Abstract
The present study aimed to evaluate suitable protein sources of formulated diet to replace prey fish. Regarding the test diet, the two dietary treatments (FM: Peruvian anchovy fish meal, and ETFM: enzyme-treated Chilean fish meal) were employed. Prey fish (PF; spangled emperor fish Lethrinus nebulosus) was used as a control. FM and ETFM diets are fed together with a limited amount of PF to Pacific bluefin tuna (PBT) larvae having an initial mean total length of 17.9 mm and body weight of 52.8 mg at 20 days post-hatching during the weaning period. Survival and growth were compared after 10 days of the feeding trial. Survival was significantly higher in the PF group than the FM and ETFM group. The PF group had significantly greater growth performance than the other groups. Between FM and ETFM groups, fish in the FM group showed significantly better growth than the ETFM group. Regarding the essential amino acids of the carcass, similar amino acid content was recorded in all treatments. However, carcass docosahexaenoic acid in the PF group was significantly higher than in the other groups. These results suggest that Peruvian anchovy meal without enzyme digestion can be used as the protein source in the formulated diet for juvenile PBT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The high market value of Pacific bluefin tuna (PBT) stocks has led to intensified fishing pressure that, in turn, has resulted in a drastic reduction in population abundance in all ocean waters where this fish is found. High prices sustained by strong market demand also create opportunities for tuna ranching, which is arguably the most profitable form of fish farming in the world (Buentello et al. 2016a, b). Therefore, the demand for this species is gradually increasing. The first effort for farming PBT was carried out in Japan during the 1970s (Miyashita 2002). In 2002, the full-cycle culture of PBT in captivity was achieved by the Fisheries Laboratory of Kindai University with the first successful spawning of first-generation broodstock (Sawada et al. 2005).
Farmed PBT was first commercially harvested (900 t) in 1993 in Japan, and tuna farming was similarly adopted globally (Tada 2010). The total production of PBT in Japan gradually increased from 2000 t in 2000 to 13,413 t in 2016 (JFA 2015; MAFF 2016). Further, PBT farming production was the third-highest in marine fish aquaculture of Japan after yellowtail Seriola quinqueradiata and red sea bream Pagrus major, showing a tendency to increase the production of PBT over the years (MAFF 2016). Because juvenile PBT are grown to a market size of 30–50 kg over around three years by farming (Masuma et al. 2011), a large amount of mature PBT was caught in the ocean. However, management for purse seine fisheries targeting PBT operated by fishing vessels were set in 2015 as follows: voluntarily restricting the annual catch to less than 4500 t for the small pelagic purse seine fishery catching juvenile PBT of less than 30 kg in body weight which operates in the East China Sea and Sea of Japan (JFA 2015). Therefore, large commercial companies, among others, have invested in PBT hatcheries and are now producing commercial quantities of juveniles (Masuma et al. 2011).
At present, production of juvenile PBT in commercial hatcheries still depends on the supply of live prey such as rotifer, Artemia, yolk-sac larvae and minced fish meat. However, this practice is difficult, costly, laborious and time-consuming to maintain efficiently and often nutritionally incomplete (Seoka et al. 2007; Haga et al. 2010). Even though several efforts have been made to advance and stabilize the production technique for this species, the survival rate from hatch to juvenile is still less than 2% (Sawada et al. 2005; Buentello et al. 2016a, b). One reason for the lower survival rate could be the limited supply of prey fish such as spangled emperor Lethrinus nebulosus and Japanese parrotfish Oplegnathus fasciatus. It is necessary to replace these feed items with formulated diets for larvae and juvenile PBT.
PBT does not readily digest fish meal compared with the closely related species chub mackerel Scomber japonicus (Takii et al. 2007a); however, they do show high digestibility for enzyme-treated Chilean fish meal (Takii et al. 2007b). Further, Ji et al. (2008) solved this problem by enhancing the digestibility of fish meal by enzyme-treatment. In 2008, a feed company (Marubeni Nisshin Feed Co., Ltd., Tokyo, Japan) developed a formulated diet based on enzyme-treated Chilean fish meal, and it has been widely distributed in PBT seed production. The merits of the enzyme-treated fish meal include higher digestibility and absorption of dietary protein components due to their low molecular weight. However, the enzyme-treated fish meal depends on high-quality fish meal from Chile. Also, due to the limited supply of Chilean fish meal, the fish meal price increased (Oil World Annual 2019). With this situation, the availability of high-quality enzyme-treated Chilean fish meal has decreased.
However, there is as yet no information available on the utility of Peruvian anchovy meal and enzyme-treated Chilean fish meal in the diet of PBT larvae and juveniles. The main objective of the present study was to investigate whether a formulated diet could replace prey fish without compromising the growth of PBT. Commercially available Peruvian anchovy meal and enzyme-treated Chilean fish meal were used, and the resultant growth performance and changes in fish body composition were evaluated.
