Abstract
Saccharina angustata (Hidakakombu), commonly known as kelp, is an edible macroalgae mainly grown in the Hidaka region, Hokkaido. Hidakakombu is graded based on its shape and color. Low-grade Hidakakombu has low value and is distributed at a low price. Therefore, a method for adding value to low-grade Hidakakombu is needed. In this study, low-grade Hidakakombu was fermented by Lactiplantibacillus pentosus SN001 to add value. Fermentation of Hidakakombu was confirmed by an increase in the number of L. pentosus SN001 cells and a decrease in pH. Fermentation with pyridoxal 5′-phosphate (PLP) increased the GABA content. L. pentosus SN001 in fermented Hidakakombu remained viable in simulated gastric and intestinal juices. Hidakakombu fermented by L. pentosus SN001 was shown to be a source of GABA and probiotics. Therefore, fermentation by L. pentosus SN001 was found to be an effective means of adding high value to low-grade Hidakakombu.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fermentation has been a common method of food preservation since ancient times (Ogrodowczyk and Drabińska 2021). Fermentation can extend the shelf life of a product (Gupta and Abu-Ghannam 2012). The process makes use of the microbial conversion of sugars to acids such as lactic acid, acetic acid, and propionic acid or to ethanol (Bruhn et al. 2019). In recent years, fermented foods have become increasingly popular due to their nutritional qualities and health benefits, besides extending the shelf life of foods and improving palatability (Ogrodowczyk and Drabińska 2021). Within this category, products containing lactic acid bacteria and probiotics have been gaining attention (Gupta and Abu-Ghannam 2012).
Among microorganisms, lactic acid bacteria are one of the γ-aminobutyric acid (GABA) producers. Several lactic acid bacteria have been evaluated for GABA production; L. plantarum (Cagno et al. 2010), L. lactis (Li and Cao 2010), L. brevis (Li and Cao 2010), L. helveticus (Li and Cao 2010), L. paracasei (Li and Cao 2010), and L. pentosus (Phuengjayaem et al. 2021) have been reported. GABA production in fermented foods using lactic acid bacteria has been reported in many studies, including juice (Wang et al. 2021), yogurt (Hussin et al. 2020), red kidney bean, and barley grain (Saraphanchotiwitthaya and Sripalakit 2018). Studies on these fermented foods have used L. plantarum and L. brevis, whereas there are few studies on fermented foods using L. pentosus. GABA is an inhibitory neurotransmitter in the brain cortex (Shelp et al. 1999) and is synthesized mainly via decarboxylation of glutamate by glutamate decarboxylase (GAD) (Capitani et al. 2003). GAD is a pyridoxal 5′-phosphate (PLP)-dependent enzyme, which catalyses the irreversible α-decarboxylation of L-glutamate to GABA. GABA has an antihypertensive effect (Hayakawa et al. 2004; Inoue et al. 2003) and ameliorates conditions such as diabetes (Adeghate and Ponery 2002), insomnia (Okada et al. 2000), and depression (Okada et al. 2000). Due to these physiological functions, there is growing commercial demand for GABA (Lee et al. 2010), and foods fortified with GABA using lactic acid bacteria including raspberry juice (Kim et al. 2009), grape must (Cagno et al. 2010), and kimchi (Seok et al. 2008) have been reported. In seaweed fermentation, an increase in GABA content due to fermentation has been reported in Nori (Tsuchiya et al. 2007) and sea tangle extract (Kim et al. 2018).
Hidakakombu, known as kelp, is mainly grown in the Hidaka region, Hokkaido. Many Japanese traditional foods such as dashi, Kombu rolls, and Tsukudani (food boiled down in soy sauce) are made from Hidakakombu. The grade of Hidakakombu is determined by its shape and color. Low-grade Hidakakombu accounts for more than 80% of the total production of Hidakakombu. Low-grade Hidakakombu has low value and is distributed at a low price. Therefore, a method for adding value to low-grade Hidakakombu is needed. Antihypertensive and probiotic effects of L. casei 001-fermented Hidakakombu have been reported as a high-value addition of Hidakakombu (Sekine et al., 2021). This study was focused on GABA converted from glutamate, which is abundant in Hidakakombu, as another method for adding value. Lactiplantibacillus pentosus SN001, a GABA-producing bacterium with few reported studies, was selected as the fermentative species. The probiotic effect of L. pentosus SN001 was also examined using the same method as for L. casei 001.
