Abstract
We examined the sea-entry conditions (i.e., timing and body size) of juvenile chum salmon that improve their survival during their coastal residency. On 25 June 2013, we sampled 365 juvenile chum salmon [57.5–98.6 mm fork length (FL)] off Konbumori, eastern Hokkaido, ⁓ 20 km east of the Kushiro River mouth, which originated from a hatchery in the Kushiro River. Sea-entry conditions of these Konbumori juveniles back-calculated using otolith daily increment analysis were compared with data from 373 juveniles released from the same hatchery that were captured at the mouth of Kushiro River (i.e., just before sea entry) from April to July 2013. Most of the Konbumori fish were estimated to have entered the sea from 25 May to 5 June, when coastal surface temperatures constantly exceeded 5 °C, which is considered favorable for juveniles. The estimated FLs at sea entry of the Konbumori fish were larger than FLs of fish sampled at the river mouth during a comparable period, which suggests that size-selective mortality existed. Back-calculated post-sea-entry growth rates of fish with larger FL at sea entry, particularly those with FL > 65 mm, tended to be high enough for survival among the Konbumori fish. Assuming growth-dependent mortality, this case study suggests that the release of larger-sized juveniles under favorable coastal temperature conditions improves their survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pacific salmon Oncorhynchus spp. are important fishery resources in the North Pacific. Species of this genus typically undergo massive mortality during their early life stages (Beamish 2018), and chum salmon Oncorhynchus keta are no exception to this, particularly just after their entry into the sea (Healey 1982; Bax 1983; Fukuwaka and Suzuki 2002; Wertheimer and Thrower 2007). Mortality patterns of chum salmon during coastal residency are thought to be size selective (Healey 1982; Tucker et al. 2016). Juvenile chum salmon originating from Japan migrate north to the central Sea of Okhotsk, where they remain for their first summer and autumn (Chistyakova and Bugaev 2016; Urawa et al. 2018). Recent studies have suggested that these juveniles experience growth-dependent mortality (Sogard 1997) along Japanese coasts (Honda et al. 2017, 2019). For juvenile chum salmon originating from the Japanese Pacific coast, a median growth rate in length of 0.65 mm/day or more after sea entry seemed necessary for them to reach a fork length (FL) of more than 90 mm upon reaching waters off eastern Hokkaido (i.e., Konbumori; Fig. 1); juveniles with growth rates below 0.45 mm/day experienced difficulty in reaching Konbumori, particularly if they originated from areas > 200 km southwest of Konbumori (Honda et al. 2017). These growth rates were determined from otolith daily increment analysis, a method that, in addition to estimating growth rate, enables us to estimate the body size of an individual at the point of sea entry and its timing (Saito et al. 2007, 2009). Origin-based growth characteristics can be determined by concurrently using the otolith thermal marking technique (Urawa et al. 2001) and otolith daily increment analysis.
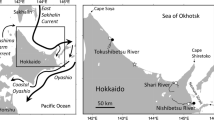
Study site in eastern Hokkaido [bold frame (left panel); enlarged map (right panel] and location of the release site [Tsurui Salmon Field Station (open circle)] of juvenile chum salmon in the Kushiro River system, and sampling sites at the Kushiro River mouth (open star) and off Konbumori (solid star). Gray line (right panel) shows the 50-m-depth contour
Although the biomass of wild chum salmon is not insignificant (Miyakoshi et al. 2012a; Morita et al. 2013, 2019), Japanese chum salmon fishery resources have been sustained by hatchery programs (Morita et al. 2006; Miyakoshi et al. 2013; Kitada 2014). Assuming growth-dependent mortality, release strategies such as releasing fish with a specific body size at a specific time is considered desirable to potentially increase post-sea-entry growth rates. Japanese hatchery programs have recommended that juveniles be released at target periods when coastal sea surface temperatures (SST) range from 5 to 13 °C and the juveniles weigh more than 1 g (approximately 50 mm FL) (Seki 2013). These release conditions are based on empirical rules and the results of scientific research (Seki and Shimizu 1996; Saito and Nagasawa 2009), and are particularly applied because juvenile chum salmon occur frequently along Japanese coasts at SST ranging from 5 to 13 °C (Kobayashi 1977; Irie 1990).
