Abstract
The feeding habits and prey selection of Gymnocypris firmispinatus in the Anning River were investigated with respect to fish size, season and sex. Gut contents of 305 individuals ranging in size from 57 to 193 mm total length were analyzed, and 16.0% of the guts were found to be empty. The vacuity index indicated that the feeding intensity of the fish roughly followed a seasonal trend, with minimum food intake in winter. However, statistically insignificant variation in the vacuity index was observed between size classes and sexes. Overall, 46 prey taxa belonging to five orders (Ephemeroptera, Plecoptera, Trichoptera, Coleoptera and Diptera) were identified in the guts of 141 fish. G. firmispinatus fed almost exclusively on aquatic insects, of which Baetis sp. and Simulium sp. were the predominant prey species, followed by Diamesa sp. and Glossosoma sp. G. firmispinatus is a generalist feeder that relies upon a wide trophic spectrum. The multivariate analysis revealed that fish size was the principal factor affecting diet. Small individuals fed primarily on small ephemeropteran larvae and dipteran larvae, whereas larger individuals preferably consumed bigger trichopteran larvae. In terms of its prey, G. firmispinatus showed strong positive selection for dipteran larvae and trichopteran larvae, and negative selection for ephemeropteran larvae, plecopteran larvae and coleopteran larvae in all seasons. This study provides evidence that the observed diet of G. firmispinatus can be explained by prey selection rather than random feeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species of the Schizothoracinae, which are generally characterized by a long life span, slow growth, late maturity and low fecundity, are dominant in the Qinghai–Tibet Plateau ichthyofauna (Chen and Cao 2000). An endemic species of the Qinghai–Tibet Plateau, Gymnocypris firmispinatus is only distributed in the Jinsha River and its tributaries. This small fish usually resides in rivers at elevations of about 2000–3000 m, where the water temperature is very low, even in summer (Chen and Cao 2000; Ma et al. 2019a).
Recently constructed cascade hydropower developments block the continuous flow of water in these rivers and destroy their ecological integrity, e.g., they seriously impact the spawning migration of adult G. firmispinatus and the feeding migration of juveniles (Ru et al. 2016). Sand excavation also destroys the habitats of these fish. Moreover, overfishing has exacerbated the decline in the G. firmispinatus population. A fundamental understanding of the ecological requirements of a fish species is needed for the development of effective conservation measures (Sánchez-Hernández and Cobo 2012). However, knowledge of the ecological requirements of G. firmispinatus is limited. Ma et al. (2018, 2019a) studied the life history traits of this species, and found that it has slow growth, low fecundity and late maturity, which suggest that it might be particularly susceptible to human activities.
Although several studies have focused on the growth and reproduction of G. firmispinatus, none have directly investigated its feeding habits. Knowledge of the feeding ecology of a species, which is closely related to its population dynamics, is useful for a deeper understanding of factors such as resource partitioning, habitat preferences, prey selection, predation, evolution, competition and energy transfer within and between ecosystems (Braga et al. 2012). The study of the feeding habits of a species provides valuable information on its possible distribution and niche in a food web (Wootton 1990). Studies on feeding ecology are also prerequisites for elucidating niche overlaps between and within species, and understanding the intensity of inter- and intraspecific interactions in fish communities (Abid et al. 2013).
Size-related changes in diet are seen in many fishes (La Mesa et al. 2007; Huo et al. 2014; Kati et al. 2015). Understanding what causes a shift in diet is vital for the elucidation of trophic roles and fish bioenergetics because dietary changes affect body growth and competition through resource partitioning (Werner and Gillian 1984; Sánchez-Hernández and Cobo 2018). The diet of a fish alters as it grows. This is most likely due to morphological and physiological changes that occur during growth. Beside its size, season is another potential factor affecting the diet of a fish (Huo et al. 2014), e.g. seasonal variation in water temperature and food resources (Hovde et al. 2002). Many fishes are opportunistic feeders, and their food spectrum relies on prey items available in their environment, which alters seasonally. These shifts, which are reflected in the composition and diversity of a diet, indicate the dietary adaptability of a species (Zander 1996).
