Abstract
Most marine mollusks exhibit osmoregulatory mechanisms to cope with hypo- or hyperosmotic stress. In the present study, we elucidated the effects of exposure to hyposmotic and hyperosmotic stress on bay scallop Argopecten irradians. The Na+, K+, and Cl− ions of bay scallop hemolymph traversed in a similar direction as that of surrounding salinity. Moreover, the gill Na+/K+-ATPase was affected by changes in salinity. We further evaluated the heat shock protein (HSP)70 mRNA expression in the digestive diverticula and glucose levels in the hemolymph of bay scallops. Sudden changes in salinity were found to affect the HSP70 mRNA expression and glucose levels. In 55% salt water (SW) at 48–72 h, HSP70 mRNA expression and glucose levels were reduced compared to the control. HSP70 mRNA was present in the cells of digestive diverticula tissue at higher levels in 120% SW at 72 h than in the control. A TUNEL assay revealed that salinity changes increased cell apoptosis. Thus, exposure to 55% SW or more for more than 24 h may threaten the survival of marine mollusks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As an effect of global warming, the marine environment is undergoing rapid changes due to the rise in sea level and water temperature. Over the past 20 years, glaciers and ice sheets have been rapidly disappearing as a result of warming and heavy rains, thereby leading to changes in ocean salinity (Philippart et al. 2011). In addition, various changes occur in the marine environment, such as salinity changes due to the influx of fresh water. Salinity is one of the most important ecological factors that affect the metabolism, osmotic regulation, stress, and circadian rhythms of marine ecosystems (Mantel and Farmer 1983). The salinity of seawater undergoes periodic changes due to natural influences such as tidal differences, evaporation of surface seawater, and seasonal rainfall (Cheng et al. 2002; Huong et al. 2010).
The various bivalve mollusks inhabiting marine ecosystems are poikilosmotic animals with the same internal and external osmotic pressure. Depending on the species, salinity may range from 4–5% to 75–80% (Berger and Kharazova 1997). In hypotonic conditions, these expand rapidly through osmotic control; moreover, the recovery period varies depending on the species, but the normal volume gradually recovers over time (Pierce et al. 1982). Scallops, like other invertebrates, have an open circulatory system, wherein a body cavity called the “hemocoel” directly transports oxygen and nutrients, and no clear distinction is observed between the blood and interstitial fluid, which is called hemolymph (Monahan-Earley et al. 2013). The hemolymph is similar to the blood of vertebrates and circulates inside the body in direct contact with animal tissues (Sowers et al. 2006), thus indicating that all cells are surrounded by the hemolymph. Therefore, ions in the hemolymph act directly on the surface of tissue cells.
In general, the osmotic regulation of marine bivalves does not differ significantly from that of vertebrates; concentrations of H2O, ions, and bio-organic molecules are the main factors related to osmotic pressure (Amende and Pierce 1980). The osmotic concentration of a cell is the sum of the various ion concentrations, proteins, and other organic compounds inside the cell; Na+, K+, and Cl− are the major osmotically active solutes of the hemolymph (Cheng et al. 2002). The transport of most ions across membranes is mediated by specialized membrane transport proteins. Membrane transport of Na+ and Cl− can be mediated by several channel proteins, including sodium channels and electroneutral cation-Cl cotransporters, among many others (Jia et al. 2018). In particular, Na+/K+-ATPase (NKA) is an enzyme found in the membranes of all animal cells. NKA is an ion channel that maintains the membrane potential by using the energy derived from ATP hydrolysis to export three sodium ions and import two potassium ions across the plasma membrane against an electrochemical gradient.
Furthermore, according to studies in vertebrates including fish and crustaceans, NKA is known to be sensitive to salinity changes (Mancera and McCormick 2000; Pham et al. 2016); however, the response of NKA to salinity changes based on species remains inconsistent, and the detailed mechanism has not yet been clearly elucidated. The expression of the NKA α-subunit in Litopenaeus vannamei was found to increase rapidly when salinity decreased (Pan et al. 2014); however, in the case of Fundulus heteroclitus, it was reported that NKA activity in the gills increased when salinity increased (Mancera and McCormick 2000).
Heat shock proteins (HSPs) play a pivotal role in normal growth and development, as well as in regulating the organism’s stress response (Hartl 1996; Lindquist and Craig 1988). When an external environmental factor deviates from the range suitable for an organism to inhabit, a series of stress responses such as generation of HSP are induced within the organism. In particular, when an organism is subjected to environmental stress, the expression of HSP is increased markedly, thereby enabling the maintenance of cell homeostasis and survival by resisting the stress factor (Kiang and Tsokos 1998; Zhang et al. 2012). The synthesized HSP performs various roles to protect cells from lethal damage caused by factors such as initial water temperature stress (Brown et al. 2004). However, when HSP is overexpressed, it negatively affects the activity of immune substances, and thus adversely affects the immunity of the organism by interfering with antigen recognition (Song et al. 2015).