In this study, we focused on GABA in fermented Hidakakombu, which has not been analyzed in previous reports, and found a method for adding value to low-grade Hidakakombu from a perspective other than the antihypertensive effect. The purpose of this study is to contribute to the high value addition of low-grade Hidakakombu by GABA production.
Materials and methods
Materials and reagents
Hidakakombu was obtained from Yokoi Kombu (Tokyo, Japan) by drying the washed Hidakakombu and grinding it into a powder in a stone mortar (Hidakakombu powder). The kelp used in this study was the lowest value, “grade 6”. L. pentosus SN001 was used from our laboratory stock. KH2PO4, Na2HPO4, HCl, NaOH, PLP, Tris, α-ketoglutaric acid, 2-mercaptoethanol, GABA, trypsin, pancreatin, bile powder, and NaCl were purchased from Fujifilm Wako Pure Chemicals Co. (Osaka, Japan). Cellulase was purchased from Yakult Pharmaceutical Industries, Ltd. (Tokyo, Japan). GABase was purchased from Sigma-Aldrich (St. Louis, MO, USA). NADP+ was purchased from Oriental Yeast Co., Ltd. (Tokyo, Japan). Cell Counting Kit-8 was purchased from Dojin Chemical Research Institute (Kumamoto, Japan). Pepsin was purchased from Nacalai Tesque (Kyoto, Japan). Plate Count Agar with BCP used for viable cell counting was purchased from Nissui Pharmaceutical Co. (Tokyo, Japan).
Fermentation of Hidakakombu by L. pentosus SN001
L. pentosus SN001 was pre-cultured in ILS medium. ILS medium was prepared based on the composition of Fujimura et al. (2021). For culture, Hidakakombu powder (5 g) was added to 100 mL 33 mM phosphate buffer solution (KH2PO4, Na2HPO4, pH 5.0) and adjusted to pH 5.0 with 1 M HCl. Fifty milligrams of cellulase was added and incubated (45 °C, 120 rpm, 24 h). Then, it was adjusted to pH 6.8 with 1 M NaOH and autoclaved (121 °C, 15 min). The autoclaved medium and pre-cultured L. pentosus SN001 (109 CFU) was cultured at 30 °C. Samples were collected after 0, 1, 2, 3, 4, and 5 days to measure the number of viable cells and pH. The cultures were lyophilized and used for subsequent experiments.
Measurement of GABA content
GABA content was measured according to Yoshihashi et al. (2009) and Jeong et al. (2019), with slight modifications. Lyophilized sample was dissolved in H2O at 10 mg/mL, extracted (50 °C, 125 spm, 1 h), and then allowed to stand for 22 h. The extracted sample was centrifuged (5 °C, 13,000×g, 10 min). The supernatant was lyophilized to obtain the assay sample. The GABA content in L. pentosus SN001-fermented Hidakakombu was determined by an enzymatic method using GABase. To 100 µL of the assay sample, 90 µL of mixed reagent [10 mM α-ketoglutaric acid, 2 mM 2-mercaptoethanol, 0.5 mM NADP+, and 0.25 U/mL GABase dissolved in 100 mM Tris buffer (pH 8.9)] was added, and the mixture was incubated at 30 °C for 15 min. Then, 10 µL of Cell Counting Kit-8 was added, and the absorbance at 450 nm was measured immediately. The concentration of the sample was set at 10 mg/mL, and GABA was used as the standard. The absorbance was measured by a microplate reader.
Measurement of GABA content of fermented Hidakakombu with PLP
A total of 0.22 µm filter-sterilized PLP was added to the autoclaved medium prepared as described in the section “Fermentation of Hidakakombu by L. pentosus SN001”. The concentration of PLP was 0, 20, 50, 100 µM. The mixture and pre-cultured L. pentosus SN001 (109 CFU) was cultured at 30 °C. The cultures were lyophilized for measurement of GABA content.