Even though the numbers of chum salmon fry/juveniles released from Japanese hatcheries have remained constant since the early 1980s (Urawa et al. 2018), and techniques used in hatchery programs have improved (Seki 2013), return rates of chum salmon adults to Japanese waters have in recent years, and in particular between 2016 and 2018, declined to levels similar to those of the early 1980s [Hokkaido National Fisheries Research Institute, Salmon information: https://salmon.fra.affrc.go.jp/zousyoku/sakemasu.html, Accessed 28 Jan 2020; North Pacific Anadromous Fish Commission (NPAFC) Catch Statistics: https://npafc.org/statistics/, Accessed 28 Jan 2020]. Why this decline has occurred remains to be clarified, but the more severe environmental conditions that juveniles encounter during their early marine life stages before reaching the offshore waters of the Sea of Okhotsk are suspected to play a role (Miyakoshi 2018; Saito and Fukuwaka 2018).
If the characteristics of sea-entry conditions (timing and body size) of juvenile chum salmon that improve post-sea-entry survival/growth, and the relationship between these characteristics and the corresponding coastal environment, were better understood, hatchery programs could be ameliorated by adjusting release strategies to improve juvenile survival. Because Japanese rivers are relatively short and most juvenile chum salmon are thought to migrate to the sea within a short period after hatchery release (< 10 days) (Mayama et al. 1982; Torao et al. 2010; Miyakoshi et al. 2012b), sea-entry conditions are considered to be closely linked to release conditions (i.e., decisions taken on the timing of release and the size of juveniles at release). Nonetheless, it is extremely difficult to identify sea-entry conditions that enhance juvenile chum salmon survival without a detailed understanding of population structure at the time of sea entry throughout the entire migratory period (Beacham et al. 2018). That is, the characteristics of individuals that survive can only be determined once the entire picture of the initial population structure before massive mortality takes place is clarified, and subsequently, the characteristics of dead fish among the population have been identified, and vice versa. However, in situ field observations at the moment of death, or even finding a dead juvenile salmon in the marine realm, is nearly impossible, except for remains found in the stomach contents of predatory animals (Beamish et al. 1992; Okado et al. 2020). Thus, we must first understand the timing and size-class composition of juveniles just before or after sea entry (i.e., near a river mouth) throughout their entire migratory periods in a specific river. Second, we need information on the sea-entry condition of juveniles sampled at a specific point during their ocean migration route, which we can obtain by using otolith daily increment analysis. Finally, by comparing these two data sets for a single cohort, we can determine the sea-entry characteristics of surviving fish and fish mortality by the time of the latter sampling point.
Collecting these sorts of data requires intensive field surveys and also involves considerable expense since the entire period of sea entry of juveniles covers several months. Additionally, in waters where juveniles that originate from different regions co-occur, the target cohort must be labeled for identification, for example, with an otolith thermal mark. For these reasons, few studies targeting juvenile Pacific salmon examine the relationship between sea-entry conditions and subsequent survival/growth (Woodson et al. 2013; Claiborne et al. 2014). We report new data for a considerable number of juvenile chum salmon sampled at a river mouth and in coastal waters, which were released from the Kushiro River, eastern Hokkaido, in 2013 (i.e., the 2012 brood year). The number of adult returns (fish of ages-2–5) to the Kushiro River for the 2012 brood year was estimated to be approximately 23,000, and was smaller than the number of adult returns of the 1980–2011 brood years (approximately 181,000, on average) (Hokkaido National Fisheries Research Institute, unpublished data, 2020). We assumed that using data obtained from poor-return stocks is effective to evaluate the survival conditions of juvenile, since they presumably experienced more severe, and thus more distinctive, environmental conditions during their coastal residency.