In bivalve mollusks, this change in the external environment also affects the survival of the organism, because the change in salinity reduces the activity and energy gain of the organism; however, it simultaneously increases the energy requirement to maintain cell volume and avoid osmotic shock (Akberali and Trueman 1985; Hauton et al. 2016). Gluconeogenesis occurs when the organism consumes energy through glucose to increase antioxidant defense against stress induced by various external environmental factors (Silvestre et al. 2021). That is, when the stress caused by the external environment is increased, the energy requirement is also increased; to solve this issue, gluconeogenesis is activated and the glucose level is increased.
The bay scallop Argopecten irradians, tested in this study, is a commercially important shellfish species in Korea and China, as well as the Atlantic coast, and mainly inhabits coastal areas. Therefore, bay scallops are easily exposed to salinity changes caused by the large influx of freshwater from land. However, few studies have reported the physiological aspects related to osmotic regulation and stress response based on the salinity changes in bay scallops (Duggan 1975). Basic biological studies have reported the growth and possibility of survival in the range of 15–30‰ in relation to a saline environment (Mercaldo and Rhodes 1982; Shriver et al. 2002).
Therefore, in the present study, changes in osmotic control and stress response were observed after exposing bay scallops to various salinity changes. The changes in osmoregulatory and stress-related substances were investigated for a short period of time (72 h). NKA is most widely distributed in the chloride cells located in the gill lamella and plays a role in the active transport of ions (Hirose et al. 2003). Therefore, changes in the ion concentration (Na+, K+, and Cl−) of the hemolymph and the expression of NKA mRNA in the gill tissues were observed in relation to the regulation of osmotic pressure. In bivalve mollusks, an active stress response has been reported in the digestive diverticula (Pan et al. 2006). Therefore, in situ hybridization was performed to confirm the expression of HSP70 mRNA in the digestive diverticula, the region where HSP70 is actually expressed in tissues, and the differences in expression. The effect of stress on apoptosis and the extent of cell death at different salinity levels were tested using terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) assay to evaluate the degree of cell apoptosis.
Materials and methods
Ethics statement
All experiments were carried out in strict accordance with the guidelines and ethical principles of the Experimental Animal Welfare Ethics Committee of Korea.
Experimental species and conditions
Bay scallops A. irradians (average shell length 57.0 ± 4.1 mm) were obtained from a commercial market (Tongyeong, Korea). Before commencement of the experiment, bay scallop individuals were acclimated for a week in five 400-L circulation filter tanks containing filtered and aerated seawater (temperature at 17 ± 2 °C, salinity at 35 ‰). Fifty percent of the total volume of seawater was changed daily.
To explore the effects of relative hypo- and hyperosmotic stress on A. irradians, control salinity [35‰ (100% seawater, SW)], hyposalinity [19.25 (55% SW), 24.5 (70% SW), and 29.8 (85% SW) ‰], and hypersalinity [42‰ (120% SW)] solutions were used in the present study; these solutions were prepared from local tap water to which appropriate amounts of Instant Ocean synthetic sea salt (Aquarium Systems, USA) were added. In the salinity acclimation experiment, A. irradians were suddenly transferred from 100% SW to 55, 70, 85, and 120% SW for 3 days (72 h). During the experiment, no scallops were observed to feed and no mortality was observed. Scallops from each replicate treatment group were randomly sampled at 0, 6, 12, 24, 48, and 72 h after exposure to osmotic stress. The gill and digestive diverticula tissues collected from each scallop were then stored at −80 °C for total RNA extraction.
To prepare cryosections, the digestive diverticula tissues from scallops in the control and 120% SW groups at 72 h were washed in phosphate-buffered saline (PBS; pH 7.4) and then immersed overnight in 4% (w/v) paraformaldehyde (PFA). Next, these tissues were washed with PBS for 1 min to drain the excess 4% PFA. Thereafter, they were transferred into 30% sucrose and preserved overnight at 4 °C. The tissues were subsequently embedded using a frozen section compound (FSC 222, Leica Biosystems, Wetzlar, Germany) and stored at −20 °C for subsequent use. Sectioning (8 μm) was done using a cryostat device (CM 3050; Leica, Wetzlar, Germany) and the sections were placed on electrostatically charged slides (Superfrost Plus; VWR International, Radnor, PA, USA). Slides were allowed to air-dry for 30 min and were then stored at −20 °C until further use.
Hemolymph analysis
In this analysis, 1 mL of hemolymph was collected from the adductor muscle using a 1-mL syringe within 3 min of removing the scallops from the tank. Thereafter, the hemolymph was separated via centrifugation (4 °C, 750×g, 3 min) and the collected hemolymph was stored at −80 °C for further analysis. Hemolymph glucose, Na+, K+, and Cl– levels were analyzed using a biochemistry auto-analyzer (series 7180; Hitachi, Tokyo, Japan).