Simulated gastric juice tolerance test
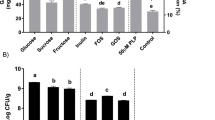
Simulated gastric juice tolerance was tested according to Tsuda (2015), with slight modifications. Thirty milliliters of ILS medium was adjusted to pH 2.0, 3.0, and 4.0 with 1 M HCl. Pepsin was added to each [final concentration 0.04% (w/v)]. A total of 1.5 g of L. pentosus SN001-fermented Hidakakombu was then added and adjusted again to pH 2.0, 3.0, and 4.0 with 1 M HCl to make simulated gastric juice (pH 2.0, 3.0, and 4.0). The samples were incubated in the simulated gastric juice at 37 °C. The samples were collected after 0, 1, 2, and 4 h, and the number of viable cells was measured (Fig. 1).
Simulated intestinal juice tolerance test
Simulated intestinal juice tolerance was tested according to Tsuda (2015), with slight modifications. Thirty milliliters of ILS medium was mixed with bile [final concentration 2% (w/v)], and L. pentosus SN001-fermented Hidakakombu incubated in simulated gastric juice (pH 3.0, 2 h) was added. The mixture was adjusted to pH 7.0 with 1 M NaOH. Then trypsin and pancreatin were added [final concentration 0.01% (w/v)] to obtain simulated intestinal juice. The samples were incubated anaerobically in simulated intestinal juice at 37 °C for 0, 9, 12, 15, and 18 h, and the number of viable cells was measured (Fig. 1). AnaeroPack-Anaero (Mitsubishi Gas Chemical Company, Inc., Tokyo, Japan) was used for anaerobic culture.
Results
Fermentation of Hidakakombu by L. pentosus SN001
The daily changes in number of viable cells, pH, and GABA content of L. pentosus SN001-fermented Hidakakombu are shown in Figs. 2 and 3. The viable cell counts peaked at day 2 of incubation (1.38 × 108 CFU/mL), and pH gradually decreased. The GABA content increased over time with the progression of fermentation.
GABA content of fermented Hidakakombu with PLP
An attempt was made to enhance GABA production by the addition of PLP in the day 2 incubation culture, which had the maximum number of viable cells. The GABA content of the fermented Hidakakombu was increased by the addition of PLP, as shown in Fig. 4. PLP addition effectively increased GABA production, but did not inhibit cell growth. Since the increase in GABA content with the addition of 100 µM PLP was significant (17. 0-fold compared to no addition), L. pentosus SN001-fermented Hidakakombu for 2-day incubation with 100 µM PLP was used for the simulated gastric juice and intestinal juice tolerance test.
Simulated gastric juice and intestinal juice tolerance test
The viable cell counts of L. pentosus SN001-fermented Hidakakombu in simulated gastric and intestinal juice tolerance tests are shown in Fig. 5. In the simulated gastric juice (pH 2.0), the number of viable L. pentosus SN001 cells decreased, and no L. pentosus SN001 cells were observed after 1 h. In simulated gastric juice (pH 3.0 and pH 4.0), the number of viable L. pentosus SN001 cells decreased, but more than 102 CFU/mL remained. In addition, L. pentosus SN001 grew to 107 CFU/mL at 18 h after exposure to simulated intestinal juice.