In short, studying the feeding habits of G. firmispinatus can contribute to an understanding of its population dynamics, and also provide essential information on dietary energy flows for the elucidation of food webs in lotic streams. Hence, we examined the trophic ecology of G. firmispinatus in the Anning River. The specific aims of this study were to evaluate: (1) the feeding intensity and diet composition of G. firmispinatus, and the effects of body length, and seasonal and sexual factors on its gut contents; (2) the feeding strategy of this species; and (3) its prey selection amongst the macroinvertebrate communities of the Anning River according to season.
Materials and methods
Study area
The study took place in the Anning River, a tributary of the Yalong River. Located in the subtropical monsoon climatic zone, the Anning River has a mean annual air temperature of 17–19 °C and annual rainfall exceeding 1240 mm/year (Ning 2009). The water flow over the stony beds of the river and its tributaries is fast. Many species of macroinvertebrates reside amongst the cobbles and boulders that mainly comprise the river bed (Ma et al. 2019b). G. firmispinatus is adapted to its habitat amongst the cobble crevices of the lotic streams, and it feeds mainly on aquatic insects and their larvae (Chen and Cao 2000; Ma et al. 2017, 2018).
Sample collection
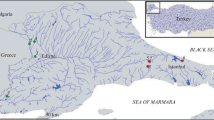
Due to fragmentation of the main stem of the Anning River by the construction of hydropower stations, G. firmispinatus has mostly moved into the river’s tributaries (Ma et al. 2018). Thus, specimens were sampled from tributaries, and specifically from those that originated from the middle of the river. Sampling was undertaken monthly from July 2015 until June 2016 (Fig. 1). Table 1 gives basic information on the study sites, such as length and width of the sampling area, and water temperature.
Benthic invertebrates, a potential food source of Gymnocypris firmispinatus, were sampled before Gymnocypris firmispinatus were sampled by electrofishing. At each site, two or three quantitative specimens were collected with a Surber sampler (30 × 30 cm, 500-μm mesh) (Morse et al. 1994). Specimens were sorted and preserved in 80% ethanol, identified to the lowest possible taxon under a dissecting microscope (Morse et al. 1994; Epler et al. 2001), and the abundance of each item was calculated.
Fish were sampled monthly from ca. 10:00 to 12:00 a.m. by using backpack electrofishing gear (Hailibao, China). G. firmispinatus was the dominant fish species in the tributaries of the middle Anning River, followed by Paracobitis variegatus and Triplophysa spp. occurred less frequently, and Schizothorax wangchiachii, Schizothorax kozlovi and several other fish species were occasionally present in the catch. G. firmispinatus (305 individuals) were randomly selected from the catch and killed immediately by soaking in an overdose of MS-222. These fish were transported in a coolbox to the laboratory, where they were measured [total length (TL), millimeters] and weighed (wet weight, to the nearest 0.1 g). The fish were dissected and their guts excised. The foregut content samples were removed and preserved in 80% ethanol. Sex was determined by macroscopic examination of gonadal morphology. Based on the observations of gonadal development and/or secondary sexual characteristics and according to Ma et al. (2018), the fish were assigned to one of two size classes (small individuals, class I ≤ 100 mm; large individuals, class II > 100 mm), to examine differences in feeding intensity and diet composition.
Feeding intensity
To evaluate the rhythm of feeding intensity, the vacuity index V = Ne/Ns × 100% was computed, where Ne is the number of empty guts, and Ns is the total number of guts examined. A χ2-test (cross-tabulation analysis) was used to determine differences in the vacuity index with regard to sampling month, fish size and sex (La Mesa et al. 2007). The analysis was performed using SPSS 16.0 and OriginPro 2016 at P < 0.05.