RNA extraction and cDNA synthesis
Total RNA was extracted from the digestive diverticula tissue using TRI Reagent® (Molecular Research Center, Inc., Cincinnati, OH, USA) according to the manufacturer’s instructions. The total RNA monitored (260:280 ratios), which indicates purity, was all between 1.8 and 2.0. The total RNA (2 μg) was reverse-transcribed in 20 μL, using an oligo-d(T)15 anchor, M-MLV [Moloney Murine Leukemia Virus] reverse transcriptase (Promega, Madison, WI, USA) and gDNA Eraser (TaKaRa Bio, Shiga, Japan), according to the manufacturer’s protocol. Synthesized complementary DNA (cDNA) was preserved at −20 °C until further use.
Quantitative polymerase chain reaction (qPCR)
qPCR was performed to determine the relative expression levels of NKA, HSP, and ribosomal protein S18 (RPS18) mRNA using cDNA reverse-transcribed from the total RNA extracted from the digestive diverticula. The primers used for qPCR are summarized in Table 1. PCR amplification was performed using a Bio-Rad CFX96™ Real-Time PCR Detection System (Bio-Rad) and iQ™ SYBR Green Supermix (Bio-Rad), with the following conditions: 0.5 μL of cDNA, 0.26 μM of each primer, 0.2 mM dNTP, SYBR Green, and Taq polymerase in buffer (10 mM Tris–HCl [pH 9.0], 50 mM KCl, 1.4 mM MgCl2, and 20 nM fluorescein) to a total volume of 25 μL. The qPCR protocol was as follows: 95 °C for 5 min, followed by 40 cycles of 95 °C for 20 s, and 55 °C for 20 s. A total of three candidate reference genes, including RPS18 (accession no. AF526232), beta-actin (AY335441), and ribosomal protein L3 (RPL3, AF526233), were evaluated using BestKeeper software (Peqlab, Erlangen, Germany; Pfaffl et al. 2004). The analysis showed a strong correlation (0.452 < r < 0.639) for all candidates. RPS18 had the highest correlation among them and was used as the reference gene. As internal controls, the experiments were duplicated with RPS18, and all data were expressed relative to the corresponding RPS18 threshold cycle (ΔCt) levels. The calibrated ΔCt values (ΔΔCt) for each sample and the internal controls were calculated using the 2−ΔΔCt method.
In situ hybridization
For in situ hybridization (ISH), the primer pairs were designed for a 480-base-pair (bp) amplification fragment obtained from the digestive diverticula. The amplicon was ligated using the pGEM-T Easy Vector system (Promega), and the plasmid was sequenced to confirm the identity and orientation of the product. Digoxigenin (DIG)-labeled oligonucleotides for antisense and sense RNA probes were synthesized by in vitro transcription with T7 RNA polymerase (New England Biolabs, Ipswich, MA, UK) and SP6 RNA polymerase (Promega) using a DIG RNA labeling mix (Roche, 68,298 Mannheim, Germany) according to the manufacturer’s protocol.
ISH was carried out according to a method described previously (Sharker et al. 2020). Briefly, the tissue sections were pre-hybridized with hybridization buffer and yeast total RNA (50 μL) for 2 h and then further hybridized with the RNA probe at 65 °C overnight. After hybridization, the tissue sections were incubated with alkaline phosphatase-conjugated anti-digoxigenin-Ap, Fab fragment antibody (diluted 1:500 in blocking solution [Roche]) at 4 °C overnight. Subsequently, the sections were washed with PBS with Tween, rinsed in alkaline Tris buffer, and finally treated with the labeling mix (2 mL alkaline Tris buffer, 9 μL nitroblue tetrazolium, and 7 μL 5-bromo-4-chloro-3-indoly phosphate disodium salt) to observe the color. Sections were viewed using a stereomicroscope (SMZ1500, Nikon, Tokyo, Japan). Finally, all images were quantified using the ImageJ platform (National Institutes of Health, Bethesda, MD, USA).
Digestive diverticula histology
Digestive diverticula tissues from 55, 100, and 120% SW were sampled at 12 h and fixed in Bouin’s solution, and then dehydrated in increasing concentrations of ethanol solution, clarified in xylene, and embedded in paraffin. Five-micrometer sections were selected and stained with hematoxylin and eosin (H&E) for observation. To compare with ISH images, frozen sections after air drying were fixed, washed, and stained with hematoxylin and alcoholic eosin. The stained slides were observed under a light microscope (Eclipse Ci, Nikon, Melville, NY, USA), and the images were captured using a digital camera (Eclipse Ci).
Detection of apoptotic cells
Apoptotic cells in the digestive diverticula tissues were detected by TUNEL stain, using an ApopTag® Peroxidase In Situ Apoptosis Detection Kit (Sigma-Aldrich) following the manufacturer’s specifications. In brief, tissue histological sections were permeabilized using a mixture containing 10 mM citrate buffer (pH 6.0) and 0.1% Triton X-100 and incubated with TUNEL reaction mixture containing terminal deoxynucleotidyl transferase and fluorescein-dUTP at 37 °C for 1 h. The apoptotic cells in the slides were detected under an optical microscope (×200 magnification; Eclipse Ci, Nikon). Finally, all images were quantified using Image-Pro Plus 6.0 software (Media Cybernetics, Inc., USA) to calculate the area of apoptosis (the brown cells) and were compared to those observed in the digestive diverticula cells.
