Abstract
The Japanese eel Anguilla japonica is a prominent and highly valued species in the aquaculture industry in Japan. Sex determination and sex differentiation in eels are significantly affected by environmental factors; however, the molecular mechanisms involved in sex differentiation in eels are largely unknown. In this study, to investigate the gonadal expression profiles of sex-specific genes during and after sexual differentiation in Japanese eel, we induced elvers into predominantly phenotypic males or females by rearing on a control diet or estradiol-17β-treated diet, respectively, during the sex differentiation period. Quantitative real-time PCR showed that forkhead box L2A (foxl2a) and cytochrome P450 aromatase (cyp19a1) were more highly expressed in ovaries than in testes, whereas the expression levels of anti-Müllerian hormone (amh) and gonadal soma-derived factor (gsdf) were significantly higher in testes than in ovaries. Furthermore, foxl2a and cyp19a1 displayed female-specific expression early in the sex differentiation process, while after slightly more growth amh and gsdf displayed male-specific expression during sex differentiation. Together, these results suggest that these genes have important roles in sexual differentiation and development in this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many vertebrates, sex is determined by the genotype, and sex differentiation is governed by biochemical pathways. Many poikilothermic vertebrates, however, exhibit plasticity in sex differentiation, and the phenotypic sex can be affected by environmental conditions during sensitive periods of gonadal development (Nakamura 2010). For instance, in many reptiles, fishes, and amphibians, sex determination is affected by a combination of genetic and environmental cues (e.g. temperature, pH, relative density, social interactions) early during development (Francis and Barlow 1993; Francis 1984; Rubin 1985; Baroiller et al. 1999).
Water temperature, as a key environmental factor during gonadal development, may override genetic sex determination mechanisms, altering the resultant phenotypic sex of many teleosts. Previously, it has been reported that genetically female progenies of Japanese flounder Paralichthys olivaceus (Kitano et al. 1999), medaka Oryzias latipes (Sato et al. 2005), and zebrafish Danio rerio (Ribas et al. 2017) could be sex-reversed into functional males when reared in high water temperatures during the critical periods of gonad differentiation. However, many aspects of the molecular mechanisms of environmental sex determination in teleosts remain unclear.
Sex determination in eels is highly influenced by the environment, although it is not yet known to what extent genetic factors contribute to the final sexual phenotype. Research on sex determination and differentiation in eels has been conducted since the 1980s. Environmental factors shown to affect the sex ratios of anguillid species include temperature and pH, density, and social interactions (Davey and Jellyman 2005). Several studies have examined gonadal morphological changes during sex differentiation in the European eel Anguilla anguilla (Colombo and Grandi 1996; Geffroy et al. 2013, 2016). It is generally accepted that eel gonadal development is related more to body length than to age (Colombo et al. 1984; Colombo and Grandi 1996; Oliveira and McCleave 2000; Jellyman 2001). Though previous studies of gonadal development in the European eel provide information on morphological sex differentiation in eels, the molecular mechanisms involved in sex differentiation are largely unknown.
In teleosts, sex determination and differentiation are mainly governed by the expression of sex-specific genes. The genes chosen for consideration in this study are considered to be related to the process of sex differentiation in fish. Forkhead box L2 (Foxl2) is a member of the winged-helix/forkhead family of transcription factors and is involved in ovarian development in teleosts, including Japanese medaka (Nakamoto et al. 2006) and black porgy Acanthopagrus schlegelii (Wu et al. 2008). Anti-Müllerian hormone (Amh), also known as Müllerian-inhibiting substance, functions in regulating germ cell proliferation and/or differentiation in male gonad development in different fish species (Yoshinaga et al. 2004; Baron et al. 2005; Haugen et al. 2012), including the Japanese eel Anguilla japonica (Miura et al. 2002). Gonadal soma-derived factor (Gsdf) is a member of the transforming growth factor-β (TGF-β) family and was shown to play a vital role in the development of both the testis and the ovary in the medaka (Shibata et al. 2010; Zhang et al. 2016). In addition, the gsdf mRNA expression pattern is related to testes development in gonochoristic fish and to sex change in protogynous fish (Horiguchi et al. 2013). Consequently, gsdf is often used in researching sex differentiation in teleosts (Kitano et al. 2012; Kaneko et al. 2015; Robledo et al. 2015).