Materials and methods
Experimental diets
Two test diets were formulated with two kinds of fish meal (FM: super prime grade Peruvian anchovy meal, and ETFM: enzyme-treated Chilean fish meal) provided by Marubeni Nisshin Feed Co., Ltd., Tokyo, Japan. Spangled emperor larvae Lethrinus nebulosus was used as a control diet (PF prey fish). Fertilized eggs of spangled emperor fish were kept in 200 L tanks, and hatched before feeding. Defatted horse mackerel meal, blood meal, DHA70E, chicken egg lecithin, fish oil, taurine, α-starch, monobasic calcium phosphate, vitamin mixture, mineral mixture, choline chloride, vitamin E, sodium ascorbic acid and inosine-5′-monophosphate (IMP) were commonly included in test diets. These ingredients were mixed with 50% distilled water (w/w) at 40 °C for 2 h using a mixer, and pelleted through the meat chopper machine (AB-120, AKIRA Co., Hyogo, Japan). The size of the pellet (ca. Ø 750 µm) was adjusted using sieves. The diets (sinking pellets) were freeze-dried in a freeze dryer (RLE-206II, Kyowa Vacuum Engineering, Co., Ltd., Saitama, Japan) for 12 h, then were stored in a freezer at −30 °C until use.
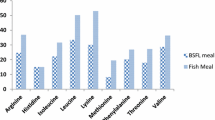
The moisture of the formulated diets was 5.2–9.2%, and that in the control diet (PF) was 93.3%. The crude protein and lipid content of formulated diets was approximately 60% and 18%, respectively (Table 1). These values were determined based on the nutritional requirements of PBT juveniles by Biswas et al. 2009a. The ETFM diet contained the highest total amino acid (482.6 g/kg, dry-weight), followed by PF and FM diet (PF: 480.6 g/kg, dry-weight., FM: 448.6 g/kg, dry-weight). Regarding free amino acid content, the FM diet had threefold higher histidine level (4.4 g/kg, dry-weight) than the other diets (ETFM: 1.6 g/kg, dry-weight., PF: 1.3 g/kg, dry-weight, Table 2). PF had the highest docosahexaenoic acid (DHA) level (17.8% of total fatty acid), followed by ETFM and FM diets (ETFM: 10.1% of total fatty acid, FM: 9.6% of total fatty acid, Table 3).
Fish, experimental design, and sampling protocol
PBT was allowed to spawn in cages, and fertilized eggs were raised in tanks at Amami Fish Farm, Amami, Kagoshima, Japan. Before the experiment, PBT larvae were fed rotifers Brachionus rotundiformis and Artemia nauplii until they reached 10 mm in total length. Then, they fed yolk-sac larvae of spangled emperor fish Lethrinus nebulosus up to 18 mm in total length. A total of 1800 PBT larvae with an initial size of 17.9 ± 2.6 mm and body weight of 52.8 ± 2.6 mg (20 days post hatching, dph) were transported and randomly distributed into six 500-L circular polycarbonate experimental tanks, resulting in 300 fish per tank, and subjected for three different feeding treatments with duplicate (Table 4).
The first 3 days of the trial were designated for weaning the PBT (20–22 dph), and the subsequent 7 days were designated for sole feeding of the test diets (23–29 dph). During the weaning period, formulated diet (FM or ETFM; provided hourly between 07:00 and 19:00) and PF (provided three times daily at 07:00, 13:00 and 19:00) were fed by hand. During the period of sole feeding, fish were fed diets 72 times a day (6 times/h), 10–15 g diet/tank (Table 4). The amount of PF was provided to apparent satiety but reduced gradually, with decreasing ratios of PF: PBT larvae (ind.: ind.) of 140:1, 100:1, and 60:1, to promote the weaning of PBT larvae onto the test diets. Fish in the PF group were fed yolk-sac larvae to apparent satiety throughout the feeding trial. The feeding trial was terminated on the seventh day of the sole feeding of test diet period to obtain sufficient numbers of survivors for chemical analysis. The flow rate of seawater was adjusted to 1200% of the tank volume per day. The average water temperature and oxygen concentration were maintained at 27.3 ± 0.3 °C and 7.1 ± 0.4 mg/L, respectively (Table 4). Black tape was placed on the tank walls in a lattice arrangement to avoid collision with tank walls (Ishibashi et al. 2013).
Prior to each sampling, feeds were withheld from the fish for 12 h to ensure excretion of intestinal content. One hundred fish were sampled at the start of the feeding trial and kept frozen at −80 °C until analysis. Fish were also sampled three times at 4, 7, and 10 days after the initiation of the feeding trial (24, 27, and 30 dph) to evaluate growth performance. Five and 40 fish were collected at 4, 7, and 10 days (24, 27, and 30 dph), respectively for the measurement of total and fork length. Body depth was also determined using an electronic micrometer (DT-150, Niigata-Seiki Co., Ltd., Niigata, Japan). At the same time, the wet weight of the whole body was weighed using a balance (AUW220D, Shimadzu Co., Kyoto, Japan). The carcasses were stored at −80 °C and were transferred to the Laboratory of Fish Nutrition, Tokyo University of Marine Science and Technology for chemical analysis.
The tank bottom was cleaned daily to maintain good water quality, and the number of dead fish collected was recorded to estimate the survival rate. The number of surviving fish was calculated by subtracting the total number of dead fish from the initial number of fish. Weight gain (WG), specific growth rate (SGR), condition factor (CF), and survival rate were calculated using the following formulae:
WG (%) = [final wet weight (g) − initial wet weight (g)]/initial wet weight (g) × 100.