Statistical analysis
All data were analyzed using SPSS version 25.0 software (IBM SPSS Inc., Armonk, NY, USA). For all the parameters analyzed, exposure to various concentrations and samples at different times of exposure were compared using two-way analysis of variance (ANOVA). ANOVA assumptions were checked using Levene’s test for homogeneity of variance and the Kolmogorov–Smirnov test for data normality. Tukey’s post hoc test for multiple comparisons was used to indicate significance. The significance level adopted was 95% (P < 0.05). Values are expressed as the mean ± standard deviation (SD).
Results
Expression of Na+/K+-ATPase mRNA
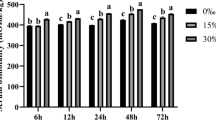
To evaluate the effect of salinity changes on NKA expression, we examined the NKA mRNA levels after hypo- and hyperosmotic stress treatment at different time intervals (Fig. 1). The trend in NKA mRNA expression was similar for increases or decreases in salinity. Moreover, the mRNA expression levels of NKA in the 120% SW group (hyperosmotic treatment) were higher than those of 100% SW at all time intervals. The levels peaked at 12 h and decreased slightly at 24–72 h, with values similar to those observed at 6 h. In the group with 55–85% SW (hyposmotic treatment), the decreased salinity led to reduced NKA mRNA expression. As the exposure time elapsed, the NKA expression level further reduced or remained similar to that observed at 6 h (P < 0.05).
Changes in the expression levels of Na+/K+-ATPase (NKA) mRNA in the gill of Argopecten irradians after the sudden change in salinity for 72 h. Values with different characters differ significantly when exposed to various exposure times in scallop within the same salinity concentration (P < 0.05). The numbers indicate significant differences between the salinity concentrations within the same exposure time (P < 0.05). All values represent the means ± SD (n = 5)
Expression of HSP70 mRNA
Sudden changes in salinity significantly affected the mRNA expression levels of HSP70 (Fig. 2). In the groups with 85 and 120% SW, the HSP70 mRNA expression levels increased with an increase in exposure time, and the expression was consistent with levels observed at 24–72 h. The hyposmotic treatment group revealed various changes in the mRNA expression based on the degree of reduced salinity. In 70% SW, these expression levels increased markedly at 24 h, and then decreased slightly. In 55% SW, the HSP70 mRNA expression levels attained a peak value at 12 h and subsequently decreased; however, at 48–72 h, these levels were reduced compared to those of the control.
Changes in the expression levels of HSP70 mRNA in the digestive diverticula of Argopecten irradians after the sudden change in salinity for 72 h. Values with different characters differ significantly when exposed to various exposure times in scallops within the same salinity concentration (P < 0.05). The numbers indicate significant differences between the salinity concentrations within the same exposure time (P < 0.05). All values represent the means ± SD (n = 5)
In order to confirm the actual expression of HSP70 mRNA in the digestive diverticula tissues, we performed ISH targeting control and 120% SW groups at 72 h, since they had the highest values in the qPCR analysis (Fig. 3). A high-intensity HSP70 signal was detected in basophilic cells and cytoplasmic granules of the digestive diverticula tissues. ISH of HSP70 mRNA confirmed that the expression of HSP70 mRNA was higher in 120% SW at 72 h than in the control.
Distribution of HSP70 mRNA in the digestive diverticula demonstrated by in situ hybridization of (a–c) control and (d–f) scallops exposed to 120% SW for 72 h. (a, b, d, e) Positive signals with an antisense probe are detected in the digestive diverticula cells (black arrows); (c, f) hybridization with the HSP70 mRNA sense riboprobe showing no hybridization signal; (b, e) magnification of the black square of a, d. (g) Quantification of in situ hybridization images of the cells of A. irradians exposed to salinity changes. (h, i) H&E staining using the frozen sections from A. irradians of (h) control and (i) scallops exposed to 120% SW for 72 h. Bpc basophilic cells, G cytoplasmic granules, L lumen. Scale bar = 100 µm
Hemolymph glucose levels
Hemolymph glucose levels increased with salinity changes and exposure time, except in 55% SW (Fig. 4). In 70, 85, and 120% SW groups, the glucose levels increased with exposure time, and the expression was remarkably similar to that at 24–72 h (85% SW) and 48–72 h (120% SW). In 55% SW, the hemolymph glucose levels decreased from 24 h onwards, and at 24–72 h, these levels were reduced compared to those of the control.