Aromatase (encoded by cyp19a1) converts androgens into estrogens and plays an important role in ovarian development in vertebrates (Nakamura 2010). cyp19a1 is suspected to play an essential role in ovarian development in teleosts (Kitano et al. 1999). Because of teleost-specific genome duplication, most teleosts have duplicated genes for aromatase, named cyp19a1a and cyp19a1b, which are expressed predominantly in the gonads and brain, respectively (Tchoudakova and Callard 1998; Kishida and Callard 2001). cyp19a1 encoding aromatase has been cloned in Japanese eel (Ijiri et al. 2003) and European eel (Tzchori et al. 2004). However, eels have a single form of aromatase, which is expressed both in the gonads and in the brain (Jeng et al. 2012). Differential expression of aromatase according to sex has been studied in the European eel by Tzchori et al. (2004) and Geffroy et al. (2013). Their results showed that cyp19a1 gene expression in gonads was higher in females than in males. However, Jeng et al. (2018) found no significant difference in the transcript levels of cyp19a1 in the gonads of males and females of Japanese eels. Thus, little is known concerning aromatase gene expression during the early stages of gonad differentiation in the Japanese eel.
In this study, to investigate the gonadal expression profiles of sex-specific genes during early sex differentiation in Japanese eel, we first induced elvers into predominantly either phenotypic males or females, by rearing on a control diet or estradiol-17β (E2)-treated diet, respectively, during the sex differentiation period. Next, we examined the gene expression patterns in the gonads by quantitative real-time polymerase chain reaction PCR (qPCR).
Materials and methods
Animals
Elvers (A. japonica) (experiment 1: body length = 5.79 ± 0.21 cm; body weight = 0.14 ± 0.02 g; experiment 2: body length = 5.78 ± 0.23 cm; body weight = 0.13 ± 0.02 g) were purchased from the Isshiki Unagi Cooperative Association of Fisheries and transferred to the aquaculture station of the Aichi Fisheries Research Institute. The experimental fish were acclimated to a freshwater pond environment (an indoor 0.8-ton fiber-reinforced plastic [FRP] tank) with natural lighting and continuous aeration. Fish were kept under warm-temperature conditions (28–30 °C) until the experiments.
Experimental treatments
In experiment 1, control (n = 100) and E2-treated (n = 100) elvers were reared in separate 0.8-ton FRP tanks. Control fish were fed a commercial eel feed powder mixed with water and ethanol. The fish were feminized by feeding a diet containing E2 at the elver stage (Chiba et al. 1993; Colombo and Grandi 1995); E2 (Sigma-Aldrich Corp., St. Louis, MO, USA) was dissolved in ethanol, then mixed with water and the commercial eel feed powder to obtain the E2-treated diet (20 mg/kg feed). Fish were fed the diet 6 days a week. Fish were collected from both groups after 120 days, when most had reached 30 cm. Gonads from individual eels were collected for gonadal histology and gene expression analysis.
In experiment 2, the controls (n = 100) and E2-treated elvers (n = 100) were reared according to the same procedure as in experiment 1. The elvers were feminized by feeding an E2-containing diet (10 mg/kg feed) because the mortality of E2-treated fish in experiment 1 was high (data not shown). To investigate expression of the sex-specific genes in the undifferentiated gonads, fish treated with or without E2 for 45 days were used. These eels were collected and divided into three body-length groups (Small, < 15.0 cm; Medium, 15–19.9 cm; Large, 20–24.9 cm) for gonadal histology and gene expression analysis. The remaining fish from each group were collected after 120 days, when most had attained a length of at least 30 cm.
Quantitative real-time PCR (qPCR)
Total RNA from the gonadal tissues of the eels was extracted by homogenization in ISOGEN (Nippon Gene, Tokyo, Japan) as previously described (Kitano et al. 2012). Next, reverse transcription was performed at 37 °C for 30 min, using PrimeScript RT Master Mix (TaKaRa, Shiga, Japan). qPCR was performed on a LightCycler 480 system (Roche, Mannheim, Germany) using SYBR Green I Master Mix (Roche). The PCR conditions were 95 °C for 5 min, then 45 cycles of 95 °C for 5 min, 59 °C for 10 s, and 72 °C for 10 s. Finally, melting-curve analysis was performed after thermo-cycling to evaluate the specificity of the amplification and to verify the absence of primer dimers. Relative gene expression levels were calculated using the ΔΔCT method (Livak and Schmittgen 2001). The copy number values of each gene were normalized against elongation factor 1 alpha (ef1α). The primers used are listed in Table 1.