SGR (%) = [ln final wet weight (g) − ln initial wet weight (g)] × 100/time (days).
CF = wet weight (g)/fork length3 (cm) × 1000.
Survival rate (%) = number of fish at harvest/number of fish stocked × 100.
PBT juveniles show continuous cruise swimming and circling in round shape tanks. Diets were fed just ahead of their swimming direction. They did not feed diets that floated on the water surface or had fallen to the bottom of the tank, but only fed diets drifting in the water column. With this situation, we had to maintain frequent feeding by hand in order to achieve satiation feeding of diets. However, it was difficult to collect uneaten diets to determine the exact feed intake. Therefore, we had to give up determining the daily feed intake in the present study. The present feeding trial followed a standard operating procedure approved by the animal care and use committee of the Tokyo University of Marine Science and Technology, Japan.
Chemical analyses
Diets and fish samples were analyzed by standard methods for moisture, crude protein and ash (AOAC 2000), and all chemical analyses were performed in triplicate and averaged. Crude lipid was determined by using a mixture of chloroform and methanol (2:1, v/v) for extraction (Folch et al. 1957). The fatty acid methyl ester was prepared, according to Morrison and Smith (1964). It was diluted with n-hexane and injected onto a gas chromatograph (GC-2025, Shimadzu, Tokyo, Japan) equipped with a SUPERCO-WAX10 column (30 m × 0.32 mm; Sigma-Aldrich Co., Ltd., St. Louis, MO, USA). The column oven temperature was increased from 170 to 250 °C at a rate of 2 °C/min and then maintained at 250 °C. The area percent of each peak was determined with a digital integrator (C-R8A Chromatopac; Shimadzu Co., Kyoto, Japan). Each fatty acid methyl ester in samples was identified by comparison with those prepared from fish meal. Total amino acid content was determined using an automatic amino acid analyzer (JLC-500/v; JEOL Co., Tokyo, Japan), following the methods of Boonyoung et al. (2012). For free amino acid analysis, samples were homogenized for 90 s (Polytron PT10-35; Kinematica AG, Tokyo, Japan), and deproteinized with 10 mL of 2% (w/v) sulfosalicylic acid (Wako Pure Chemical Industries, Osaka, Japan). Next, the samples were centrifuged twice in a high-speed refrigerated centrifuge (SRX-201; Tommy Co., Tokyo, Japan) at 1610×g for 15 min at 4 °C. The upper layers were decanted and passed through a 0.45 µm membrane filter (Millipore, Corp., Darmstadt, Germany), and injected into the automatic amino acid analyzer.
Statistical analyses
Growth performance and initial and final carcass composition data were analyzed by using the IBM SPSS 19 software package for Windows (SPSS Inc., Chicago, IL, USA). Data were evaluated for assumptions, including normality and homogeneity of variance, using the Shapiro–Wilk and Levene’s tests, respectively, and no violation was detected (P > 0.05). Statistical analyses of data were conducted using ANOVA with a 95% significance level (P < 0.05). When a significant treatment effect was detected, the Tukey’s honestly significant difference (HSD) test was used to assess significant differences among means.
Results
Survival and growth of PBT juveniles
The survival rate decreased during the period from 1 to 4 days (weaning period: 1–3 days, 20–22 dph) in all treatment groups. Thereafter, however, the rate in all groups remained (Fig. 1). During the whole test period, the highest survival at the final day was recorded in the PF group (52.2%), followed by FM and ETFM groups (FM: 24.0%, ETFM: 17.3%, Fig. 1). During the post-weaning period (4–10 days, 23–30 dph), also in the same manner, the highest survival rate on the final day was recorded in the PF group (91.9%) followed by the FM and ETFM groups (FM: 63.9%, ETFM: 52.4%, Fig. 2). The survival rates in the FM and ETFM groups remained in this period. However, the rate in the PF group decreased after 8 days. Survival was significantly higher in the PF group than the FM and ETFM group (P < 0.05, Fig. 2).
The PF group attained the greatest total length and body weight, and these parameters were significantly higher than all groups (P < 0.05, Figs. 3, 4). Among the test diet groups, significantly higher total length and body weight were observed in the PBT juvenile fed FM diet than those fed ETFM diet (P < 0.05, Figs. 3, 4). However, no significant difference was found in body depth among the PF, FM and ETFM groups (P > 0.05, Fig. 5). Regarding CF, fish fed FM and ETFM diets showed significantly higher CF than those fed PF (P < 0.05, Table 5). Significantly higher final body weight, weight gain and SGR were observed in fish fed the PF than those fed the ETFM diet (Table 5). However, they were not significantly different from those of the FM group.