Levels of glucose in the hemolymph of Argopecten irradians after the sudden change in salinity for 72 h. Values with different characters differ significantly when exposed to various exposure times in scallop within the same salinity concentration (P < 0.05). The numbers indicate significant differences between the salinity concentrations within the same exposure time (P < 0.05). All values represent the means ± SD (n = 5)
H&E and TUNEL staining
H&E analysis showed that the nucleus was stained blue, and the cytoplasm, collagen fibers, and hemocytes were varying degrees of red (Fig. 5a). Histological analysis of samples showed that the epithelial thickness of tubules was reduced and was distinguishable from the digestive gland. TUNEL assay was used to detect apoptotic cells in the digestive diverticula (Fig. 5b). The apoptotic cells increased in number after the sudden change in salinity compared to those in the control (Fig. 5c).
(a) H&E staining and (b) TUNEL assay using the digestive diverticula sections from Argopecten irradians after the sudden changes in salinity at 100% SW for 0 h, 100% SW for 12 h, 55% SW for 12 h, and 120% SW for 12 h. Dark-colored cells indicated by black arrows are apoptotic. Bpc basophilic cells, G cytoplasmic granules, L lumen. Scale bars = 100 µm. (c) Quantification of TUNEL assay images of the cells of A. irradians exposed to salinity changes. Changes in areas of apoptotic cells relative to tissue area (%)
Discussion
The salinity of the environment which aquatic organisms inhabit is not constant. Therefore, osmotic pressure control, a basic physiological process, is essential to adapt to the difference in ion concentration outside and inside the body in most aquatic animals; however, as the inhabiting environment varies for each organism, the osmotic pressure control mechanism differs from species to species, and a complex mechanism is observed in each species (Kültz 2015; Urbina and Glover 2015). Therefore, it is necessary to study the osmotic regulation and salinity stress of various organisms living in the aquatic environment. The present study was conducted on the bay scallop, a species that inhabits near-sea areas and is frequently exposed to salinity changes.
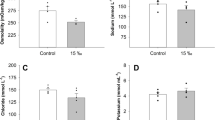
In this study, the bay scallops were exposed to hypo- (55, 70, and 85% SW) and hypersaline (120% SW) environments for 72 h. The results showed that the concentration of Na+, K+, and Cl− ions in the hemolymph was salinity-dependent; that is, the concentration of each ion increased and decreased with an increase and decrease in the salinity concentration, respectively, of the exposed environment (Online resource 1). This trend was described by Cheng et al. (2002), wherein Taiwan abalone Haliotis diversicolor was exposed to various salinity changes of 23–38 ppt; in this case, the increase in salinity and the increasing and decreasing trend of each ion were similar. Lin et al. (2016) reported that when the hard clam Meretrix lusoria was exposed to low and high salinity environments, the Na+ and Cl− ion concentrations decreased and increased, respectively.
This indicates that the abrupt change in salinity directly altered the ion concentration in the hemolymph surrounding the tissue, thereby affecting the balance between the intracellular and extracellular potentials. In addition, this change in balance may have been influenced by changes in cell volume and transmembrane transport of water molecules due to the complexity of osmotic regulation.
Although these ions are individually transported by ion channels, NKA is a multichannel protein capable of transporting Na+ and K+ simultaneously. NKA is a major ion transporter and is essential for osmotic regulation. In this study, the expression of NKA mRNA in the gill tissues increased with an increase in the salinity concentration of the exposed environment, and it subsequently decreased as the salinity concentration decreased.
Sun et al. (2021) reported that the NKA activity of Pinctada fucata increased remarkably with increasing salinity, similar to the change in osmotic pressure. Moreover, when Gadus morhua was moved from 33 to 9‰, the expression of NKA mRNA in the gills was markedly reduced; however, this expression level increased markedly when the animal was moved from 9 to 33‰ (Larsen et al. 2012). Thus, presumably, NKA affected the osmotic regulation of the hemolymph by participating in the active transport of Na+ and K+ through the cell membrane of the gills in the bay scallop as well.
HSP plays a pivotal role in preventing irreversible protein denaturation, and is regulated by various environmental factors; moreover, HSP is involved in stress and immune responses (Gestal et al. 2008). Therefore, it is very important to investigate the function and expression of HSP70 under various stress environments. In this study, it was observed that the expression levels of HSP70 mRNA increased with changes in salinity. Notably, in 55% SW, the group with lowest salinity, a rapid decrease in the expression level was observed from 24 h after exposure. Nie et al. (2017) reported that the expression of HSP70 was increased when the Manila clam was exposed to a low-salinity environment, and HSP expression was also found to increase with an increase or decrease in salinity in Crassostrea hongkongensis and Pinctada martensii (Fu et al. 2011; Li et al. 2016). The aforementioned results indicate that the expression of HSP70 mRNA can be induced under saline stress, and presumably, the increase in HSP70 expression satisfies the need for more HSP70 to promote the refolding of proteins that otherwise become abnormal proteins.