Histological analysis
The samples of eel body segments or gonadal tissues were fixed in Bouin’s solution overnight at 4 °C, then dehydrated, embedded in paraffin, and serially sectioned at 5 μm as previously described (Kitano et al. 2012). Sections were stained with hematoxylin and eosin. Finally, gonadal status was determined by examination of the tissue sections under a light microscope, using the procedure described by Jeng et al. (2018) to characterize the gonad histological structure.
Statistical analysis
Experimental results were tested using Levene’s test for homogeneity of variance. Data were compared using Student’s t test or analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test, using SPSS Statistics software (IBM Corp., Armonk, NY, USA).
Results
Tissue observation and gene expression patterns for gonads after sex differentiation
Eels raised to at least 120 days were sacrificed and gonadal sex was determined. Among the control fish, 91% (n = 69) were males and 9% (n = 7) were undifferentiated; conversely, among the E2-treated fish, 97% (n = 64) were females and 3% (n = 2) were undifferentiated (Fig. 1a). Spermatogonia were observed in males of the control group (Fig. 1b, c), and oocytes were observed in females of the E2-treated group (Fig. 1d, e). The expression levels of vasa, foxl2a, cyp19a1, amh, gsdf, and ef1α were analyzed by qPCR. The germ cell marker vasa was expressed in both testes and ovaries (Fig. 1f). Expression of the female-specific genes foxl2a and cyp19a1 was significantly higher in ovaries than in testes (Fig. 1g, h). Conversely, expression of the male-specific genes amh and gsdf was significantly higher in testes than in ovaries (Fig. 1i, j).
Results of experiment 1. Time course of the feminization experiment through an estradiol-17β-treated diet, and a pie chart showing the sex ratio of Japanese eels raised to at least 120 days (a). Histological sections of gonads after hematoxylin and eosin staining: testis (b, c) and ovary (d, e). Quantitative real-time PCR analysis of the expression of each gene in the gonads of fish (n = 5) (f–j). Relative expression levels of the target genes were normalized to that of ef1α: vasa (f), foxl2a (g), cyp19a1 (h), amh (i), and gsdf (j). *p < 0.05; **p < 0.01; ***p < 0.001; SG spermatogonia, OC oocyte
Tissue observation and gene expression patterns for undifferentiated gonads
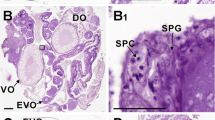
Eels raised to 45 days were collected for gonadal histology; all were found to have undifferentiated gonads. Of the eels reared to at least 120 days, 88% (n = 30) were males and 12% (n = 4) had undifferentiated gonads in the control group, whereas 94% (n = 48) were females and 6% (n = 3) had undifferentiated gonads in the E2-treated group (Fig. 2a). For analysis of their gene expression profiles, the eels raised to 45 days were divided into three body-length groups (Small, Medium, and Large). Germ cells were observed in the gonads of individuals in all length groups of the controls and the E2-treated fish (Fig. 2b–g). Analysis of gene expression by qPCR revealed that the germ cell marker vasa was expressed in the gonads of both the controls and the E2-treated fish; however, in the Large groups, expression of vasa was significantly higher in the E2-treated fish than in the controls (Fig. 2h). In all length groups, expression of the female-specific gene foxl2a was significantly higher in gonads of the E2-treated eels than in those of control fish (Fig. 2i). In both the Medium and Large groups, expression of cyp19a1 was significantly higher in the E2-treated fish than in the control fish (Fig. 2j). In contrast, expression levels of the male-specific genes amh and gsdf were significantly higher in the gonads of the controls than in the E2-treated fish in the Large group only (Fig. 2k, l).