Materials and methods
Sampling juvenile chum salmon
About 8.742 million juvenile chum salmon were released from the Tsurui Salmon Field Station (Fig. 1), which is about 31 km upstream of the Kushiro River mouth, between 15 April and 29 May 2013 (i.e., 2012 brood-year stock; Online Resource 1). The fish could be identified by the same thermal hatch code, 2-2-3-3H, in their sagittal otoliths. Additionally, about 34.739 million juvenile chum salmon without a thermal hatch code were released into this river system during 2013 from other salmon hatcheries. Naturally spawning chum salmon have also been reported in this system (Miyakoshi et al. 2012a). Juveniles originating from rivers in this region are thought to migrate to the Sea of Okhotsk following the easternmost coast of Hokkaido Island (Irie 1990; Honda et al. 2017; Urawa et al. 2018).
Juvenile chum salmon were sampled about 400 m upstream of the Kushiro River mouth (Fig. 1) on 17 days between 22 April and 5 July (Online Resource 2). On each sampling date, a beach seine (18 m wide, 1.3–1.8 m deep, with a 4.6-m-long, 4-mm-mesh bag) was pulled with the assistance of a small boat. The daily total sampled area was approximately 2000 m2 and was consistent between sampling dates. In total, 7976 juvenile chum salmon were caught, of which 1398 were retained for subsequent analysis (other fish were released immediately on-site after counting). FLs of retained specimens were immediately measured (samples were not frozen) to the nearest 0.1 mm, and their sagittal otoliths removed. Otoliths were later mounted on glass slides using Buehler Thermoplastic Cement (Buehler, Lake Bluff, USA). Each otolith was then polished using 0.3- to 12.0-µm Buehler FibrMet Abrasive Discs until the thermal hatch code was readable under light microscopy; the presence (or absence) of the code was noted, and was verified by two experts. Measurements were made at magnification 200×. From the 1398 specimens, the 2-2-3-3H hatch code was found in 373 fish (40.0–72.4 mm FL; Online Resource 2).
Juvenile chum salmon were collected from coastal waters off Konbumori (Fig. 1), about 20 km east of the Kushiro River mouth, by a two-boat surface trawl. The trawl net was 25 m long, 4 m deep, with a 3-m-long and 4-mm-mesh bag, and was towed for 10–20 min at a speed of 2.5–3.5 kN, parallel to the shore. Fish collection was conducted seven times, on 4, 11, 18, and 25 June, and 9, 16, and 23 July 2013, at five stations (Stns 1–5), each at a different distance from shore (0.6, 1.2, 4.0, 8.0 and 12.0 km, respectively; 42°50–56′N, 144°33–35′E); Stn 5 was surveyed once only, on 9 July 2013. Of the 1773 juvenile chum salmon caught, 1686 (95.1%) were collected at Stn 1, and 52 (2.9%) at Stn 2, on 25 June 2013. Fish were placed into an icebox and transported to the laboratory where they were stored at − 18 °C until measurement. After thawing the fish, their FL was measured and otolith hatch code checked (as described previously). Of the original 1773 juveniles, 473 had the Kushiro River hatch code (2-2-3-3H), of which one (58.7 mm FL) was caught on 16 July 2013 at Stn 2 and the others (57.5–98.6 mm FL) on 25 June at Stns 1 (n = 471) and 2 (n = 1).