Total length of PBT juveniles fed different diets (FM fish meal diet, ETFM enzyme-treated fish meal diet, PF prey fish) during the rearing period. Different superscript letters indicate significant differences among the dietary groups (P < 0.05, day 4, n = 10; day 7, n = 10; day 10, n = 80; 20–30 dph)
Body weight of PBT juveniles fed different diets (FM fish meal diet, ETFM enzyme-treated fish meal diet, PF prey fish) during the rearing period. Different superscript letters indicate significant differences among the dietary groups (P < 0.05, day 4, n = 10; day 7, n = 10; day 10, n = 80; 20–30 dph)
Body depth of PBT juveniles fed different diets (FM fish meal diet, ETFM enzyme-treated fish meal diet, PF prey fish) during the rearing period. Different superscript letters indicate significant differences among the dietary groups (P < 0.05, day 4, n = 10; day 7, n = 10; day 10, n = 80; 20–30 dph)
Proximate chemical composition of PBT
Significantly higher moisture content was found in the PF group (P < 0.05, Table 5). However, no significant difference was found between the PF and ETFM groups (P > 0.05, Table 5). No significant difference was observed in protein content among the groups (P > 0.05, Table 5). On the other hand, the highest carcass lipid content was observed in PBT in the FM and ETFM groups (Table 5). Carcass lipid content was significantly higher in test diet groups than that of the PF group (P < 0.05, Table 5). Among the essential amino acids, similar amino acid content was recorded in all groups (Table 6). There were no significant differences among the test diet groups (P > 0.05, Table 6).
Percentage of the 18:1n-9, 18:2n-6 and 20:1 of carcass lipid were significantly higher in the fish-fed test diet groups than those fed PF (Table 7). Percentages of 20:5n-3 (eicosapentaenoic acid, EPA) of the carcasses of the FM- and ETFM-fed groups were significantly higher than the PF group. However, carcass 22:6n-3 (docosahexaenoic acid, DHA) in the PF group was significantly higher than other groups. The highest ratio of DHA/EPA was obtained in the PF group, followed by the ETFM and FM groups; the high DHA/EPA ratio in the latter diets resulted from lower EPA (Table 7). The Σn-6 was significantly higher in the FM and ETFM diet groups than PF group. In contrast, Σn-3 and n-3/n-6 ratios were lower in the PF group but higher in the diet group (Table 7).
Discussion
A part of mortality was caused by extrinsic factors related to responses of juvenile PBT to rearing conditions, inevitable trauma caused by collision against tank wall, jumping out of a tank and biting on the caudal or pelvic fin. Further, a high frequency of collision was reported to be observed after PBT reached 4 cm in total length (Miyashita 2002; Sawada et al. 2005). The PBT juveniles used in the present study did not reach this size 10 days (30 dph) after initiation of the feeding. These phenomena indicate that rearing trials on juvenile PBT should be shorter. Also, many previous studies demonstrated that acceptable growth could be obtained even in less than two weeks, and that estimation of suitable feed by comparing growth performance was capable (Miyashita 2002; Takii et al. 2007b; Seoka et al. 2007, 2008; Biswas et al. 2009a, b; Cho et al. 2016). In the present study, the survival and growth of PBT were significantly different in the 10 days of the feeding trial (30 dph). When one tries to determine the nutrient requirements for fish, the juvenile is used because of faster growth and higher nutrient requirements. Yellowtail Seriola quinqueradiata is known as a relatively high-growth species and a SGR of around 5.7 was recorded for juveniles under optimal conditions (Watanabe et al. 2000). However, PBT juveniles grow faster than yellowtail, and SGR of 18.6–19.3, roughly double that of yellowtail, was recorded (Ji et al. 2008; Biswas et al. 2009b). For this reason, even a shorter period of growth trial for 10 days provides reproducible results and is thus sufficient for determining a suitable diet for PBT.
Fish fed PF showed a decrease in survival after 8 days of the beginning of the feeding trial compared to those fed a formulated diet. According to Sawada et al. (2000) during hatchery production, PBT larvae are fed yolk-sac larvae such as spangled emperor fish Lethrinus nebulosus or Japanese parrotfish Calotomus japonicas until they reach 10 to 40 mm of total length. Then, they were supplied with minced sand lance Ammodytidae personatus from 40 mm total length onward. Because of the highly piscivorous nature of PBT, it is essential to offer a sufficient amount of PF larvae and/or minced sand lance to avoid the risk of cannibalism (Miyashita 2002). In the present study, the mean total length of the PF group was longer than 40 mm at 8 days. It was thought that the increased number of PBT experiencing suboptimal nutritional condition occurred due to insufficient nutrients in PF in this stage. However, fish fed formulated diets (Peruvian fish meal, FM; enzyme-treated Chilean fish meal, ETFM) tended to mitigate the mortality from the 5 days after initiation of the feeding trial. One possible explanation is that formulated diet contains higher protein and lipid content than PF in a dry matter basis; it could provide sufficient nutrient compared to PF.
Ji et al. (2008) reported the dietary utility of enzyme-treated fish meal for juvenile PBT. In their trial 1, the growth and feed performance of juvenile PBT fed a Chilean enzyme-treated fish meal-based diet were significantly higher than those fed Peruvian fish meal diets (Peruvian vs Chilean: final mean body weight, 3.92 g vs 4.95 g; SGR, 13.9% vs 17.2%; FE, 74.9% vs 126%, respectively). In the present study, however, juvenile PBT fed a Peruvian fish meal diet did not show inferior growth compared to those fed an enzyme-treated diet. It should be noted that there was difference in the fish size between the studies. Ji et al. (2008) reported that fishes with an initial weight of 1.48 g were grown for 7 days and reached 4–5 g of the final weight. However, the fish used in the present study (initial weight: 52.8 mg) reached 587.5–800.9 mg of the final weight. In the logical context, it could be considered that because our experimental fish were smaller in size at the initiation of feeding trials, the fish fed Peruvian fish meal have achieved sufficient growth. However, normally smaller fish had less digestive ability. Apparently, Peruvian fish meal does not contain higher levels of peptide than enzyme-treated Chilean fish meal. Thus, it is very unlikely that smaller PBT could efficiently utilize less digestive Peruvian fish meal. Another explanation is feeding frequency. Our trial examined a weaning diet and extensive feeding was conducted to achieve sufficient feeding on a formulated diet. Unfortunately, we could not determine the exact amount of successfully fed diet for PBT; higher feed frequency could be one of the possible causes of the difference in terms of growth between Ji et al. (2008) and our present study.
