA, Harran A (2011) Unusual presence of wormian (sutural) bones in human skulls. Folia Morphol (Warsz) 70:291–294
Malhotra VK, Agrawal SK, Tiwari SP (1980) Os orbitale. Acta Anat 106:290–292
Manjunath KY (2013) Supernumerary bones in the walls of the bony orbit. People’s J Sci Res 6:7–12
Marathe R, Yogesh A, Pandit S, Joshi M, Trivedi G (2010) Inca-interparietal bones in neurocranium of human skulls in Central India. J Neurosci Rural Pract 1:14–16
Marti B, Sirinelli D, Maurin L, Carpentier E (2013) Wormian bones in a general paediatric population. Diagn Interv Imaging 94:428–432
Murlimanju BV, Prabhu LV, Ashraf CM, Kumar CG, Rai R, Maheshwari C (2011) Morphological and topographical study of wormian bones in cadaver dry skulls. J Morphol Sci 28:176–179
Olry R (1994) Os dans Incas, os épactal, os wormien: histoire d’une trilogie mal comprise. Acta Belgica Historia Medicinae 7:137–141
Opperman LA (2000) Cranial sutures as intramembranous bone growth sites. Dev Dyn 219:472–485
Parker CA (2009) Wormian bones. BiblioLife, Charleston, pp 19–24
Patil M, Sheelavant S (2012) Sexual dimorphism among the wormian bones in adult human skulls. J Indian Acad Forensic Med 34:124–127
Pryles CV, Khan AJ (1979) Wormian bones: a marker of CNS abnormality? Am J Dis Child 133:380
Riveiro ME, Von Tschudi JJ (1853) Peruvian antiquities. Putnam, New York, pp 38–39
Romero-Reverón R, Arráez-Aybar LA (2015) Ole Worm (1588–1654)—anatomist and antiquarian. Eur J Anat 19:299–301
Sanchez-Lara PA, Graham JM Jr, Hing AV, Lee J, Cunningham M (2007) The morphogenesis of wormian bones: a study of craniosynostosis and purposeful cranial deformation. Am J Med Genet A 143:3243–3251
Saxena SK, Chowdhary DS, Jain SP (1986) Interparietal bones in Nigerian skulls. J Anat 144:235–237
Schoenwolf GL, Bleyl SB, Brauer PR, Francist-West PH (2009) Development of the pharyngeal apparatus and face. Larsen’s human embryology. Elsevier, Philadelphia, pp 545–549
Semler O, Cheung MS, Glorieux FH, Rauch F (2010) Wormian bones in osteogenesis imperfect: correlation to clinical findings and genotype. Am J Med Genet 152A:1681–1687
Singh PJ, Gupta CD, Arora AK (1979) Incidence of interparietal bones in adult skulls of Agra Region. Anat Anz 145:528–531
Soames RW (1995) Skeletal system. In: Williams PL (ed) Gray’s anatomy: the anatomical basis of medicine and surgery, 38th edn. Churchill Livingstone, New York, pp 606–607
Udupi S, Srinivasan JK (2011) Interparietal (Inca) bone: a case report. Int J Anat Var 4:90–92
Vedula D, Rani R, Vijayalakshmi K, Thu KM, Rao VB, Viswakanth B (2015) Incidence of wormian bones in the North Coastal Andhra Pradesh. IOSR J Dent Med Sci 14:53–57
Vishali N, Ebenraj TJ, Rojomon TC (2014) A rare existence of significant number of wormian bones in the lambdoid suture. Int J Sci Res 3:671–677
Walulkar S, Ksheersagar D, Walulkar M (2012) The study of wormian bones in human skulls in Vidarbha region. PJMS 2:18–21
Yucel F, Egilmez H, Akgun Z (1998) A study of interparietal bone in man. Turk J Med Sci 28:505–509
Acknowledgements
The authors express heartfelt gratitude to all the clinical tutors and technicians of the Department of Anatomy, ESI-PGIMSR and ESIC Medical College, Joka, Kolkata, India for their unconditional support throughout the study. We are grateful to the authorities of ESI-PGIMSR and ESIC Medical College, Joka for their kind cooperation during the course of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standard
The authors hereby declare that the study was conducted only after approval had been obtained from the Ethical Committee of ESI-PGIMSR & ESIC Medical college, Joka, Kolkata, India, whose guidelines are in accordance with the Declaration of Helsinki (1964) and all subsequent revisions.
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Ghosh, S.K., Biswas, S., Sharma, S. et al. An anatomical study of wormian bones from the eastern part of India: is genetic influence a primary determinant of their morphogenesis?. Anat Sci Int 92, 373–382 (2017). https://doi.org/10.1007/s12565-016-0342-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-016-0342-1