Diet composition and niche breadth
A total of 141 fish (TL = 32–193 mm) were selected for the diet composition analyses. The gut contents of each specimen were washed out into a petri dish. Each prey item was classified and identified to the lowest feasible taxonomic level (Morse et al. 1994; Epler 2001; Zhou 2003), then counted individually. After absorbing excess water with blotting paper, the prey item was weighed (0.1 mg) on an electronic balance (Huo et al. 2014).
The dietary contribution of each prey item was determined by using frequency of occurrence (O%), percentage by number (N%), percentage by weight (W%), index of relative importance (IRI) = O% (N% + W%), and IRI% (Pinkas et al. 1971; Cortés 1997). The feeding niche breadth of G. firmispinatus was calculated by the Shannon–Wiener diversity index (H′) = − ∑ Pi (ln Pi) (Shannon 1948), and species evenness by Pielou’s index (J) = H′/ln S (Pielou 1966), where Pi is the proportion of each prey species in a sample and S is the number of prey taxa.
To evaluate the feeding strategy of G. firmispinatus, Amundsen’s graphical method was applied to the dataset of prey type as Ai = (∑Gi/∑Gti) × 100, where Ai is the prey-specific abundance of prey i, Gi the gut contents (weight) comprising prey i, and Gti the total gut contents of only those fish with prey i in their guts (Amundsen et al. 1996).
Diet variation with season, fish size and sex
Size, seasonal and sex-related changes in feeding habits were examined by multivariate analysis. A cluster analysis was applied to the dataset comprising W% values of prey type computed for each group of fish according to size class (class I ≤ 100 mm TL, class II > 100 mm TL), season (spring, summer, autumn, winter) and sex (male, female); 16 groups of fish were obtained in this way (La Mesa et al. 2007). The Bray–Curtis similarity matrix was constructed for this fish group-prey dataset, and hierarchical agglomerative clustering was applied to the similarity matrix (La Mesa et al. 2007). PRIMER 5.0 software (Clarke and Warwick 1994) was used for the statistical analyses.
Prey selection
Prey selection was computed by using Ivlev’s selectivity index (I) = (ri − pi)/(ri + pi), where ri is the proportion of a prey category in the gut contents of a fish, and pi is its proportional availability in the river (Ivlev 1961). The selectivity index ranges from − 1 (strong negative selection) to 1 (strong positive selection).
Results
In total, 305 individual fish were examined. The male:female sex ratio was 1.03:1, and the TL of males and females ranged from 57 to 145 mm and 58–193 mm TL, respectively. The sex of 23 of the individuals (32–71 mm TL) could not be determined.
Feeding intensity
Of the total number of guts studied, 49 (16.0%) were empty. There was a significant difference in the vacuity index among the fish according to the month in which they were sampled (χ2 = 20.402, p = 0.040); the highest index was for fish caught in January (38.5%) and the lowest for September (0%; Fig. 2). No significant differences were observed between the two size groups (χ2 = 3.573, p = 0.059) or between the sexes (χ2 = 0.307, p = 0.579).
Diet composition and niche breadth
In the gut contents of the 141 G. firmispinatus examined, 46 prey items belonged to one of five orders (Ephemeroptera, Plecoptera, Trichoptera, Coleoptera and Diptera) (Table 2). H′ and J of the total samples were 2.67 and 0.70, respectively. There were some differences in H′ and J over the seasons. The highest H′ was recorded in winter (2.55), and the highest J in summer and winter (0.76), while the lowest values of the two indices were for spring (H′ = 2.02, J = 0.61). The H′ of males was higher than that of females (Table 3).
Most dietary samples of G. firmispinatus included a variety of aquatic insects. Some tiny sands were also observed among the foregut contents. Ephemeropteran larvae, trichopteran larvae, and dipteran larvae were the most abundant groups in the diets in terms of number, weight and IRI, followed by plecopteran larvae, while coleopteran larvae were occasionally present. Based on O%, N%, and IRI%, the principal prey were Baetis sp. and Simulium sp., followed by Diamesa sp. and Glossosoma sp., but in terms of W%, the primary prey were Epeorus sp. (13.92%) and Glossosoma sp. (12.22%), followed by Baetis sp. (9.45%) and Simulium sp. (7.64%) (Table 2).