Viable cell counts of L. pentosus SN001-fermented Hidakakombu in simulated gastric juice (a) and intestinal juice (b). a: pH 4.0(●), pH 3.0 (■), pH 2.0 (▲) (mean ± SD, n = 4). Refer to Fig. 1 for experimental conditions
Discussion
Fermentation of Hidakakombu by L. pentosus SN001
The number of viable cells of L. pentosus SN001-fermented Hidakakombu increased to a maximum of 1.38 × 108 CFU/mL on day 2 of the culture, and pH gradually decreased to 3.92. Lactic acid bacteria produce lactic acid in addition to proliferation through fermentation, which reduces the pH. Therefore, it was clear that the Hidakakombu was fermented by L. pentosus SN001. There are many advantages of fermenting seaweed, such as enhanced suppression of blood pressure elevation (Tsuchiya et al. 2007; Sekine et al. 2021), antioxidant effects (Rianingsih and Sumardianto 2020), and anti-inflammatory effects (Lin et al. 2016), in addition to the increase in GABA production and probiotics. L. pentosus SN001-fermented Hidakakombu may have these functionalities, suggesting that fermentation of Hidakakombu is a very effective means of adding value by enhancing functionality. The GABA content in the fermented Hidakakombu increased daily with the progression of fermentation. GABA is synthesized via the decarboxylation of glutamate by GAD (Capitani et al. 2003), and glutamate may be added as a source of raw material for GABA production (Hasegawa et al. 2018; Gharehyakheh et al. 2019; Hussin et al. 2020). However, the addition of excess glutamate may inhibit GABA production (Hasegawa et al. 2018). In addition, artificial addition of glutamate has not been positively accepted by consumers (Lee et al. 2010). In this study, glutamate, which is naturally occurring in kelp, was used as a source for the production of GABA. It was suggested that glutamate, which is abundantly contained in Hidakakombu, was converted to GABA.
GABA content of fermented Hidakakombu with PLP
One factor that increases GABA production is the addition of PLP. PLP functions as a coenzyme of GAD, which catalyzes GABA production (Capitani et al. 2003). Therefore, since the addition of PLP is expected to increase the production of GABA, PLP was added to Hidakakombu before fermentation. The GABA content of the fermented Hidakakombu with 100 µM PLP was 17 times that of the kelp without PLP. Therefore, it is clear that GABA production in lactic acid fermentation of Hidakakombu was increased by the addition of PLP. These results suggest that L. pentosus SN001-fermented Hidakakombu is a source of GABA. However, the addition of PLP above 100 μM had little additional effect on the production of GABA. This may be because GAD from L. pentosus SN001, which is an apoenzyme, was mostly converted to a holoenzyme by the addition of 100 μM PLP. In other reports of GABA production in lactic acid bacteria, GABA production ceased to increase beyond a threshold of PLP 20 µM in L. plantarum NDC75017 and PLP 10 µM in L. paracasei (Komatsuzaki et al. 2005; Shan et al. 2015). Therefore, PLP 100 μM was the concentration determined to work most effectively for GABA production in Hidakakombu fermentation with L. pentosus SN001. The addition of more than 100 μM PLP would have resulted in a slow increase in holoenzyme production, which is why no significant increase in GABA content was observed.
Simulated gastric juice and intestinal juice tolerance test
Probiotics are defined as live microorganisms that, when administered in sufficient quantities, confer health benefits to the host (Hill et al. 2014). Therefore, probiotics need to be resistant to stomach acid and bile, which have strong bactericidal effects. This tolerance is important for growth after passing through the stomach alive and reaching the intestinal tract (Sekine et al. 2021). Simulated gastric and intestinal juice tolerance tests have been conducted to validate the probiotic effect of lactic acid bacteria (Tsuda 2015; Lim et al. 2018; Sekine et al. 2021). These studies confirmed the survival of lactic acid bacteria in simulated gastric juice and their growth in simulated intestinal juice, suggesting a probiotic effect. The pH of the stomach is maintained at approximately 2.0 and increases to 4.0 when food is ingested (Sagawa et al. 2009). Therefore, pH of simulated gastric juice was set at pH 2.0, pH 3.0, and pH 4.0, assuming fasting and food intake in the stomach. In this study, the number of viable cells of L. pentosus SN001 decreased, but more than 102 CFU/mL remained in simulated gastric juice (pH 3.0). Thus, we used the samples incubated in simulated gastric juice at pH 3.0 for the intestinal juice test. Since the gastric digestion time is reported to be 2–4 h (Pennacchia et al. 2004), and there was no change in the viable cell count in 2–4 h, we set the time for gastric juice processing at 2 h in the intestinal juice test. In simulated intestinal juice, L. pentosus SN001 grew to 107 CFU/mL at 18 h. The rate of increase in the number of lactic acid bacteria in the simulated intestinal fluid was comparable to that of L. casei 001-fermented Hidakakombu. Therefore, the results suggested that L. pentosus SN001 had a probiotic effect, passing through the stomach and growing in the intestine. Hidakakombu contains dietary fibers such as alginate and fucoidan. These dietary fibers function as prebiotics that provide important components for the growth of lactic acid bacteria when they reach the intestine (Charoensiddhi et al. 2020). When L. pentosus SN001-fermented Hidakakombu is ingested, the lactic acid bacteria (probiotics) and Hidakakombu (prebiotics) are exposed to the digestive juices together. Since the nutrients in Hidakakombu assist the growth of the lactic acid bacteria, fermented kelp can be a symbiotic food. From this perspective, the combination of Hidakakombu and L. pentosus SN001 was suggested to be useful as a probiotic food.