Results of experiment 2. Time course of the feminization experiment through an estradiol-17β-treated diet, and a pie chart showing the sex ratio of Japanese eels raised to 45 days (a). Histological sections of undifferentiated gonads after hematoxylin and eosin staining in eels of body-length groups Small (b, e), Medium (c, f), and Large (d, g). Quantitative real-time PCR analysis of the expression of each gene in the gonads of fish (n = 5) (h–l). Relative expression levels of the target genes were normalized to that of ef1α: vasa (h), foxl2a (i), cyp19a1 (j), amh (k), and gsdf (l). *p < 0.05; **p < 0.01; ***p < 0.001; GC germ cell
Discussion
In this study, we raised elvers of Japanese eel for at least 120 days to ensure a feminizing effect through E2-treated diets; gonadal histological analysis showed that almost all E2-treated fish were females, whereas no females were found among the controls. Using a similar rearing method to achieve feminization, Jeng et al. (2018) likewise found only males in the control group of Japanese eels after 6 months, and Colombo and Grandi (1995) produced a large proportion of males among laboratory-raised European eel. In the current study, based on a histological characterization of gonads following Jeng et al. (2018), the gonads of both the control and E2-treated fish raised to only 45 days (experiment 2) were all undifferentiated.
The expression of vasa increased with gonadal development in the controls and E2-treated fish. Robledo et al. (2015) reported that in turbot Scophthalmus maximus, vasa expression starts to increase at an early stage of gonadal development, but with higher expression in females than in males. Our results suggest that vasa is a germ cell-specific marker in eels, as in some other teleosts. Foxl2a is a paralog of foxl2 and has an important function in ovarian differentiation in rainbow trout Oncorhynchus mykiss (Baron et al. 2004). In this study, the sexually dimorphic expression of foxl2a in ovaries was similar to that found in other teleosts. We found a significant difference in cyp19a1 gene expression between Japanese eel testes and ovaries, a result that agrees with a study conducted with European eel (Geffroy et al. 2013). However, Jeng et al. (2018) reported no difference between males and females in cyp19a1 expression in the gonads of Japanese eel. When we used the primer described by Jeng et al. (2018), we likewise found no differences between cyp19a1 expression in testes and ovaries (data not shown); therefore, we designed a new primer and used it for qPCR. Expression of foxl2a and cyp19a1 in E2-treated fish increased during gonadal development, suggesting the involvement of these genes in ovarian development. In a previous study, transient transfection assay showed that the Japanese flounder Foxl2 can directly activate the cyp19a1 gene transcription in vitro (Yamaguchi et al. 2007), and therefore Foxl2 may also induce cyp19a1 expression in eels. In contrast, the male-specific genes amh and gsdf were significantly more highly expressed in testes than in ovaries. We also found higher amh and gsdf expression in the undifferentiated gonads of males compared with females, which is consistent with the well-described dimorphic expression in European eel (Geffroy et al. 2016). Thus, our data align with the results of previous studies, and together, the results suggest that amh and gsdf are required for testis development in eels.
Cortisol is the major glucocorticoid produced by the adrenal cortex in vertebrates, and its production can be induced by stress. It was previously reported that cortisol suppresses the expression of foxl2a and cyp19a1, but induces the expression of amh, which causes masculinization of XX fish (Yamaguchi et al. 2010). Furthermore, Kitano et al. (2012) reported that exposure to cortisol inhibited the expression of cyp19a1 and induced the expression of gsdf in XX gonads of medaka during gonadal sex differentiation. We hypothesize that masculinization of cultured eels may be prompted by suppression of female-specific genes (foxl2a and cyp19a1) and induction of male-specific genes (amh and gsdf). We intend to conduct future research that focuses on the involvement of cortisol in masculinization in cultured eels.
In conclusion, in Japanese eel, foxl2a and cyp19a1 display female-specific expression during early sex differentiation, while after slightly more growth, amh and gsdf significantly display male-specific expression during the early stages of sex differentiation. Together, these results suggest that these genes play important roles in sexual differentiation and development in eels.
