Abstract
This study presents a novel method for the rapid and precise detection of mackerels belonging to the genus Scomber, which are prevalent in the Japanese market and are subject to labeling regulations owing to allergenic concerns. We developed loop-mediated isothermal amplification (LAMP) methods targeting the ribosomal DNA sequences of four Scomber species: S. japonicus, S. australasicus, S. scombrus, and S. colias. By incorporating hydroxynaphthol blue for colorimetric detection, our method enables visual inspection of results, eliminating the need for complex instruments. Optimization of LAMP conditions and verification of specificity demonstrated reliable detection of Scomber DNA with a minimum detection limit of 1–100 pg, depending on the Scomber species, and enabling efficient detection within 30–60 min using a simple incubator. No cross-amplification occurred among closely related species. Additionally, we successfully detected Scomber DNA in processed foods, demonstrating the usefulness and practicality of our LAMP methods. Despite relatively lower sensitivity compared with real-time polymerase chain reaction (PCR), our LAMP methods offer advantages in simplicity, rapidity, and ease of use, making the methods suitable for broader application beyond specialized laboratories. Thus, our LAMP methods provide a practical solution for monitoring food labeling compliance, thereby enhancing consumer confidence and regulatory efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Japan, a food labeling system for specific allergenic substances became mandatory by law on 1 April 2002 (Akiyama et al. 2011). Scomber is among the food items recommended for labeling in the Japanese Ministerial Notification (Akiyama and Adachi 2021). The implementation of the food labeling system has significantly ensured dietary safety for individuals with food allergies. To guarantee the accuracy of food labels and enhance consumer confidence in the food labeling system, it is crucial to establish an accurate method for detecting allergenic food materials, such as Scomber fish, in processed foods.
Meanwhile, owing to the globalization of food distribution, Atlantic mackerel Scomber scombrus and Atlantic chub mackerel S. colias have entered the Japanese market in recent years, alongside chub mackerel S. japonicus and blue mackerel S. australasicus caught in the sea around Japan. While whole fish can be identified on the basis of morphological characteristics before processing, the loss of features, such as fins, skin, and head, as well as color changes, during processing may make it challenging for consumers to ascertain whether the processed food they are consuming contains Scomber species or matches the species indicated on the food label. Therefore, to safeguard consumer safety and their right to know, a reliable detection method is necessary to identify the species of mackerel present in processed foods.
Polymerase chain reaction (PCR) is currently the most prevalent method for detecting Scomber species, utilizing various approaches, such as conventional PCR (Aranishi and Okimoto 2004; Infante et al. 2006; Infante and Manchado 2006), PCR-restriction enzyme fragment length polymorphism method (Aranishi 2005), multiplex PCR (Catanese et al. 2010), and real-time PCR (Velasco et al. 2013), to detect one or several specific species of Scomber. Recently, we have developed accurate and sensitive detection methods for Scomber fish on the basis of real-time PCR with a detection limit of 1 pg. These methods utilize primers targeting the internal transcribed spacer regions of nuclear ribosomal DNA, enabling the individual identification of four Scomber species and the comprehensive detection of Scomber fish regardless of species. (Cui et al. 2023). However, PCR-based detection methods, while highly sensitive, require thermal cycling, involving repeated temperature changes between specific temperature ranges (denaturation, annealing, and extension), which can be time-consuming and necessitate specialized equipment (Zhu et al. 2020). Therefore, we turned our attention to loop-mediated isothermal amplification (LAMP) (Notomi et al. 2000). This is because LAMP operates at a constant temperature, typically around 60–65 °C, eliminating the need for complex thermal cycling equipment and reducing the overall assay time (Notomi et al. 2015; Parida et al. 2008). Additionally, LAMP can be detected visually without the need for specialized equipment, using techniques, such as turbidity, colorimetric change, or fluorescence (Scott et al. 2020), making it suitable for point-of-care applications.