Otolith daily increment analysis
For juveniles sampled from Konbumori, the sagittal otolith not used in hatch code identification was used for daily increment analysis, unless one otolith of a pair was lost, broken, or replaced by vaterite. Consequently, otoliths from 365 specimens were used (57.5–98.6 mm FL), all from fish sampled at Stn 1 on 25 June. Otolith daily incremental analyses followed methods used by Honda et al. (2017), wherein data for 18 of the 365 specimens used in this study were also reported; these data were reused without change. Extracted sagittal otoliths were mounted on glass slides using thermoplastic cement, then polished on both sides using 0.3–3.0-µm abrasive discs until the core was exposed. Sea-entry timing was determined based on the presence of a “sea-entry check,” a distinctive growth increment that forms on an otolith when a fish migrates from freshwater to marine environments (Saito et al. 2007). For 37 specimens (mean ± SD, 75.6 ± 8.4 mm FL) for which this check was unclear, or for which multiple check-like increments were observed, we estimated the point of sea entry on the otolith by analyzing the otolith’s strontium (Sr) to calcium (Ca) weight percent ratio using an electron probe micro-analyzer (JXA-8230; JEOL, Tokyo) following Honda et al. (2017). The point of sea entry was defined when a three-point running average (the measurements were spaced at 2- μm intervals) of the Sr:Ca ratio exceeded 4.0 × 10-3 (see Honda et al. 2017, for details). The number and width of otolith daily growth increments beyond the sea-entry check/point were counted and measured using an otolith measurement system (ARP/W + RI version 5.30; Ratoc System Engineering, Tokyo). Increment measurements were performed along a line from the core to either the dorsal or ventral side, depending on where the increments were most clear. Sea-entry date was back-calculated by subtracting the number of daily growth increments counted from the date on which a fish was sampled. The relationship between otolith radius (R, micrometers) and FL (millimeters) is described in Eq. (1) as follows:
where a and b are the intercept and slope of the regression line fitted to log-transformed FL and R, respectively. To make the curve fit data for both R and FL when fish hatched (RH and FLH), and R and FL for fish when captured (RC and FLC), a and b were calculated individually by minimizing the sum-of-squares residuals between observed and predicted FLH and FLC (using the least-square method), using the Solver Add-in with Microsoft Excel®. We assume RH to be 141.13 µm for fish measured along the ventral-side line, and 111.11 µm along the dorsal-side line, while FLH for all fish was set at 20.44 mm (Saito et al. 2009). Using Eq. (1), we were able to back-calculate FL at sea entry and daily growth in FL (millimeters).
Data analysis
We compared back-calculated dates and FLs of fish at sea entry caught at Konbumori (hereafter, Konbumori fish) with dates and FLs of fish caught at the Kushiro River mouth (river-mouth fish). Then, mean daily growth in FL from the estimated sea-entry date to the date of collection (i.e., 25 June 2013 for all specimens) was calculated individually and used as an index of growth rate in the analyses. We did this because daily growth in FL of juvenile chum salmon during their early marine life is generally linearly correlated with daily periodicity (Salo 1991; Vega et al. 2017). Honda et al. (2017) used the exact same back-calculation method as that used in the current study, and found an individual linear-like growth trajectory in Japanese juvenile chum salmon. The FL measurement for Konbumori fish was done post-thawing—a process known to shorten fish FL (Kasugai 2013). Nonetheless, in the case of measurements of juvenile chum salmon (n = 50) collected at the Chitose and Shizunai Rivers in Hokkaido, the difference in FL before (34–77 mm, x) and after (32–74 mm, y) freezing and thawing was small, and can be described by y = 0.99x − 2.1 (Morita, unpublished data). A similar equation with a slope of > 0.93 was reported by Armstrong and Stewart (1997) for juvenile Atlantic salmon Salmo salar of FL 32–139 mm. As all the thawed fish were obtained from Konbumori, the back-calculated FLs at sea entry might be slight underestimates, but we decided not to correct our estimated FL.