References
Baroiller JF, Guiguen Y, Fostier A (1999) Endocrine and environmental aspects of sex differentiation in fish. Cell Mol Life Sci 55:910–931
Baron D, Cocquet J, Xia X, Fellous M, Guiguen Y, Veitia RA (2004) An evolutionary and functional analysis of FoxL2 in rainbow trout gonad differentiation. J Mol Endocrinol 33:705–715
Baron D, Houlgatte R, Fostier A, Guiguen Y (2005) Large-scale temporal gene expression profiling during gonadal differentiation and early gametogenesis in rainbow trout. Biol Reprod 73:959–966
Chiba H, Iwatsuki K, Hayami K, Yamauchi K (1993) Effects of dietary estradiol-17ß on feminization, growth and body composition in the Japanese eel (Anguilla japonica). Comp Biochem Physiol 106A:367–371
Colombo G, Grandi G, Rossi R (1984) Gonad differentiation and body growth in Anguilla anguilla L. J Fish Biol 24:215–228
Colombo G, Grandi G (1995) Sex differentiation in the European eel: histological analysis of the effects of sex steroids on the gonad. J Fish Biol 47:394–413
Colombo G, Grandi G (1996) Histological study of the development and sex differentiation of the gonad in the European eel. J Fish Biol 48:493–512
Davey AJH, Jellyman DJ (2005) Sex determination in freshwater eels and management options for manipulation of sex. Rev Fish Biol Fish 15:37–52
Francis RC (1984) The effects of bidirectional selection for social dominance on agonistic behaviour and sex ratios in the paradise fish (Macropodus opercularis). Behaviour 90:25–45
Francis RC, Barlow GW (1993) Social control of primary sex differentiation in the Midas cichlid. Proc Natl Acad Sci USA 90:10673–10675
Geffroy B, Guiguen Y, Fostier A, Bardonnet A (2013) New insights regarding gonad development in European eel: evidence for a direct ovarian differentiation. Fish Physiol Biochem 39:1129–1140
Geffroy B, Guilbaud F, Amilhat E, Beaulaton L, Vignon M, Huchet E, Rives J, Bobe J, Fostier A, Guiguen Y, Bardonnet A (2016) Sexually dimorphic gene expressions in eels: useful markers for early sex assessment in a conservation context. Sci Rep 6:34041
Haugen T, Almeida FFL, Andersson E, Bogerd J, Male R, Skaar KS, Schulz RW, Sørhus E, Wijgerde T, Taranger GL (2012) Sex differentiation in Atlantic cod (Gadus morhua L.): morphological and gene expression studies. Reprod Biol Endocrinol 10:1–13
Horiguchi R, Nozu R, Hirai T, Kobayashi Y, Nagahama Y, Nakamura M (2013) Characterization of gonadal soma-derived factor expression during sex change in the protogynous wrasse, Halichoeres trimaculatus. Dev Dyn 242:388–399
Ijiri S, Kazeto Y, Lokman PM, Adachi S, Yamauchi K (2003) Characterization of a cDNA encoding P-450 aromatase (CYP19) from Japanese eel ovary and its expression in ovarian follicles during induced ovarian development. Gen Comp Endocrinol 130:193–203
Jellyman DJ (2001) The influence of growth rate on the size of migrating female eels in Lake Ellesmere, New Zealand. J Fish Biol 58:725–736
Jeng SR, Pasquier J, Yueh WS, Chen GR, Lee YH, Dufour S, Chang CF (2012) Differential regulation of the expression of cytochrome P450 aromatase, estrogen and androgen receptor subtypes in the brain-pituitary-ovarian axis of the Japanese eel (Anguilla japonica) reveals steroid dependent and independent mechanisms. Gen Comp Endocrinol 175:163–172
Jeng SR, Wu GC, Yueh WS, Kuo SF, Dufour S, Chang CF (2018) Gonadal development and expression of sex-specific genes during sex differentiation in the Japanese eel. Gen Comp Endocrinol 257:74–85
Kaneko H, Ijiri S, Kobayashi T, Izumi H, Kuramochi Y, Wang DS, Misuno S, Nagahama Y (2015) Gonadal soma-derived factor (gsdf), a TGF-beta superfamily gene, induces testis differentiation in the teleost fish Oreochromis niloticus. Mol Cell Endocrinol 415:87–99
Kishida M, Callard GV (2001) Distinct cytochrome P450 aromatase isoforms in zebrafish (Danio rerio) brain and ovary are differentially programmed and estrogen regulated during early development. Endocrinology 142:740–750
Kitano T, Takamune K, Kobayashi T, Nagahama Y, Abe S (1999) Suppression of P450 aromatase gene expression in sex-reversed males produced by rearing genetically female larvae at a high water temperature during a period of sex differentiation in the Japanese flounder (Paralichthys olivaceus). J Mol Endocrinol 23:167–176