In the present study, we aimed to develop comprehensive and species-specific LAMP detection methods for the four Scomber species prevalent in the Japanese market. By adding hydroxynaphthol blue (HNB) to the reaction mixture, the detection results can be determined by observing the color change in the mixture (Goto et al. 2009). Our method holds the promise of achieving simpler and faster detection of Scomber species.
Materials and methods
Samples used for the detection of Scomber species
Specimens of S. japonicus and S. australasicus from Japan, as well as S. scombrus from Norway, were obtained from fish markets in Japan. A specimen of S. colias from West Africa was generously donated by Maruha Nichiro Corporation. Additionally, five fish species (Thunnus orientalis, Thunnus obesus, Katsuwonus pelamis, Seriola quinqueradiata, and Scomberomorus niphonius) belonging to the same Scombridae family but different genera than Scomber, and two fish species, Oncorhynchus keta and Salmo salar, from the Salmonidae family, were included in the study as non-Scomber species (Table 1). Species identification was conducted on the basis of package labels and/or morphological characteristics, and all samples were stored at −80 °C until DNA extraction.
A total of 14 processed foods labeled as containing Scomber species and four processed foods without Scomber labeling were purchased at markets in Tokyo, Japan. The label information of processed foods is shown in Table 2.
DNA was extracted from approximately 200 mg of fish muscle or processed food using the NucleoSpin Food DNA Extraction Kit (Macherey–Nagel, Germany) according to the manufacturer’s instructions. The quality of the extracted DNA was evaluated by Gene Quant 100 spectrophotometer (GE Healthcare, Japan) with an absorbance ratio of A260 / A280 ranging from 1.7 to 2.0 and by running a 0.8% agarose gel electrophoresis. DNA samples were stored at −20 °C until used for analysis.
Designing LAMP primers
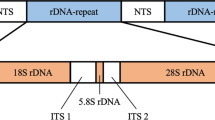
The ribosomal DNA (rDNA) sequences containing internal transcribed spacer (ITS) 1 and ITS 2 regions of four Scomber species (Cui et al. 2023) were chosen for the design of LAMP primers. The accession numbers for these four Scomber species in the DNA Data Bank of Japan (DDBJ)/GenBank/European Molecular Biology Laboratory (EMBL) database are LC773205 (S. japonicus), LC773259 (S. colias), LC773260 (S. australasicus), and LC773261 (S. scombrus). PrimerExplorer V5 software (https://primerexplorer.jp/lampv5/index.html) was used to design Scomber species-specific and comprehensive LAMP primers from ITS 1 and ITS 2 regions, respectively. Each set of primers comprises two outer primers (F3 and B3) and two inner primers. The forward inner primer, FIP, consists of the F1c and F2 sequences connected by “TTTT.” The backward inner primer, BIP, consists of the B1c and B2 sequences connected by “TTTT.” All the LAMP primers and their respective locations on the ITSs are shown in Table 3 and Online Resource Fig. S1.
LAMP conditions for detecting the Scomber species
The LAMP reaction was performed using the PC-320 PCR thermal cycler (ASTEC Co., Ltd, Japan) at a constant temperature of 65 °C for 30–60 min to determine the optimal incubation time for achieving the highest efficiency of the LAMP reaction. Each reaction mixture, with a volume of 25 µL, comprised 8 U of BcaBEST DNA Polymerase (Takara Bio Inc, Japan), 12.5 µL of 2 × BcaBEST buffer, 0.2 µM of each outer primer, 1.6 µM of each inner primer, 120 μM of HNB (DOJINDO, Japan), and 10 ng of the test DNA template, unless otherwise specified. Upon completion of the reaction, results were assessed by visually inspecting color changes: a shift in the reaction mixture color from purple to blue indicates the amplification of DNA from the corresponding LAMP primer set used, while maintaining a purple color indicates the lack of DNA amplification.