Different growth patterns were apparent in the PBT fed the formulated diet and the PF. PBT fed formulated diets showed higher growth rates compared to fish fed PF, particularly between 7 to 10 days of the feeding trial (Figs. 3, 4, and 5). Tanaka et al. (2007) suggest that the PBT juvenile grows faster from 27 to 33 dph and even more rapidly from 33 to 35 dph. They also suggests that protein content per individual PBT and protein synthesis increased during the accelerated period of growth. This difference could reflect the different moisture content between the formulated diet and PF, where around 6.5-fold higher moisture content was observed in the PF. This considerable difference might be attributable to the differences in nutrient content among the formulated diet and PF, which consequently resulted in higher dietary energy and nutrient intake in the formulated diet groups (Cho et al. 2016).
Protein source is the most important component in fish feeds, because it is the most expensive feed ingredient and highly required in carnivorous fish diets (Wilson 2002). In the present study, two kinds of test diets were used to explore optimal protein sources for formulated diet for PBT juveniles to replace PF. According to Ji et al. (2008), the ETFM is more easily digested than conventional fish meal and it has been commonly used in formulated diet for PBT juveniles (Biswas et al. 2009a, b). Satoh et al. (2002) reported that the treatment of fish meal with protease increases the amount of water-soluble protein and soluble nitrogen (N). The soluble N component is mainly peptide with a molecular weight of 10,000 or less (Haard et al. 1985). Further, Cahu et al. (1999) suggested the induction of trypsin secretion in European seabass Dicentrarchus labrax larvae fed the diet containing protein hydrolysate, demonstrating that dietary supplementation of fish protein hydrolysate facilitates the maturation of the digestive function of larval fish and enhances their growth and survival. Collectively, these reports suggested that protein sources rich in water-soluble protein/peptides are a better dietary component than intact protein for juvenile and larval fish. We, therefore, also assumed that an ETFM diet could show better results than an FM diet in the present study. However, in apparent contrast, the FM group was better than the ETFM group in terms of growth performance in the present study. Similarly, juvenile PBT fed the diet in which fish meal was replaced with a non-enzyme-treated soybean meal diet had significantly higher growth than those fed enzyme-treated soybean meal diet (Biswas et al. 2011). We thought that this difference in growth performance was derived from the nutritional content of feed ingredients because the growth difference from feeding of different diets can be finally attributed to variation of chemical components in the diets.
Histidine is one of the essential amino acids in fish species (Ketola 1982). Tuna and skipjack have large amounts of dark muscle and contain relatively higher free histidine compounds there than the other fishes. It was suggested that histidine compounds have antioxidant activities and play a role in protecting from the negative impact of reactive oxygen species generated by continuous swimming (Suyama et al. 1973). Further, it was suggested that dietary supplementation of histidine improved growth of yellowtail Seriola quinqueradiata (Yokoyama et al. 2010; Yamamoto et al. 2019). In the present study, the high free histidine level (4.4 g/kg, dry-weight) in the FM diet could have been one of the reasons for the high survival and growth. On the other hand, the low level of free histidine (1.6 g/kg, dry-weight) in ETFM could be one of causes of lower survival and growth.
Regarding the essential amino acid content of the carcass, fish fed an FM diet had a higher essential amino acid content than those fed ETFM diet and had higher histidine, isoleucine, lysine, methionine and threonine compared to the initial body. Histidine, lysine, methionine, isoleucine and threonine are classified as glycogenic amino acids and used in gluconeogenesis for energy production (Young and Ajami 2001; Brosnan 2003). Higher essential and glycogenic amino acid content in the FM diet implied the higher dietary value of this diet compared to the ETFM diet.