ISH was additionally performed in the digestive diverticula to determine where HSP70, which is normally expressed by external stressors, is actually induced by salinity changes in the digestive diverticula of the bay scallop and leads to a difference in the expression level. The subjects exposed for 3 days in the 120% SW group, which was confirmed via qPCR to exhibit the highest expression of HSP mRNA in various salinity change groups, were compared to the control. In addition, it was visually confirmed that HSP70 was expressed in basophilic cells and cytoplasmic granules of the digestive diverticula. Furthermore, in the 120% SW group, HSP mRNA expression levels in the channels and connective tissues of the digestive diverticula were increased when compared with those of the control group. The results of ISH in Pacific oyster Crassostrea gigas infected with Ostreid herpesvirus showed increased expression of the HSP70 mRNA in the connective tissues of the digestive gland compared to the control (De-la-Re-Vega et al. 2017). The results of these previous studies indicate that this process is similar to that observed when the bay scallop was exposed to changes in the salinity environment.
In general, bivalve mollusks increase their energy requirements to avoid osmotic shock in changing salinity environments (Akberali and Trueman 1985). An increase in glucose level in the absence of food indicates that gluconeogenesis was stimulated. In this study, the glucose level in the hemolymph was increased in all salinity change experimental groups except for the 55% SW group. This result was consistent with that of Haider et al. (2018), who reported an increase in carbohydrate content (in glucose equivalents) when the soft-shell clam Mya arenaria was exposed to salinity changes.
Therefore, it is presumed that gluconeogenesis is induced in the bay scallop for energy consumption, thereby resulting in increased glucose levels, even in the absence of an external energy supply, to cope with stress by increasing the body’s defense mechanism in an environment with large salinity changes. However, when exposed to 55% SW (19.25‰) for more than 24 h, the glucose level tended to decrease, similar to the expression pattern of HSP70 mRNA. This result is consistent with HSP70 expression, wherein the bay scallops were exposed to a hyposaline environment of about 20‰ or less for more than 1 day, the individual synthesized energy on its own to relieve the hyposalinity stress, and the energy demand increased to more than the energy obtained, thereby increasing the limit of self-recovery. Presumably, this occurs because energy is no longer consumed by lowering the body’s metabolism and not being able to overcome it.
Additionally, to determine cell death by osmotic stress, the cell damage in the digestive diverticula cells of the scallop was also observed. The number of apoptotic cells increased with salinity variation. Slobodskova et al. (2012) reported that when M. yessoensis was exposed to an anoxic environment, cell damage was significantly increased. The results of the present study are consistent with these studies, wherein we observed increases in cell damage in A. irradians in response to increased and decreased water salinity. Increased osmotic stress of A. irradians due to salinity change is thought to have affected the levels of cell damage.
In conclusion, this study presented endocrinological data on the osmoregulatory mechanism of bay scallop by explaining the changes in salinity stress in the digestive diverticula as well as ion concentration and osmotic ability in the gills of hemolymph in hypo- and hypersalinity environments. The ion concentration of the bay scallop revealed a similar tendency to that of the external environment, which was consistent with the expression of NAK. In addition, HSP70 mRNA expression and glucose levels were increased in response to the external stress; however, exposure to a hyposaline environment of 55% SW or more for more than 24 h led to a sharp decline in the body’s defense capability, thereby increasing the threat to survival. Although this study observed the physiological changes in bay scallops for a short period, a long-term study is further needed to observe the physiological changes in bay scallops according to salinity changes from an ecological point of view. These research data can be used as basic biological data in the research field related to salinity change.
References
Akberali HB, Trueman ER (1985) Effects of environmental stress on marine Bivalve Molluscs. Adv Mar Biol 22:101–197. https://doi.org/10.1016/S0065-2881(08)60051-6
Amende LM, Pierce SK (1980) Cellular volume regulation in salinity stressed molluscs: the response of Noetia ponderosa (Arcidae) red blood cells to osmotic variation. J Comp Physiol 138:283–289. https://doi.org/10.1007/BF00691562