The specificity of the developed LAMP method for Scomber species was assessed using DNA from the abovementioned 11 fish species (Table 1) at 65 °C for the optimal incubation times: 60 min for the comprehensive primer set, 40 min for the S. japonicus-specific and S. australasicus-specific primer sets, 50 min for the S. scombrus-specific primer set, and 35 min for the S. colias-specific primer set. Subsequently, the detection limits of the template DNA were determined by using tenfold serial dilutions of Scomber DNA (ranging from 10 ng to 1 pg) with comprehensive or species-specific LAMP primer sets. The influence of contaminating non-Scomber DNA on the detection of Scomber was evaluated by preparing LAMP reaction mixtures containing a total of 10 ng of DNA, with each Scomber species’ detection limit DNA amount supplemented with 1.43 ng of DNA from each of the seven non-Scomber fish species listed in Table 1 and conducting LAMP reactions.
Analysis of Scomber ingredients in processed foods
In total, 10 ng of DNA extracted from 18 processed food samples was analyzed using the LAMP method. The reaction conditions were as described above in the “LAMP conditions for detecting the Scomber species” section. Subsequently, the DNA extracted from the same 18 processed food samples was analyzed using a PikoReal real-time PCR system (Thermo Fisher Scientific Inc.) with our previously established comprehensive and species-specific real-time PCR detection methods for four Scomber species (Cui et al. 2023). In brief, the PCR reaction for comprehensive Scomber detection was conducted with a 10 µL reaction mixture comprising 10 ng of DNA template, 5 µL of KOD SYBR qPCR Mix (TOYOBO CO, LTD., Japan), and 0.2 µM of each forward and reverse Scomber comprehensive primer, followed by thermal cycling consisting of initial denaturation at 98 °C for 2 min, followed by 30 cycles of denaturation at 98 °C for 10 s, and annealing and extension at 68 °C for 22 s. The PCR reaction for species-specific Scomber detection was performed with a 10 µL reaction mixture containing 10 ng of DNA template, 5 µL of KOD SYBR qPCR Mix, and 0.4 µM of each species-specific forward and reverse Scomber primer, followed by thermal cycling consisting of initial denaturation at 98 °C for 2 min, followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 64 °C for 10 s, and extension at 68 °C for 15 s. Finally, the results obtained from both LAMP and real-time PCR analyses were compared to evaluate their concordance.
All experiments were conducted in two independent runs to ensure the reproducibility of results.
Results
Detection of Scomber Species by the LAMP Method
LAMP primer sets were designed targeting regions within the rDNA ITS 1 or ITS 2. Specifically, ITS 1 was utilized for primers specific to each individual Scomber species, while ITS 2 was employed for comprehensive primers covering all four Scomber species. This selection was informed by our previous study indicating that while both ITS 1 and ITS 2 contain highly specific regions for Scomber species, ITS 1 demonstrates a higher specificity for each respective Scomber species (Cui et al. 2023). The designed LAMP primers are detailed in Table 3, and their respective positions on the ITS regions are depicted in Online Resource Fig. S1.
To determine the optimal incubation time for efficient LAMP reactions, 10 ng of template DNA from each of the four Scomber species was incubated in the reaction mixture at 65 °C for 30–60 min with comprehensive or species-specific LAMP primer sets. Consequently, it was determined that the optimal incubation times were 60 min for the comprehensive primer set, 40 min for the S. japonicus-specific and S. australasicus-specific primer sets, 50 min for the S. scombrus-specific primer set, and 35 min for the S. colias-specific primer set. Figure 1 illustrates the validation of specificity for each primer set at these reaction times. With the comprehensive primer set, color changes from purple to blue were observed in the reaction mixtures of all Scomber species (Fig. 1a, numbers 1–4). Conversely, no color change was observed in the reaction mixtures of five closely related fish species belonging to the same Scombridae family, two fish species belonging to a different family, Salmonidae, or in the no-template control (Fig. 1a, numbers 5–11 and N), indicative of successful Scomber-specific detection irrespective of the Scomber species. Similarly, when using the S. japonicus-specific primer set (Fig. 1b), S. australasicus-specific primer set (Fig. 1c), S. scombrus-specific primer set (Fig. 1d), and S. colias-specific primer set (Fig. 1e), blue color changes were specifically observed for each respective Scomber species, indicating successful Scomber species-specific detection.