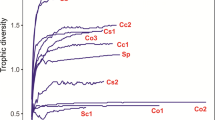
The dietary composition of G. firmispinatus is graphically presented in Fig. 3. According to the feeding strategy axis, G. firmispinatus can be considered a generalist feeder as most of its prey are present in the lower part of the plot. In relation to their contribution to niche width, the location of most prey in the diagram demonstrates the variation in utilization of prey resources between and within individual groups of fish. The population-level variation may be explained to a large extent by the TL-dependent shifts in diet. Ephemeropteran larvae were the most important prey category for small individuals (Fig. 3a), whereas trichopteran larvae were the primary prey category for large individuals (Fig. 3b).
Diet variation with season, fish size and sex
The clustering of fish groups in relation to size, sex and season is shown in Fig. 4. The diet composition was mainly determined by fish size, i.e., this had a stronger effect than season or sex. The two groups shown in Fig. 4 (A and B) mainly represent large (> 100 mm TL) and small (≤ 100 mm TL) fishes, respectively. Small individuals fed primarily on small ephemeropteran larvae and dipteran larvae, whereas bigger larvae, and more trichopteran larvae, were more frequently consumed by large individuals. There was high similarity in diets between the two size groups of fish in spring, as shown in the dendrogram, and the effect of sampling season on the diet of G. firmispinatus was generally low. Finally, the sex of a fish appeared to be the least important factor with respect to diet diversity of G. firmispinatus, since this was randomly distributed within the clustered groups.
Dendrogram of diet composition data according to season, sex and TL. a, b Main clustering groups at 50% similarity. SPR Spring, SUM summer, AUT autumn, WIN winter, M males, F females, 1 small individuals, 2 large individuals; for other abbreviations, see Fig. 3
Prey selection
The composition of the macroinvertebrate assemblage (N%) showed a high variation among seasons. Aquatic insects contributed 98.2% of the total abundance, with ephemeropterans (53.5%), dipterans (28.6%) and trichopterans (7.7%) the taxonomically richest groups. According to Ivlev’s selectivity index, trichopteran larvae and dipteran larvae were positively hunted for, while ephemeropteran larvae, plecopteran larvae and coleopteran larvae were negatively fed on by G. firmispinatus in all seasons. In details, G. firmispinatus preferred dipteran larvae to trichopteran larvae in spring, but selected trichopteran larvae more positively in winter. Coleopteran larvae were the least favoured food of the fish (Fig. 5).
Relative numerical abundance (N%) of prey categories in fish gut contents (Nf%) and in the macroinvertebrate community (Nm%) for the Anning River between July 2015 and June 2016, according to season. Dietary preferences expressed as Ivlev’s selectivity index are given above the bars for each prey category. For abbreviations, see Fig. 3, oth other
Discussion
As also seen in other fishes (Hovde et al. 2002; La Mesa et al. 2007; Ma et al. 2014), the feeding intensity of G. firmispinatus generally followed a seasonal trend. Some studies found that the highest percentage of fish with empty guts occurred during the spawning season, which was attributed to a significant decrease in food ingestion during reproduction (Hovde et al. 2002; Šantić et al. 2009). However, no specific differences in observed trends of feeding intensity were seen in the present study for the reproductive period of G. firmispinatus, which is between March and May (Ma et al. 2018). The lowest feeding intensity was observed in winter, which may have been related to the low water temperature then (Abid et al. 2013), and could indicate strong temperature-dependent regulation of food intake in G. firmispinatus, or lower metabolism in this season, which may lead to a decrease in food ingestion to a minimum (Šantić et al. 2005). In contrast, the intensity of food intake was highest in September; this intake may have contributed to the accumulation of fat, which could be an additional source of energy for the fish in winter.