Method for adding value to low-grade Hidakakombu
In this study, L. pentosus SN001 was applied to low-grade Hidakakombu, and fermentation was confirmed by an increase in viable cell count and a decrease in pH. It was suggested that GABA was produced from the glutamate derived from Hidakakombu by fermentation without the addition of glutamate. The amount of GABA produced by fermentation was greatly increased by the addition of PLP. In addition, L. pentosus SN001 showed in vitro digestion tolerance, indicating a probiotic effect of L. pentosus SN001-fermented Hidakakombu. L. pentosus SN001-fermented Hidakakombu was shown to be a source of GABA and probiotics. Seaweed is an attractive source for commercialization because of its fast growth rate and lack of need for arable land, fresh water, and fertilizers (Charoensiddhi et al. 2020). In addition, among the substrates used for fermentation, plant-derived fermented foods are gaining attention (Gupta and Abu-Ghannam 2012). They are suitable for vegetarians and lactose-intolerant people, and have the advantage of containing a wide variety of phytochemicals, minerals, and dietary fiber as well as probiotics (Gupta and Abu-Ghannam 2012). Therefore, the utilization value of Hidakakombu is expected to be enhanced by fermentation with L. pentosus SN001. In conclusion, fermentation by L. pentosus SN001 was found to be an effective method for adding high value to low-grade Hidakakombu.
References
Adeghate E, Ponery A (2002) GABA in the endocrine pancreas: cellular localization and function in normal and diabetic rats. Tissue Cell 34:1–6. https://doi.org/10.1054/tice.2002.0217
Bruhn A, Brynning G, Johansen A, Lindegaard M, Sveigaard H, Aarup B, Fonager L, Andersen L, Rasmussen M, Larsen M, Elsser-Gravesen D, Børsting M (2019) Fermentation of sugar kelp (Saccharina latissima)—effects on sensory properties, and content of minerals and metals. J Appl Phycol 31:3175–3187. https://doi.org/10.1007/s10811-019-01827-4
Cagno R, Mazzacane F, Rizzello C, Angelis M, Giuliani G, Meloni M, Servi B, Gobbetti M (2010) Synthesis of γ-aminobutyric acid (GABA) by Lactobacillus plantarum DSM19463: functional grape must beverage and dermatological applications. Appl Microbiol Biotechnol 86:731–741. https://doi.org/10.1007/s00253-009-2370-4
Capitani G, Biase D, Aurizi C, Gut H, Bossa F, Grütter M (2003) Crystal structure and functional analysis of Escherichia coli glutamate decarboxylase. EMBO J 22:4027–4037. https://doi.org/10.1093/emboj/cdg403
Charoensiddhi S, Abraham R, Su P, Zhang W (2020) Chapter four—seaweed and seaweed-derived metabolites as prebiotics. Adv Food Nutr Res 91:97–156. https://doi.org/10.1016/bs.afnr.2019.10.001
Fujimura Y, Shimura M, Nagai H, Hamada-Sato N (2021) Evaluation of angiotensin-converting enzyme-inhibitory activity in abalone viscera fermented by Lactobacillus casei 001. J Funct Foods 82:104474. https://doi.org/10.1016/j.jff.2021.104474
Gharehyakheh S, Elhami Rad A, Nateghi L, Varmira K (2019) Production of GABA-enriched honey syrup using Lactobacillus bacteria isolated from honey bee stomach. J Food Process Preserv 43:e14054. https://doi.org/10.1111/jfpp.14054
Gupta S, Abu-Ghannam N (2012) Probiotic fermentation of plant based products: possibilities and opportunities. Crit Rev Food Sci Nutr 52:183–199. https://doi.org/10.1080/10408398.2010.499779