Berger VJ, Kharazova AD (1997) Mechanisms of salinity adaptations in marine molluscs. In: Naumov AD et al (eds) Interactions and adaptation strategies of marine organisms. Springer, Dordrecht, pp 115–126
Brown HM, Briden A, Stokell T, Griffin FJ, Cherr GN (2004) Thermotolerance and HSP70 profiles in adult and embryonic California native oysters, Ostreola conchaphila (Carpenter, 1857). J Shellfish Res 23:135–141
Cheng W, Yeh SP, Wang CS, Chen JC (2002) Osmotic and ionic changes in Taiwan abalone Haliotis diversicolor supertexta at different salinity levels. Aquaculture 203:349–357. https://doi.org/10.1016/S0044-8486(01)00606-8
De-la-Re-Vega E, Sánchez-Paz A, Gallardo-Ybarra C, Lastra-Encinas MA, Castro-Longoria R, Grijalva-Chon JM, López-Torres MA, Maldonado-Arce AD (2017) The Pacific oyster (Crassostrea gigas) Hsp70 modulates the Ostreid herpes virus 1 infectivity. Fish Shellfish Immunol 71:127–135. https://doi.org/10.1016/j.fsi.2017.09.079
Duggan WP (1975) Reactions of the bay scallop, Argopecten irradians, to gradual reductions in salinity. Chesap Sci. https://doi.org/10.2307/1350947
Fu D, Chen J, Zhang Y, Yu Z (2011) Cloning and expression of a heat shock protein (HSP) 90 gene in the haemocytes of Crassostrea hongkongensis under osmotic stress and bacterial challenge. Fish Shellfish Immunol 31:118–125. https://doi.org/10.1016/j.fsi.2011.04.011
Gestal C, Roch P, Renault T, Pallavicini A, Paillard C, Novoa B, Oubella R, Venier P, Figueras A (2008) Study of diseases and the immune system of bivalves using molecular biology and genomics. Rev Fish Sci 16:133–156. https://doi.org/10.1080/10641260802325518
Haider F, Sokolov EP, Sokolova IM (2018) Effects of mechanical disturbance and salinity stress on bioenergetics and burrowing behavior of the soft-shell clam Mya arenaria. J Exp Biol. https://doi.org/10.1242/jeb.172643
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–579. https://doi.org/10.1038/381571a0
Hauton C (2016) Physiological responses: effects of salinity as a stressor to aquatic invertebrates. In: Solan M, Whiteley NM (eds) Stressors in the marine environment: physiological and ecological responses; societal implications. Oxford University Press, Oxford, pp 3–24
Hirose S, Toyoji K, Naito N, Takei Y (2003) Molecular biology of major components of chloride cells. Comp Biochem Physiol B 136:593–620. https://doi.org/10.1016/s1096-4959(03)00287-2
Huong DTT, Jasmani S, Jayasankar V, Wilder M (2010) Na/K-ATPase activity and osmo-ionic regulation in adult whiteleg shrimp Litopenaeus vannamei exposed to low salinities. Aquaculture 304:88–94. https://doi.org/10.1016/j.aquaculture.2010.03.025
Jia Y, Liu X (2018) Expression of Na+/K+-ATPase was affected by salinity change in pacific abalone Haliotis discus hannai. Front Physiol 9:1244. https://doi.org/10.3389/fphys.2018.01244
Kiang JG, Tsokos GC (1998) Heat shock protein 70 kDa: molecular biology, biochemistry and physiology. Pharmacol Ther 80:183–201. https://doi.org/10.1016/S0163-7258(98)00028-X
Kültz D (2015) Physiological mechanisms used by fish to cope with salinity stress. J Exp Biol 218:1907–1914. https://doi.org/10.1242/jeb.118695
Larsen PF, Nielsen EE, Meier K, Olsvik PA, Hansen MM, Loeschcke V (2012) Differences in salinity tolerance and gene expression between two populations of Atlantic Cod (Gadus morhua) in response to salinity stress. Biochem Genet 50:454–466. https://doi.org/10.1007/s10528-011-9490-0
Li J, Zhang Y, Liu Y, Zhang Y, Xiao S, Yu Z (2016) Co-expression of heat shock protein (HSP) 40 and HSP70 in Pinctada martensii response to thermal, low salinity and bacterial challenges. Fish Shellfish Immunol 48:239–243. https://doi.org/10.1016/j.fsi.2015.11.038
Lin CH, Yeh PL, Lee TH (2016) Ionic and amino acid regulation in hard clam (Meretrix lusoria) in response to salinity challenges. Front Physiol 7:368. https://doi.org/10.3389/fphys.2016.00368
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677. https://doi.org/10.1146/annurev.ge.22.120188.003215
Mancera JM, McCormick SD (2000) Rapid activation of gill Na+, K+-ATPase in the euryhaline teleost Fundulus heteroclitus. J Exp Zool 287:263–274. https://doi.org/10.1002/1097-010X(20000901)287:4%3c263::AID-JEZ1%3e3.0.CO;2-I
Mantel LH, Farmer LL (1983) Osmotic and ionic regulation. In: Mantel LH (ed) The biology of crustacea, vol 5. Academic Press, New York, pp 53–161
Mercaldo RS, Rhodes EW (1982) Influence of reduced salinity on the Atlantic bay scallop, Argopecten irradians, in a shallow estuary. J Shellfish Res 2:177–181
Monahan-Earley R, Dvorak AM, Aird WC (2013) Evolutionary origins of the blood vascular system and endothelium. J Thromb Haemost 11:46–66. https://doi.org/10.1111/jth.12253