The diets of fish usually shift as they grow due to the morphological changes that accompany this process (Wootton 1990). Our study showed size-related differences in food composition in G. firmispinatus, and size-related shifts in diet composition of freshwater fishes have been reported in a number of studies (Matić-Skoko et al. 2004; La Mesa et al. 2007; Kati et al. 2015). These shifts have also been reported for another species of the Schizothoracinae, Oxygymnocypris stewartii, small individuals of which fed mainly on Cobitidae and Hydropsychidae larvae, while larger individuals tended to consume Cyprinidae and Chironomidae larvae (Huo et al. 2014). However, for some other Schizothoracinae fishes (such as Schizopygopsis younghusbandi and Schizothorax o’connori), which have a protruding under jaw with sharp horns and graze mainly on algae, no significant ontogenetic shifts in diets were found (Yang et al. 2011; Ma et al. 2014). Size-related variations in diet composition possibly reflect morphological and maturational changes, especially an increase in gape size (Graeb et al. 2005; Huo et al. 2014), improvements in locomotion (Beamish 1978) and sensory capabilities (Li et al. 1985). These factors can influence the ability of a fish to catch different types and sizes of prey (Huo et al. 2014). Furthermore, large individuals tend to ingest bigger prey that take longer to catch (such as trichopteran larvae), and which represent a more useful food as they have a higher caloric value and thus sustain a high metabolic investment (La Mesa et al. 2007). A shift in diet as an organism grows has probably evolved as a strategy to decrease intraspecific competition between juveniles and adults for food (Schoener 1974; Werner 1979; Amundsen et al. 1996).
Besides the biological characteristics of a fish and the physical characteristics of its habitat, the food it selects is also affected by prey characteristics (Adams et al. 2007; Sánchez-Hernández and Cobo 2018). According to the optimal foraging theory, fishes should choose those prey taxa that maximize the net energetic gain in relation to the energetic cost of their capture, ingestion and digestion (Gerking 1994). In this study, G. firmispinatus preferred dipteran larvae and trichopteran larvae in every season (Fig. 5), which might be attributed to the following factors: (1) dipteran and trichopteran larvae move more slowly than ephemeropteran, plecopteran larvae and coleopteran larvae, which could make it easier for fish to search for and capture them; (2) the bodies of dipteran and trichopteran larvae are fairly soft (e.g. abdomen), which might make them more digestible and more profitable in terms of the energy expenditure required for their capture compared to that required for the three other types of insect larvae. These findings are broadly in accordance with those of Huo et al. (2014), who pointed out that habitat and energy intake rate may influence the composition of prey that are consumed by a fish. Although ephemeropteran larvae were very abundant in the sampling reach, they did not represent the highest proportion of prey in the gut of G. firmispinatus (Table 2). Our results on dietary preferences are similar to those of studies on other fish species that also showed that fishes do not always ingest the most abundant prey items available in their environment (Sánchez-Hernández and Cobo 2012; Kati et al. 2015).
Overall, the present study indicates seasonal trends in the feeding intensity of G. firmispinatus, which is regarded as a generalist feeder that depends upon a wide trophic spectrum. G. firmispinatus was found to feed almost exclusively on aquatic insects, with a preference for dipteran larvae and trichopteran larvae. Size-related diet variation was also found, where individuals > 100 mm TL consumed more trichopteran larvae. The present study provides valuable information for the further study of energy flows and food webs in lotic streams. In addition, this study provides a scientific basis for the further study of Schizothoracinae fishes and for their conservation in waterbodies at high altitudes.