Hasegawa M, Yamane D, Funato K, Yoshida A, Sambongi Y (2018) Gamma-aminobutyric acid fermentation with date residue by a lactic acid bacterium, Lactobacillus brevis. J Biosci Bioeng 125:316–319. https://doi.org/10.1016/j.jbiosc.2017.10.003
Hayakawa K, Kimura M, Kasaha K, Matsumoto K, Sansawa H, Yamori Y (2004) Effect of a γ-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive Wistar-Kyoto rats. Br J Nutr 92:411–417. https://doi.org/10.1079/bjn20041221
Hill C, Guarner F, Reid G, Gibson G, Merenstein D, Pot B, Morelli L, Canani R, Flint H, Salminen S, Calder P, Sanders M (2014) The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. https://doi.org/10.1038/nrgastro.2014.66
Hussin F, Chay S, Zarei M, Meor Hussin A, Ibadullah W, Zaharuddin N, Wazir H, Saari N (2020) Potentiality of self-cloned Lactobacillus plantarum Taj-Apis362 for enhancing GABA production in yogurt under glucose induction: optimization and its cardiovascular effect on spontaneous hypertensive rats. Foods 9:1826. https://doi.org/10.3390/foods9121826
Inoue K, Shirai T, Ochiai H, Kasao M, Hayakawa K, Kimura M, Sansawa H (2003) Blood-pressure-lowering effect of a novel fermented milk containing γ-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr 57:490–495. https://doi.org/10.1038/sj.ejcn.1601555
Jeong A, Yong C, Oh S (2019) Bioconversion of gamma-aminobutyric acid from monosodium glutamate by Lactobacillus brevis Bmb5. J Microbiol Biotechnol 29:1745–1748. https://doi.org/10.4014/jmb.1907.07004
Kim J, Lee M, Ji G, Lee Y, Hwang K (2009) Production of γ-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. Int J Food Microbiol 130:12–16. https://doi.org/10.1016/j.ijfoodmicro.2008.12.028
Kim D, Dasagrandhi C, Park S, Eom S, Huh M, Mok J, Kim Y (2018) Optimization of gamma-aminobutyric acid production using sea tangle extract by lactic acid bacterial fermentation. LWT 90:636–642. https://doi.org/10.1016/j.lwt.2018.01.011
Komatsuzaki N, Shima J, Kawamoto S, Momose H, Kimura T (2005) Production of γ-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol 22:497–504. https://doi.org/10.1016/j.fm.2005.01.002
Lee B, Kim J, Kang Y, Lim J, Kim Y, Lee M, Jeong M, Ahn C, Je J (2010) Antioxidant activity and γ-aminobutyric acid (GABA) content in sea tangle fermented by Lactobacillus brevis BJ20 isolated from traditional fermented foods. Food Chem 122:271–276. https://doi.org/10.1016/j.foodchem.2010.02.071
Li H, Cao Y (2010) Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids 39:1107–1116. https://doi.org/10.1007/s00726-010-0582-7
Lim J, Yoon S, Tan P, Yang S, Kim S, Park H (2018) Probiotic properties of Lactobacillus plantarum LRCC5193, a plant-origin lactic acid bacterium isolated from kimchi and its use in chocolates. J Food Sci 83:2802–2811. https://doi.org/10.1111/1750-3841.14364
Lin H, Lu W, Tsai G, Chou C, Hsiao H, Hwang P (2016) Enhanced anti-inflammatory activity of brown seaweed Laminaria japonica by fermentation using Bacillus subtilis. Process Biochem 51:1945–1953. https://doi.org/10.1016/j.procbio.2016.08.024
Ogrodowczyk A, Drabińska N (2021) Crossroad of tradition and innovation—the application of lactic acid fermentation to increase the nutritional and health-promoting potential of plant-based food products—a review. Pol J Food Nutr Sci 71:107–134. https://doi.org/10.31883/pjfns/134282