Dietary EPA, DHA and n-3 long-chain polyunsaturated fatty acids (LC-PUFA) affect the physiological process and subsequent normal growth of marine fish (Sargent et al. 1999). However, most marine fishes are unable to synthesize DHA in their body, and they require it in their food as an essential fatty acid, so they may selectively accumulate it through the food chain (Zlatanos and Laskaridis 2007). In the present study, the PF group showed greater growth, particularly in body weight, than in the other groups, and the PF diet had higher DHA by 1.8-fold, and n-3 LC-PUFA by 1.2-fold. These findings indicate that the growth of PBT juveniles is influenced by the dietary fatty acid content. Although some studies on PBT have been conducted, very little is known about the detailed lipid and fatty acid requirements of tuna. The content of saturated fatty acids (e.g. 14:0, 16:0 and 18:0) tended to increase with increasing dietary fatty acid content (Tables 3 and 7). According to Cejas et al. (2004), the fatty acid composition of lipid from fish tissue reflects the fatty acid content of the lipid in the diet. The FM group had higher 16:0 (palmitic acid) content than the ETFM group. 16:0 is known for being a predominant source of potential metabolic energy in fish during growth (Huynh et al. 2007), and it is the predominant saturated fatty acid in the main feed source of the farmed tuna (Huynh et al. 2007; Zlatanos and Laskaridis 2007). Økland et al. (2005) have demonstrated that there was an inverse relationship between the amount of the n-3 fatty acids and the total lipid content. The n-3/n-6 ratio for carcass of this experiment was 4.8 (PF group), 3.1 (FM group) and 2.1 (ETFM group), respectively. A high accumulation of n-3 LC-PUFA in fish fed PF could indicate the importance of the health benefits of these fatty acids for PBT. The higher n-3 LC-PUFA in the FM group compared to the ETFM group suggests that the relatively high n-3 LC-PUFA content seems to be a result of consuming saturates and monoenes as energy sources.
Enzyme-treated fish meal is very expensive and not commercially available for the industrial culture of PBT. This study suggests that Peruvian fish meal without enzyme digestion can be used as the protein source in formulated diets for juvenile PBT. In conclusion, here we demonstrated that the growth rate of fish in the formulated diet group in the last 3 days was better than that of the PF group, although the growth of the final fish was inferior in the fish in the formulated diet group than the PF group. However, this study lasted only 10 days. Thus, a future study is required to test the feed performance of a Peruvian fish meal-based diet in a longer feeding trial. There are few studies on the development of formulated diets for the early stage of PBT with the fish size used in the present study (Cho et al. 2016). Further research is needed in the near future.
Abbreviations
- ANOVA:
-
One-way analysis of variance
- CF:
-
Condition factor
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- ETFM:
-
Enzyme-treated fish meal
- FM:
-
Fish meal
- IMP:
-
Inosine-5′-monophosphate
- LC-PUFA:
-
Long-chain polyunsaturated fatty acids
- PBT:
-
Pacific bluefin tuna
- PF:
-
Prey fish
- SGR:
-
Specific growth rate
- WG:
-
Weight gain
References
AOAC (2000) Official methods of analysis, 16th edn. Association of Official Analyrical Chemists, Gaithersburg, MD
Biswas BK, Ji S-C, Biswas AK, Seoka M, Kim Y-S, Kawasaki K, Takii K (2009a) Dietary protein and lipid requirements for the Pacific bluefin tuna Thunnus orientalis juvenile. Aquaculture 288:114–119. https://doi.org/10.1016/j.aquaculture.2008.11.019
Biswas BK, Ji S-C, Biswas AK, Seoka M, Kim Y-S, Takii K (2009b) A suitable dietary sugar level for juvenile Pacific bluefin tuna Thunnus orientalis. Aquac Sci 57:99–108. https://doi.org/10.11233/aquaculturesci.57.99
Biswas AK, Biswas BK, Ito J, Takaoka O, Yagi N, Itoh S, Takii K (2011) Soybean meal can partially replace enzyme-treated fish meal in the diet of juvenile Pacific bluefin tuna Thunnus orientalis. Fish Sci 77:615–621. https://doi.org/10.1007/s12562-011-0363-6
Boonyoung S, Haga Y, Satoh S (2012) Preliminary study on effects of methionine hydroxyl analog and taurine supplementation in a soy protein concentrate-based diet on the biological performance and amino acid composition of rainbow trout Oncorhynchus mykiss (Wallbaum). Aquac Res 44:1339–1347. https://doi.org/10.1111/j.1365-2109.2012.03138.x
Brosnan JT (2003) Interorgan amino acid transport and its regulation. J Nutr 133:2068S-2072S. https://doi.org/10.1093/jn/133.6.2068S
Buentello A, Seoka M, Kato K, Partridge GJ (2016a) Tuna farming in Japan and Mexico. Advances in tuna aquaculture. Academic Press, Cambridge, pp 189–215
Buentello A, Seoka M, Suarez J (2016b) Nutrition of cultured tuna species. Advances in tuna aquaculture. Academic Press, Cambridge, pp 273–321