Comprehensive and species-specific Scomber detection by the LAMP method. The blue color indicates successful amplification of DNA from the respective LAMP primer sets, while the purple color indicates absence of DNA amplification. The primer sets used in the reactions are: a comprehensive primer set, b Scomber japonicus-specific primer set, c S. australasicus-specific primer set, d S. scombrus-specific primer set, and e S. colias-specific primer set. The species of template DNA used in the LAMP reactions are indicated at the top of tubes as follows: (1) S. japonicus, (2) S. australasicus, (3) S. scombrus, (4) S. colias, (5) Thunnus albacares, (6) T. orientalis, (7) T. obesus, (8) Katsuwonus pelamis, (9) Scomberomorus niphonius, (10) Oncorhynchus keta, and (11) Salmo salar. “N” indicates absence of template. The red numbers indicate reaction mixtures where a color change from purple to blue was observed
Detection limits of Scomber DNA
The detection limits of Scomber DNA by the LAMP method were evaluated using each species of Scomber DNA, serially diluted by tenfold, ranging from 10 ng to 1 pg. When using the comprehensive primer set for LAMP reactions, as shown in Fig. 2a, the minimum detectable quantity of Scomber DNA varied depending on the species. Specifically, the detection limits were found to be 100 pg for S. japonicus, S. australasicus, and S. colias, and 10 pg for S. scombrus. Therefore, the detection limit for non-specific Scomber species detection using the comprehensive primer set was determined to be 100 pg. Similarly, species-specific detection also exhibited variation in detection limits among Scomber species. As shown in Fig. 2b and summarized in Table 4, the detection limits were 1 pg for S. japonicus, 10 pg for S. australasicus, 100 pg for S. scombrus DNA, and 10 pg for S. colias. Furthermore, even when DNA other than Scomber species was mixed with the Scomber DNA in the LAMP reaction mixture, both comprehensive and species-specific Scomber detections remained detectable at these detection limit values (Fig. 3a, b).
Detection limit of Scomber DNA by the LAMP method. The color of the reaction solution is as described in Fig. 1. The primer sets used in the reactions are: a comprehensive primer set, b-S. ja, species-specific primer set for Scomber japonicus, b-S. au, species-specific primer set for S. australasicus, b-S. sc, species-specific primer set for S. scombrus, and b-S. co species-specific primer set for S. colias. DNA templates labeled S. ja, S. au, S. sc, and S. co represent DNA from S. japonicus, S. australasicus, S. scombrus, and S. colias, respectively. The amount of DNA template used in the LAMP reactions is indicated at the top of each panel. “N” indicates absence of template
Scomber-specific LAMP in reaction mixtures containing mixed DNA of Scomber and other species. The color of the reaction solution is as described in Fig. 1. The primer sets used in the reactions are: a comprehensive primer set, b-S. ja species-specific primer set for Scomber japonicus, b-S. au species-specific primer set for S. australasicus, b-S. sc species-specific primer set for S. scombrus, and b-S. co, species-specific primer set for S. colias. DNA templates labeled S. ja, S. au, S. sc, and S. co represent DNA from S. japonicus, S. australasicus, S. scombrus, and S. colias, respectively, while 7 sp denotes DNA from the seven fish species other than Scomber listed in Table 1. The amount of Scomber DNA used in the LAMP reactions is as follows: a 100 pg for all four Scomber species; b the detection limit of each target Scomber species, 1 pg for S. japonicus, 10 pg for S. australasicus, 100 pg for S. scombrus, and 10 pg for S. colias. All reaction mixtures, except for “N” (no template), contained 10 ng of DNA from the seven fish species, including 1.43 ng each
Detection of Scomber DNA in Processed Foods
DNA extracted from 14 types of processed foods labeled as containing Scomber ingredients and 4 types of processed foods without Scomber species labeling were subjected to LAMP analysis to determine the presence of Scomber DNA. Table 2 provides a detailed list of the processed foods used in this study included a variety of treatments, such as mashing, salting, grilling, marination, smoking, pickling, canning, oil immersion, roasting, drying, miso pickling, and dried broth. The code numbers in Table 2 correspond to the numbers at the top of tubes in Fig. 4. As illustrated in Fig. 4a, when using the comprehensive LAMP primer set, positive results (indicated by a color change from purple to blue) were observed