Okada T, Sugishita T, Murakami T, Murai H, Saikusa T, Horino T, Onoda A, Kajimoto O, Takahashi R, Takahashi T (2000) Effect of the defatted rice germ enriched with GABA for sleeplessness, depression, autonomic disorder by oral administration. Nippon Shokuhin Kagaku Kogaku Kaishi 47:596–603. https://doi.org/10.3136/nskkk.47.596 (In Japanese with English abstract)
Pennacchia C, Ercolini D, Blaiotta G, Pepe O, Mauriello G, Villani F (2004) Selection of Lactobacillus strains from fermented sausages for their potential use as probiotics. Meat Sci 67:309–317. https://doi.org/10.1016/j.meatsci.2003.11.003
Phuengjayaem S, Booncharoen A, Tanasupawat S (2021) Characterization and comparative genomic analysis of gamma-aminobutyric acid (GABA)-producing lactic acid bacteria from Thai fermented foods. Biotechnol Lett 43:1637–1648. https://doi.org/10.1007/s10529-021-03140-y
Rianingsih L, Sumardianto (2020) Antioxidant activity in seaweed (Sargassum sp.) extract fermented with Lactobacillus plantarum and Lactobacillus acidophilus. IOP Conf Ser Earth Environ Sci 530:012011. https://doi.org/10.1088/1755-1315/530/1/012011
Sagawa K, Li F, Liese R, Sutton S (2009) Fed and fasted gastric pH and gastric residence time in conscious beagle dogs. J Pharm Sci 98:2494–2500. https://doi.org/10.1002/jps.21602
Saraphanchotiwitthaya A, Sripalakit P (2018) Production of γ-aminobutyric acid from red kidney bean and barley grain fermentation by Lactobacillus brevis TISTR 860. Biocatal Agric Biotechnol 16:49–53. https://doi.org/10.1016/j.bcab.2018.07.016
Sekine T, Nagai H, Hamada-Sato N (2021) Antihypertensive and probiotic effects of hidakakombu (Saccharina angustata) fermented by Lacticaseibacillus casei 001. Foods 10:2048. https://doi.org/10.3390/foods10092048
Seok J, Park K, Kim Y, Bae M, Lee M, Oh S (2008) Production and characterization of kimchi with enhanced levels of γ-aminobutyric acid. Food Sci Biotechnol 17:940–946
Shan Y, Man C, Han X, Li L, Guo Y, Deng Y, Li T, Zhang L, Jiang Y (2015) Evaluation of improved γ-aminobutyric acid production in yogurt using Lactobacillus plantarum NDC75017. J Dairy Sci 98:2138–2149. https://doi.org/10.3168/jds.2014-8698
Shelp B, Bown A, McLean M (1999) Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci 4:446–452. https://doi.org/10.1016/s1360-1385(99)01486-7
Tsuchiya K, Matsuda S, Hirakawa G, Shimada O, Horio R, Taniguchi C, Fujii T, Ishida A, Iwahara M (2007) GABA production from discolored laver by lactic acid fermentation and physiological function of fermented laver. J Food Process Preserv 33:121–125. https://doi.org/10.5891/jafps.33.121 (In Japanese with English abstract)
Tsuda H (2015) Identification of lactic acid bacteria from raw milk of wagyu cattle and their tolerance to simulated digestive juice. Milk Science 64:207–214. https://doi.org/10.11465/milk.64.207
Wang D, Wang Y, Lan H, Wang K, Zhao L, Hu Z (2021) Enhanced production of γ-aminobutyric acid in litchi juice fermented by Lactobacillus plantarum HU-C2W. Food Biosci 42:101155. https://doi.org/10.1016/j.fbio.2021.101155
Yoshihashi T, Warun V, Patcha-ree T, Vipa S (2009) Method for quantification of γ-aminobutyric acid. WO 2009/128461 A
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sekine, T., Yamanushi, M. & Hamada-Sato, N. GABA production and probiotic addition in Saccharina angustata (Hidakakombu) by fermentation of Lactiplantibacillus pentosus SN001. Fish Sci 88, 429–435 (2022). https://doi.org/10.1007/s12562-022-01597-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-022-01597-y