Nie H, Liu L, Huo Z, Chen P, Ding J, Yang F, Yan X (2017) The HSP70 gene expression responses to thermal and salinity stress in wild and cultivated Manila clam Ruditapes philippinarum. Aquaculture 470:149–156. https://doi.org/10.1016/j.aquaculture.2016.12.016
Pan LQ, Ren J, Liu J (2006) Responses of antioxidant systems and LPO level to benzo(a)pyrene and benzo(k)fluoranthene in the haemolymph of the scallop Chlamys ferrari. Environ Pollut 141:443–451. https://doi.org/10.1016/j.envpol.2005.08.069
Pan L, Liu H, Zhao Q (2014) Effect of salinity on the biosynthesis of amines in Litopenaeus vannamei and the expression of gill related ion transporter genes. J Ocean Univ China 13:453–459. https://doi.org/10.1007/s11802-014-2013-y
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: bestkeeper-excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515. https://doi.org/10.1023/B:BILE.0000019559.84305.47
Pham D, Charmantier G, Boulo V, Wabete N, Ansquer D, Dauga C (2016) Ontogeny of osmoregulation in the Pacific blue shrimp. Comp Biochem Physiol B Biochem Mol Biol 196:27–37. https://doi.org/10.1016/j.cbpb.2015.12.007
Philippart CJ, Anadón R, Danovaro R, Dippner JW, Drinkwater KF, Hawkins SJ, Reid PC (2011) Impacts of climate change on European marine ecosystems: observations, expectations and indicators. J Exp Mar Bio Ecol 400:52–69. https://doi.org/10.1016/j.jembe.2011.02.023
Pierce SK (1982) Invertebrate cell volume control mechanisms: a coordinated use of intracellular amino acids and inorganic ions as osmotic solute. Biol Bull 163:405–419. https://doi.org/10.2307/1541452
Sharker MR, Nou IS, Kho KH (2020) Molecular characterization and spatiotemporal expression of prohormone convertase 2 in the Pacific abalone, Haliotis discus hannai. PloS one 15(4):e0231353. https://doi.org/10.1371/journal.pone.0231353
Shriver AC, Carmichael RH, Valiela I (2002) Growth, condition, reproductive potential, and mortality of bay scallops, Argopecten irradians, in response to eutrophic-driven changes in food resources. J Exp Mar Bio Ecol 279:21–40. https://doi.org/10.1016/S0022-0981(02)00340-4
Silvestre JA, Pires SF, Pereira V, Colaço M, Costa AP, Soares AM, Rodrigues A (2021) Meeting the salinity requirements of the bivalve mollusc Crassostrea gigas in the depuration process and posterior shelf-life period to improve food safety and product quality. Water 13:1126. https://doi.org/10.3390/w13081126
Slobodskova VV, Zhukovskaya AF, Chelomin VP (2012) DNA damage in the gill cells of the marine scallop Mizuhopecten yessoensis during anoxic stress and aerobic recovery. Ocean Sci J 47:95–100. https://doi.org/10.1007/s12601-012-0010-x
Song L, Wang L, Zhang H, Wang M (2015) The immune system and its modulation mechanism in scallop. Fish Shellfish Immunol 46:65–78. https://doi.org/10.1016/j.fsi.2015.03.013
Sowers A, Young S, Grosell M, Browdy C, Tomasso J (2006) Hemolymph osmolality and cation concentrations in Litopenaeus vannamei during exposure to artificial sea salt or a mixed-ion solution: relationship to potassium flux. Comp Biochem Physiol A Mol Integr Physiol 145:176–180. https://doi.org/10.1016/j.cbpa.2006.06.008
Sun J, Chen M, Fu Z, Yang J, Zhou S, Yu G, Ma Z (2021) A comparative study on low and high salinity tolerance of two strains of Pinctada fucata. Front Mar Sci 8:1039. https://doi.org/10.3389/fmars.2021.704907
Urbina MA, Glover CN (2015) Effect of salinity on osmoregulation, metabolism and nitrogen excretion in the amphidromous fish, Inanga (Galaxias maculatus). J Exp Mar Bio Ecol 473:7–15. https://doi.org/10.1016/j.jembe.2015.07.014
Zhang G, Fang X, Guo X, Li L, Luo R, Xu F (2012) The oyster genome reveals stress adaptation and complexity of shell formation. Nature 490:49–54. https://doi.org/10.1038/nature11413
Acknowledgements
This work was supported by a grant from the National Research Foundation of Korea (NRF) (Ministry of Education) (2021R1F1A1046310).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12562_2022_1586_MOESM1_ESM.pdf
Supplementary file1 Concentration of (a) Na+, (b) K+, and (c) Cl– in the hemolymph of Argopecten irradians after a sudden change in salinity for 72 h. Values with different characters differ significantly when exposed to various exposure times in scallop within the same salinity concentration (P < 0.05). The numbers indicate significant differences between the salinity concentrations within the same exposure time (P < 0.05). All values represent the means ± SD (n = 5) (PDF 153 KB)
Rights and permissions
About this article
Cite this article
Song, J.A., Choi, Y.J. & Choi, C.Y. Effects of salinity changes on the osmoregulatory and stress responses in the bay scallop Argopecten irradians. Fish Sci 88, 275–283 (2022). https://doi.org/10.1007/s12562-022-01586-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-022-01586-1