References
Abid S, Ouannes-Ghorbel A, Jarboui O, Bouain A (2013) Diet composition and feeding habits of the wide-eyed flounder, Bothus podas, in the Gulf of Gabes (Tunisia). Mar Biodivers 43:149–161
Adams CF, Pinchuk AI, Coyle KO (2007) Seasonal changes in the diet composition and prey selection of walleye pollock (Theragra chalcogramma) in the northern Gulf of Alaska. Fish Res 84:378–389
Amundsen PA, Gabler HM, Staldvik FJ (1996) A new approach to graphical analysis of feeding strategy from stomach contents data-modification of the Costello (1990) method. J Fish Biol 48:607–614
Beamish FWH (1978) Swimming capacity. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic Press, New York, pp 101–187
Braga RR, Bornatowski H, Vitule JRS (2012) Feeding ecology of fishes: an overview of worldwide publications. Rev Fish Biol Fish 22:915–929
Chen YF, Cao WX (2000) Schizothoracinae. In: Yue PQ (ed) Fauna Sinica Osteichthyes Cypriniformes III. Science Press, Beijing, pp 273–388 (in Chinese)
Clarke KR, Warwick RM (1994) Change in marine communities: an approach to statistical analysis and interpretation. Natural Environment Research Council, Plymouth
Cortés E (1997) A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Can J Fish Aquat Sci 54:726–738
Epler JH (2001) Identification manual for the larval Chironomidae (Diptera) of North and South Carolina. St Johns River Water Management District, Palatka
Gerking SD (1994) Feeding ecology of fish. Academic Press, San Diego
Graeb BDS, Galarowicz T, Wahl DH, Dttmers JM, Simpson MJ (2005) Foraging behavior, morphology, and life history variation determine the ontogeny of piscivory in two closely related predators. Can J Fish Aquat Sci 62:2010–2020
Hovde SC, Albert OT, Nilssen EM (2002) Spatial, seasonal and ontogenetic variation in diet of Northeast Arctic Greenland halibut (Reinhardtius hippoglossoides). ICES J Mar Sci 59:421–437
Huo B, Xie CX, Madenjian CP, Ma BS, Yang XF, Huang HP (2014) Feeding habits of an endemic fish, Oxygymnocypris stewartii, in the Yarlung Zangbo River in Tibet, China. Environ Biol Fish 97:1279–1293
Ivlev VA (1961) Experimental ecology of the feeding fishes. Yale University Press, New Haven
Kati S, Mozsár A, Árva D, Cozma NJ, Czeglédi I, Antal L, Nagy SA, Erős T (2015) Feeding ecology of the invasive Amur sleeper (Perccottus glenii Dybowski, 1877) in Central Europe. Int Rev Hydrobiol 100:116–128
La Mesa G, La Mesa M, Tomassetti P (2007) Feeding habits of the Madeira rockfish Scorpaena maderensis from the central Mediterranean Sea. Mar Biol 150:1313–1320
Li KT, Wetterer JK, Hairston NG Jr (1985) Fish size, visual resolution, and prey selectivity. Ecology 66:1729–1735
Ma BS, Xie CX, Huo B, Yang XF (2014) Feeding habits of Schizothorax oconnori Lloyd, 1908 in the Yarlung Zangbo River, Tibet. J Appl Ichthyol 30:286–293
Ma BS, Xu B, Wei KJ, Deng LJ, Gan WX, Zhu XY, Yao YH (2017) Length–weight and length–length relationships of four native fish species from the Yalong River, China. J Appl Ichthyol 33:839–841
Ma BS, Wei KJ, Xu B, Xu J, Zhu XY, Nie YY (2018) Reproductive characteristics of Gymnocypris firmispinatus in the Anning River, China. Fish Sci 84:963–974
Ma BS, Nie YY, Wei KJ, Xu B, Zhu XY, Xu J (2019a) Estimates on age, growth, and mortality of Gymnocypris firmispinatus (Cyprinidae: Schizothoracinae) in the Anning River, China. J Oceanol Limnol 37:736–744
Ma BS, Xu B, Wei KJ, Liang M, Xu J, Zhu XY (2019b) Macroinvertebrate community structure and its relation to the environmental conditions in the middle Anning River. Acta Hydrobiol Sin 43:644–654 (in Chinese with English abstract)