only in reactions containing DNA from foods labeled as Scomber-containing and a positive control containing S. japonicus-derived DNA (Fig. 4a, numbers 1–10, 15–18, and P). Figures 4b–e display the results of LAMP reactions using Scomber species-specific LAMP primer sets. Among the processed food DNA samples subjected to LAMP, only the two reaction mixtures numbered as 17 and 18, containing DNA from foods labeled as S. colias, yielded positive results when using the S. colias-specific LAMP primer set (Fig. 4e). For the remaining 12 processed food-derived DNA samples labeled as containing Scomber, without specific indication of Scomber species, positive results were obtained as follows: when using the S. japonicus-specific LAMP primer set, samples numbered as 1, 4–10, and 15 were positive (Fig. 4b); when using the S. australasicus-specific LAMP primer set, samples numbered as 15 and 16 were positive (Fig. 4c); when using the S. scombrus-specific LAMP primer set, samples numbered as 2, 3, and 16 were positive (Fig. 4d), with sample 15 showing positivity for both S. japonicus and S. australasicus, and sample 16 showing positivity for both S. australasicus and S. scombrus (Fig. 4).
Detection of Scomber species in processed foods by the LAMP method. The color of the reaction solution is as described in Fig. 1. DNA extracted from 18 processed foods listed in Table 2 was analyzed using the following LAMP primer sets: a comprehensive LAMP primers, b Scomber japonicus specific LAMP primer set, c S. australasicus specific LAMP primer set, d S. scombrus specific LAMP primer set, and e S. colias specific LAMP primer set. The numbers at the top of tubes denote the use of template DNA extracted from the corresponding processed foods listed in Table 2, with identical numbers referencing the code numbers in Table 2. Specifically, reaction tubes 1–10 and 15–18 contained template DNA derived from processed foods labeled as containing Scomber species (particularly 17 and 18 labeled as containing S. colias), tubes 11–14 contained template DNA from processed foods without Scomber species labeling. “P” indicates positive control using template DNA from S. japonicus, and “N” indicates absence of template DNA. The red numbers indicate reaction mixtures where a color change from purple to blue was observed
To validate these results, the Scomber real-time PCR detection method previously developed by us (Cui et al. 2023) was conducted using the same processed food-derived DNA as templates. The obtained results (Table 5) from both comprehensive Scomber PCR detection and each Scomber species-specific real-time PCR detection matched completely with those obtained from the LAMP detection method (Fig. 4). Thus, our developed LAMP method for detecting Scomber in processed foods was proven capable of accurately detecting both Scomber and individual Scomber species.
Discussion
In the present study, we successfully developed a novel approach utilizing loop-mediated isothermal amplification (LAMP) for the rapid and specific detection of Scomber species DNA in processed foods. Through a series of experiments, we validated the specificity, sensitivity, and practical applicability of the LAMP assay.
Our designed LAMP primer sets, targeting regions within the rDNA ITS 1 or ITS 2, were based on the previous study indicating the high specificity of these regions for Scomber species (Cui et al. 2023). The specificity of our primer sets was further confirmed through experimental validation, wherein color changes indicative of Scomber DNA presence were observed only in reactions containing Scomber DNA, while no color change was observed in reactions with closely related fish species or in the absence of template DNA (Fig. 1).
The sensitivity of the LAMP method, as demonstrated in our study, is generally considered lower than that of real-time PCR. Various studies have reported higher sensitivity levels for real-time PCR compared with LAMP in detecting specific DNA targets. For instance, Xiong et al. (2020) developed a LAMP method for Salmo salar with a minimum detection limit of 50 pg, whereas their real-time PCR method achieved a sensitivity ten times higher, with a minimum detection limit of 5 pg. Similar findings have been reported for other fish species, including Oncorhynchus mykiss, Oncorhynchus keta, and Anguilla anguilla, where LAMP detection methods exhibited detection limits ranging from 100 to 500 pg, compared with real-time PCR methods with higher sensitivity (Espiñeira and Vieites 2016; Li et al. 2022, 2013; Spielmann et al. 2019).