Kitano T, Hayashi Y, Shiraishi E, Kamei Y (2012) Estrogen rescues masculinization of genetically female medaka by exposure to cortisol or high temperature. Mol Reprod Dev 79:719–726
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Miura T, Miura C, Konda Y, Yamauchi K (2002) Spermatogenesis-preventing substance in Japanese eel. Development 129:2689–2697
Nakamoto M, Matsuda M, Wang DS, Nagahama Y, Shibata N (2006) Molecular cloning and analysis of gonadal expression of Foxl2 in the medaka, Oryzias latipes. Biochem Biophys Res Commun 344:353–361
Nakamura M (2010) The mechanism of sex determination in vertebrates-are sex steroids the key-factor? J Exp Zool 313A:381–398
Oliveira K, McCleave JD (2000) Variation in population and life history traits of the American eel, Anguilla rostrata, in four rivers in Maine. Environ Biol Fishes 59:141–151
Ribas L, Liew WC, Diaz N, Sreenivasan R, Orban L, Piferrer F (2017) Heat-induced masculinization in domesticated zebrafish is family-specific and yields a set of different gonadal transcriptomes. Proc Natl Acad Sci USA 114:E941–E950
Robledo D, Ribas L, Cal R, Sánchez L, Piferrer F, Martínez P, Viñas A (2015) Gene expression analysis at the onset of sex differentiation in turbot (Scophthalmus maximus). BMC Genomics 16:1
Rubin DA (1985) Effect of pH on sex ratio in cichlids and a poecilid (Teleostei). Copeia 1:233–235
Sato T, Endo Y, Yamahira K, Hamaguchi S, Sakaizumi M (2005) Induction of female-to-male sex reversal by high temperature treatment in medaka, Oryzias latipes. Zoolog Sci 22:985–988
Shibata Y, Paul-Prasanth B, Suzuki A, Usami T, Nakamoto M, Matsuda M, Nagahama Y (2010) Expression of gonadal soma derived factor (GSDF) is spatially and temporally correlated with early testicular differentiation in medaka. Gene Expr Patterns 10:283–289
Tchoudakova A, Callard GV (1998) Identification of multiple CYP19 genes encoding different cytochrome P450 aromatase isozymes in brain and ovary. Endocrinology 139:2179–2189
Tzchori I, Degani G, Hurvitz A, Moav B (2004) Cloning and developmental expression of the cytochrome P450 aromatase gene (CYP19) in the European eel (Anguilla anguilla). Gen Comp Endocrinol 138:271–280
Wu GC, Tomy S, Nakamura M, Chang CF (2008) Dual roles of cyp19a1a in gonadal sex differentiation and development in the protandrous black porgy, Acanthopagrus schlegeli. Biol Reprod 79:1111–1120
Yamaguchi T, Yamaguchi S, Hirai T, Kitano T (2007) Follicle-stimulating hormone signaling and Foxl2 are involved in transcriptional regulation of aromatase gene during gonadal sex differentiation in Japanese flounder, Paralichthys olivaseus. Biochem Biophys Res Commun 359:935–940
Yamaguchi T, Yoshinaga N, Yazawa T, Gen K, Kitano T (2010) Cortisol is involved in temperature-dependent sex determination in the Japanese flounder. Endocrinology 151:3900–3908
Yoshinaga N, Shiraishi E, Yamamoto T, Iguchi T, Abe S, Kitano T (2004) Sexually dimorphic expression of a teleost homologue of Müllerian inhibiting substance during gonadal sex differentiation in Japanese flounder, Paralichthys olivaceus. Biochem Biophys Res Commun 322:508–513
Zhang X, Guan G, Li M, Zhu F, Liu Q, Naruse K, Herpin A, Nagahama Y, Li J, Hong Y (2016) Autosomal gsdf acts as a male sex initiator in the fish medaka. Sci Rep 6:19738
Acknowledgements
This research was supported by grants from the Bio-oriented Technology Research Advancement Institution, project NARO 13 (Research program on development of innovative technology). Cynthia Kulongowski (MSc), with the Edanz Group (https://en-author-services.edanzgroup.com/ac), edited a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Inaba, H., Hara, S., Horiuchi, M. et al. Gonadal expression profiles of sex-specific genes during early sexual differentiation in Japanese eel Anguilla japonica. Fish Sci 87, 203–209 (2021). https://doi.org/10.1007/s12562-020-01491-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-020-01491-5