Matić-Skoko S, Antolić B, Kraljević M (2004) Ontogenetic and seasonal feeding habits of the annular seabream (Diplodus annularis L.) in Zostera sp. beds, eastern Adriatic Sea. J Appl Ichthyol 20:376–381
Morse JC, Yang L, Tian L (1994) Aquatic insects of China useful for monitoring water quality. Houhai University Press, Nanjing
Ning X (2009) Studies on characteristics of annual runoff in Anning River. Master’s thesis, Sichuan Normal University, Chengdu (in Chinese with English abstract)
Pielou ECJ (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144
Pinkas L, Oliphant MS, Iverson ILK (1971) Food habits of albacore, bluefin tuna, and bonito in California waters. Fish Game Fish Bull 152:1–105
Ru HJ, Zhang Y, Li YF, Wang HM, Shen ZW, Wu XX, Li R, Sheng Q, Ni ZH (2016) Community composition and status of fish resources in Anning River. J Hydroecol 37:68–74 (in Chinese with English abstract)
Sánchez-Hernández J, Cobo F (2012) Ontogenetic dietary shifts and food selection of endemic Squalius carolitertii (Actinopterygii: Cypriniformes: Cyprinidae) in River Tormes, central Spain, in summer. Acta Ichthyol Piscat 42:101–111
Sánchez-Hernández J, Cobo F (2018) Modelling the factors influencing ontogenetic dietary shifts in stream-dwelling brown trout (Salmo trutta Linnaeus, 1758). Can J Fish Aquat Sci 75:590–599
Šantić M, Jardas I, Pallaoro A (2005) Feeding habits of horse mackerel, Trachurus trachurus (Linnaeus 1758), from the central Adriatic Sea. J Appl Ichthyol 21:125–130
Šantić M, Podvinski M, Pallaoro A, Jardas I, Kirincic M (2009) Feeding habits of megrim, Lepidorhombus whiffiagonis (Walbaum, 1792), from the central Adriatic Sea. J Appl Ichthyol 25:417–422
Schoener TW (1974) Resource partitioning in ecological communities. Science 185:27–39
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27:379–423
Werner EE (1979) Niche partitioning by food size in fish communities. In: Stroud RH, Clepper H (eds) Predator–prey systems in fisheries management. Sport Fishing Institute, Washington, DC, pp 311–322
Werner EE, Gillian JF (1984) The ontogenetic niche and species interactions in size-structured populations. Annu Rev Entomol 15:393–425
Wootton RJ (1990) Ecology of teleosts fishes. Chapman and Hall, New York
Yang XF, Xie CX, Ma BS, Huo B, Huang HP, Zhang HJ, Xu J (2011) Feeding habits of Schizopygopsis younghusbandi younghusbandi. Freshwater Fish 41:40–44 (in Chinese with English abstract)
Zander CD (1996) The distribution and feeding ecology of small-size epibenthic fish in the coastal Mediterranean Sea. In: Eleftheriou A, Ansell AC, Smith J (eds) Biology and ecology of shallow coastal waters. Olsen and Olsen, Fredensborg, pp 369–376
Zhou CF (2003) A taxonomic study on mayflies from mainland China (Insecta: Ephemeroptera). Doctor’s thesis, Nankai University, Tianjin (in Chinese with English abstract)
Acknowledgments
The authors would like to thank Meng Liang and Yuanyuan Nie for their help in sample collection. Financial support was provided by the Central Public-interest Scientific Institution Basal Research Fund, the Chinese Academy of Fishery Sciences (2018JBF14), and the National Natural Science Foundation of China (51809280).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, B., Xu, B., Wei, K. et al. Feeding habits of the cyprinid Gymnocypris firmispinatus in the Anning River, China. Fish Sci 86, 749–758 (2020). https://doi.org/10.1007/s12562-020-01445-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-020-01445-x