In our study, the sensitivity of the LAMP method was lower compared with previous real-time PCR methods developed for Scomber species detection (Cui et al. 2023). However, our LAMP method demonstrated sufficient detection sensitivity for Scomber DNA in commercially processed foods subjected to various treatments, indicating its practical utility (Fig. 4 and Table 5). The determination of detection limits revealed varying sensitivities depending on the Scomber species and the primer sets used. While the comprehensive LAMP primer set exhibited a minimum detection limit of 100 pg for most Scomber species, species-specific primer sets enabled detection at lower concentrations, ranging from 1 to 100 pg (Fig. 2 and Table 4). Importantly, these detection limits were achieved even in the presence of DNA from other fish species (Fig. 3), indicating the robustness of our method in complex sample matrices.
Accurate detection of allergenic food materials, such as mackerels of the genus Scomber, is essential for ensuring food safety and compliance with labeling regulations, particularly in countries, such as Japan, where stringent food labeling laws are enforced to protect consumers with food allergies (Akiyama et al. 2011). The Japanese government has established mandatory foods for labeling and recommended foods for labeling (including Scomber mackerel), with a labeling threshold of 10 µg protein/g food (corresponding to the allergen soluble protein weight/food weight) (Akiyama and Adachi 2021). However, the quantification of DNA from processed foods is influenced by various factors (e.g., heat, cutting, oxidation–reduction, pressure, microbial fermentation, storage conditions, and temperature during food processing), causing variations in DNA yield and inconsistent correlation with protein content. Moreover, the diverse raw materials in processed foods make it challenging to quantify the amount of Scomber-derived protein present. To address these issues, we recently developed comprehensive and species-specific Scomber detection methods using real-time PCR with a detection limit of 1 pg for each (Cui et al. 2023). This study demonstrated that using DNA extracted from mixed foods, Scomber species could be detected if the mixed food contained 10 µg of Scomber meat per gram. Given that 10 µg of Scomber meat contains significantly less than 10 µg of Scomber-derived protein, this method, while not perfect, is expected to detect Scomber species at levels well below the allergen labeling threshold regulated by the Japanese government. Compared with these real-time PCR methods, the LAMP method in the present study exhibits lower detection sensitivity for Scomber species, ranging from 1 to 100 pg (Fig. 2 and Table 4) and did not examine the detection limits of Scomber DNA in mixed foods containing Scomber meat. However, it successfully detects Scomber species even from various processed foods, including dried broth (Table 2), suggesting its practical applicability for detecting Scomber species in processed foods. Additionally, the LAMP method allows for immediate visual confirmation of Scomber presence through a color change from purple to blue upon reaction completion, offering simplicity and reducing detection time by approximately 30–60 min compared with our real-time PCR methods (Cui et al. 2023). LAMP also operates at a constant temperature, eliminating the need for complex thermal cycling equipment required for real-time PCR, thus lowering equipment costs. The cost of reagents and disposable labware for LAMP is comparable to real-time PCR. Therefore, LAMP is advantageous for its simplicity, rapidity, cost-effectiveness, isothermal operation that shortens assay time, and visual detection capability, making it suitable for on-site testing and resource-limited settings.
In conclusion, our study demonstrates the effectiveness and practicality of loop-mediated isothermal amplification (LAMP) as a rapid and specific method for detecting Scomber species in processed foods. The high specificity, simplicity, and rapidity of the LAMP method position it as a valuable tool for food safety monitoring and regulatory compliance efforts. Despite its lower sensitivity compared with real-time PCR, the advantages of LAMP, including ease of use and rapid results interpretation, ensure its suitability for practical applications in various operational settings. The present study contributes to advancing food safety initiatives and safeguarding consumer health by providing a reliable method for detecting allergenic ingredients in processed foods.