Cahu CL, Zambonino Infante JL, Quazuguel P, Le Gall MM (1999) Protein hydrolysate vs. fish meal in compound diets for 10-day old sea bass Dicentrachus labrax larvae. Aquaculture 171:109–119. https://doi.org/10.1016/S0044-8486(98)00428-1
Cejas JR, Almansa E, Jerez S, Bolanos A, Samper M, Lorenzo A (2004) Lipid and fatty acid composition of muscle and liver from wild and captive mature female broodstocks of white seabream Diplodus sargus. Comp Biochem Physiol Part A 138:91–102. https://doi.org/10.1016/j.cbpc.2004.03.003
Cho JH, Haga Y, Kamimura Y, Akazawa A, Itoh A, Satoh S (2016) Production performance of Pacific bluefin tuna Thunnus orientalis larvae and juveniles fed commercial diets and effects of switching diets. Aquac Sci 64:359–370. https://doi.org/10.11233/aquaculturesci.64.359
Folch J, Lees M, Sloan Stanley GH (1957) A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–507. https://doi.org/10.1016/S0021-9258(18)64849-5
Haard NF, Kariel N, Herzberg G, Feltham LAW, Winter K (1985) Stabilisation of protein and oil in fish silage for use as a ruminant feed supplement. J Sci Food Agr 36:229–241. https://doi.org/10.1002/jsfa.2740360402
Haga Y, Naiki T, Takebe T, Kumon K, Tanaka Y, Shiozawa S, Nakamura Y, Ishida S, Ide K, Masuma S, Takeuchi T (2010) Effect of feeding yolk-sac larvae of spangled emperor Lethrinus nebulosus at different ages on survival and growth of Pacific bluefin tuna Thunnus orientalis. Aquac Sci 58:491–499. https://doi.org/10.11233/aquaculturesci.58.491
Huynh MD, Kitts DD, Hu C, Trites AW (2007) Comparison of fatty acid profiles of spawning and non-spawning Pacific herring, Clupea harengus pallasi. Comp Biochem Physiol Part B 146:504–511. https://doi.org/10.1016/j.cbpb.2006.11.023
Ishibashi Y, Izumi T, Kurata M, Okada T (2013) Effects of the tank wall pattern on the survival rate, bone injury, and stress response of Pacific bluefin tuna, Thunnus orientalis. Aquacult Eng 56:13–17. https://doi.org/10.1016/j.aquaeng.2013.03.004
JFA (2015) Japanese Fisheries Agency. Marino-Forum 21 General Incorporated Association. http://www.yousyokugyojyou.net/index4.htm.
Ji SC, Takaoka O, Biswas AK, Seoka M, Ozaki K, Kohbara J, Ukawa M, Shimeno S, Hosokawa H, Takii K (2008) Dietary utility of enzyme-treated fish meal for juvenile Pacific bluefin tuna Thunnus orientalis. Fish Sci 74:54–61. https://doi.org/10.1111/j.1444-2906.2007.01475.x
Ketola HG (1982) Amino acid nutrition of fishes: requirements and supplementation of diets. Comp Biochem Physiol Part B 73:17–24. https://doi.org/10.1016/0305-0491(82)90197-3
MAFF (2016) Ministry of Agriculture, Forestry and Fisheries. Statistics Division Annual Report of Fisheries and Aquaculture Industry Statistics. Chiyoda, Tokyo, Japan, p 22 (in Japanese)
Masuma S, Takebe T, Sakakura Y (2011) A review of the broodstock management and larviculture of the Pacific northern bluefin tuna in Japan. Aquaculture 315:2–8. https://doi.org/10.1016/j.aquaculture.2010.05.030
Miyashita S (2002) Studies on the seedling production of the Pacific bluefin tuna Thunnus thynnus orientalis. Bull Fish Lab Kindai Univ. 8:1–171. https://ci.nii.ac.jp/naid/10026139005/
Morrison WR, Smith LM (1964) Preparation of fatty acid methylesters and dimethyl acetals from lipids with boron trifluoridemethanol. J Lipid Res 5:600–60. https://doi.org/10.1016/S0022-2275(20)40190-7
Oil World Annual (2019) “Fish Oil and Meal World”. Oil World Annual. ISTA Mielke GmbH., Hamburg, Germany. No. 138. http://www.oilworld.biz/t/sample/sample_25.pdf
Økland HMW, Stoknes IS, Remme JF, Kjersta M, Synnes M (2005) Proximate composition, fatty acid and lipid class composition of the muscle from deep-sea teleosts and elasmobranchs. Comp Biochem Physiol Part B 140:437–443. https://doi.org/10.1016/j.cbpc.2004.11.008
Sargent J, McEvoy L, Esteves A, Bell G, Bell M, Henderson J, Tocher D (1999) Lipid nutrition of marine fish during early development: current status and future directions. Aquaculture 179:217–229. https://doi.org/10.1016/S0044-8486(99)00191-X
Satoh K, Sanada Y, Hitaka E, Kimoto K (2002) Dietary effects of the protease treatments of fish meal on the growth, feed efficiency, and protein digestibility of yellowtail during low temperature season. Aquac Sci 50:219–226. https://doi.org/10.11233/aquaculturesci1953.50.219 (in Japanese with English abstract)
Sawada Y, Miyashita S, Aoyama M, Kurata M, Mukai Y, Okada T, Murata O, Kumai H (2000) Rotifer-size selectivity and optimal feeding density of bluefin tuna Thunnus orientalis larvae. Aquac Sci 48:169–177. https://doi.org/10.11233/aquaculturesci1953.48.169
Sawada Y, Okada T, Miyashita S, Murata O, Kumai H (2005) Completion of the Pacific bluefin tuna Thunnus orientalis (Temminck et Schlegel) life cycle. Aquac Res 36:413–421. https://doi.org/10.1111/j.1365-2109.2005.01222.x
Seoka M, Kurata M, Hatanaka Y, Biswas AK, Ji S-C, Kumai H (2007) Possible nutrients in affecting the larval growth of Pacific bluefin tuna Thunnus orientalis. Aquac Sci 55:55–64. https://doi.org/10.11233/aquaculturesci1953.55.55
Seoka M, Kurata M, Tamagawa R, Biswas AK, Biswas BK, Yong ASK, Kim Y-S, Ji S-C, Takii K, Kumai H (2008) Dietary supplementation of salmon roe phospholipid enhances the growth and survival of Pacific bluefin tuna Thunnus orientalis larval and juveniles. Aquaculture 275:225–234. https://doi.org/10.1016/j.aquaculture.2007.12.027
Suyama M, Yoshizawa Y (1973) Free amino acid composition of the skeletal muscle of migratory fish. Nippon Suisan Gakkaishi 39:1339–1343. https://doi.org/10.2331/suisan.39.1339
Suyama M, Hirano T, Suzuki T (1986) Buffering capacity of free histidine and its related dipeptides in white and dark muscles of yellowfin tuna. Nippon Suisan Gakkaishi 52:2171–2175. https://doi.org/10.2331/suisan.52.2171
Tada M (2010) Challenges and opportunities for the full cycle farmed tuna in Japan. In: Miyashita S, Takii K, Sakamoto W, Biswas A (eds) Joint International Symposium of Kindai University and Setouchi Town on The 40th Anniversary of Pacific Bluefin Tuna Aquaculture. Kindai University Press, Setouchi Town, pp 40–44
Takii K, Seoka M, Izumi M, Hosokawa H, Shimeno S, Ukawa M, Kohbara J (2007a) Apparent digestibility coefficient and energy partition of juvenile Pacific bluefin tuna Thunnus orientalis and chub mackerel Scomber japonicas. Aquac Sci 55:571–577. https://doi.org/10.11233/aquaculturesci1953.55.571
Takii K, Seoka M, Ohara N, Nasu T, Oda S, Miyashita S, Ukawa M, Shimeno S, Hosokawa H (2007b) Dietary utility of Chilean fish meal and Pollack liver oil for juvenile Pacific bluefin tuna. Aquac Sci 55:579–585. https://doi.org/10.11233/aquaculturesci1953.55.579
Tanaka Y, Gwak W-S, Tanaka M, Sawada Y, Okada T, Miyashita S, Kumai H (2007) Ontogenetic changes in RNA, DNA and protein contents of laboratory-reared Pacific bluefin tuna Thunnus orientalis. Fish Sci 73:378–384. https://doi.org/10.1111/j.1444-2906.2007.01345x
Watanabe K, Ura K, Yada T, Kiron V, Satoh S, Watanabe T (2000) Energy and protein requirements of yellowtail for maximum growth and maintenance of body weight. Fish Sci 66:1053–1061. https://doi.org/10.1046/j.1444-2906.2000.00168.x
Wilson RP (2002) Amino acids and proteins. In: Halver JE, Hardy RW (eds) Fish Nutrition, 3rd edn. Academic Press, San Diego, pp 143–179
Yamamoto T, Matsunari H, Oku H, Murashita K, Yoshinaga H, Furuita H (2019) Supplemental effects of histidine to fishmeal-based and low-fishmeal diets on the growth performance and tissue free amino acid levels of fingerling yellowtail Seriola quinqueradiata. Aquac Sci 67:367–375. https://doi.org/10.11233/aquaculturesci.67.367
Yokoyama S, Sotoyama Y, Nawata T, Ishikawa M, Koshio S (2010) Histidine supplementation to plant protein used diet improves growth and freshness of muscle in yellow tail Seriola quinqueradiata. In: 14th International Symposium on Fish Nutrition and Feeding. Qindao, China, Program & Abstract, p 150
Young VR, Ajami AM (2001) Glutamine: The emperor or his clothes? J Nutr 131:2449S-2459S. https://doi.org/10.1093/jn/131.9.2449S
Zlatanos S, Laskaridis K (2007) Seasonal variation in the fatty acid composition of three Mediterranean fish-sardine (Sardina pilchardus), anchovy (Engraulis incrasicholus) and picarel (Spicara smaris). Food Chem 103:725–728. https://doi.org/10.1016/j.foodchem.2006.09.013
Acknowledgements
This work was financially supported in part by a project on the development of production technology of healthy Pacific bluefin tuna juveniles from Maruha Nichiro Co. The costs of this study were partly defrayed by a grant, “Technological Development Project for Optimal Broodstock Selection and Maintenance for Pacific Bluefin Tuna Aquaculture, a Part of Natural Resources- and Eco-Friendly Pacific Bluefin Tuna Culture Technological Development Project,” given to S.S. and Y.H. through the Fisheries Research Agency by the Fisheries Agency of Japan.
Author information
Authors and Affiliations
Contributions
J-HC: conceptualization, methodology, data curation, investigation, writing—original draft, visualization, writing—review & editing. YH: conceptualization, supervision, project administration, funding acquisition, writing—review & editing. YK: investigation, validation, resources. AI: conceptualization, funding acquisition, project administration. SS: conceptualization, funding acquisition, project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cho, JH., Haga, Y., Kamimura, Y. et al. Peruvian fish meal has comparative potential to enzyme-treated Chilean fish meal as protein source of diet for larvae and juvenile Pacific bluefin tuna Thunnus orientalis. Fish Sci 88, 161–172 (2022). https://doi.org/10.1007/s12562-021-01573-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-021-01573-y