References
Akiyama H, Adachi R (2021) Japanese food allergy-labeling system and comparison with the international experience; detection and thresholds. Food Safety 9:101–116
Akiyama H, Imai T, Ebisawa M (2011) Japan food allergen labeling regulation—history and evaluation. Adv Food Nutr Res 62:139–171
Aranishi F (2005) PCR-RFLP analysis of nuclear nontranscribed spacer for mackerel species identification. J Agric Food Chem 53:508–511
Aranishi F, Okimoto T (2004) PCR-based detection of allergenic mackerel ingredients in seafood. J Genet 83:193–195
Catanese G, Manchado M, Fernández-Trujillo A, Infante C (2010) A multiplex-PCR assay for the authentication of mackerels of the genus Scomber in processed fish products. Food Chem 122:319–326
Cui W, Sano Y, Koyama H, Kurose K (2023) Comprehensive and species-specific detection of mackerels of the genus Scomber in processed foods using SYBR Green-based real-time PCR. Fisheries Sci 89:875–887
Espiñeira M, Vieites JM (2016) Genetic system for an integral traceability of European eel (Anguilla anguilla) in aquaculture and seafood products: authentication by fast real-time PCR. Eur Food Res Technol 242:25–31
Goto M, Honda E, Ogura A, Nomoto A, Hanaki K (2009) Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques 46:167–172
Infante C, Manchado M (2006) Multiplex-polymerase chain reaction assay for the authentication of the mackerel Scomber colias in commercial canned products. J AOAC Int 89:708–711
Infante C, Crespo A, Zuasti E, Ponce M, Pérez L, Funes V, Catanese G, Manchado M (2006) PCR-based methodology for the authentication of the Atlantic mackerel Scomber scombrus in commercial canned products. Food Res Int 39:1023–1028
Li X, Li J, Zhang S, He Y, Pan L (2013) Novel real-time PCR method based on growth hormone gene for identification of Salmonidae ingredient in food. J Agr Food Chem 61:5170–5177
Li H, Kong J, Xie R, Yu W, Chen A (2022) Comparative rapid identification of Salmo salar, Oncorhynchus mykiss, and Oncorhynchus keta components based on loop-mediated isothermal amplification and quantitative polymerase chain reaction. Aquaculture 550:737835
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28:e63–e63
Notomi T, Mori Y, Tomita N, Kanda H (2015) Loop-mediated isothermal amplification (LAMP): principle, features, and future prospects. J Microbiol 53:1–5
Parida M, Sannarangaiah S, Dash PK, Rao P, Morita K (2008) Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev Med Virol 18:407–421
Scott AT, Layne TR, O’Connell KC, Tanner NA, Landers JP (2020) Comparative evaluation and quantitative analysis of loop-mediated isothermal amplification indicators. Anal Chem 92:13343–13353
Spielmann G, Ziegler S, Haszprunar G, Busch U, Huber I, Pavlovic M (2019) Using loop-mediated isothermal amplification for fast species delimitation in eels (genus Anguilla), with special reference to the European eel (Anguilla anguilla). Food Control 101:156–162
Velasco A, Sánchez A, Martínez I, Santaclara FJ, Pérez-Martín RI, Sotelo CG (2013) Development of a Real-Time PCR method for the identification of Atlantic mackerel (Scomber scombrus). Food Chem 141:2006–2010
Xiong X, Huang M, Xu W, Cao M, Li Y, Xiong X (2020) Tracing Atlantic Salmon (Salmo salar) in processed fish products using the novel loop-mediated isothermal amplification (LAMP) and PCR assays. Food Anal Method 13:1235–1245
Zhu H, Zhang H, Xu Y, Laššáková S, Korabečná M, Neužil P (2020) PCR past, present and future. Biotechniques 69:317–325
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were reported by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, W., Koyama, H. & Kurose, K. Rapid and specific detection of mackerels of the genus Scomber using loop-mediated isothermal amplification (LAMP). Fish Sci (2024). https://doi.org/10.1007/s12562-024-01812-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12562-024-01812-y