Generalized additive models (GAMs) were used to interpret how the sea-entry condition (date and FL) of Konbumori fish affected their subsequent growth. Growth rate was set as an objective variable, and back-calculated sea-entry (Julian) date and sea-entry FL were set as predictor variables, with wrapping in a non-parametric smoothing function. Gamma-distribution with a log link was used for model error distribution. All GAMs were fitted using R version 3.5.2 (R Core Team 2018) and the statistical packages mgcv (Wood 2019) and MuMIn (Bartoń 2018). Predictor variables and the best model were selected based on generalized cross validation and Akaike’s information criterion (AIC) values, and the lowest AIC model chosen. To select the best model from all possible combinations of factors, model selection for GAMs was done using the dredge function in MuMIn. One fish with a back-calculated FL of 81.44 mm at sea entry was considered an outlier (its FL exceeded the mean by + 5σ); it was excluded only from this analysis to meet the assumptions for model building. If a distinctive trend was apparent, we focused on relationships between growth rates, and dates and FL at the time of sea entry, taking favorable and unfavorable growth rates in this region into consideration (> 0.65 and < 0.45 mm/day, respectively) (Honda et al. 2017).
Coastal SST has been used for decades as a criterion for the timing of juvenile chum salmon release and sea entry (Seki 2013). Back-calculated sea-entry timing was compared with coastal SST (degrees Celsius), which was calculated daily by averaging hourly data from a water temperature logger (Starmon mini; Star-Oddi, Gardabaer, Iceland) deployed at 3-m depth at Konbumori Stn 2 (42°56.53′N, 144°34.57′E) from 30 April to 31 July 2013. For comparison with historical values, daily SST anomalies (difference in average SST from 1994 to 2012) were calculated.
Results
Juvenile chum salmon were captured continuously at the Kushiro River mouth from early May to early June 2013 (Fig. 2); the ratio of thermal-marked fish increased from 3 June because many juveniles were released in late May (Online Resources 1, 2). Estimated sea-entry dates of Konbumori fish occurred over a shorter period of time, between 15 May and 15 June (with 5–95th percentiles between 25 May and 5 June; hereafter, main period of sea entry) (Fig. 2). Although FLs of river-mouth fish (mean ± SD, 54.3 ± 5.9 mm FL) and back-calculated FLs at sea entry for Konbumori fish (58.9 ± 4.4 mm FL) were generally similar, they showed a particular difference in early June (Fig. 2). The FLs of river-mouth fish sampled on 24 and 27 May combined (55.7 ± 4.5 mm FL), and 3 (57.2 ± 6.2 mm FL) and 6 June (50.1 ± 4.9 mm FL), were significantly smaller than those of Konbumori fish, which, for estimated sea-entry dates 24–27 May had an average FL of 59.7 ± 5.2 mm, and for 3–6 June, 59.5 ± 4.1 mm (Wilcoxon rank sum test, p < 0.01; Fig. 3). The values of river-mouth fish were also smaller than those of Konbumori fish with estimated sea-entry dates between 28 May and 2 June, 58.6 ± 4.3 mm FL (p < 0.01, Fig. 3). Additionally, many individuals with < 50 mm FL were found among river-mouth fish sampled on 24 and 27 May (12.0%), 3 June (11.6%) and 6 June (59.3%), while 0%, 1.6% and 1.5% of Konbumori fish were estimated to migrate to the sea at < 50 mm FL from 24 to 27 May, 28 May–2 June, and 3–6 June, respectively (Fig. 3).
Fork length distributions of juvenile chum salmon captured on 24 and 27 May 2013 combined, and 3 and 6 June, at the Kushiro River mouth (open bars) and corresponding distributions using back-calculated fork lengths at sea entry from 24 to 27 May, 3–6 June and the in-between period (28 May–2 June) for those captured at Konbumori (solid bars). Asterisk indicates significant difference as determined by Wilcoxon rank sum test at α = 0.01
The mean estimated growth rate ± SD (range) of Konbumori fish was 0.57 ± 0.10 (0.33–1.05) mm/day. As a result of model selection for GAMs, the full model was selected as the best model (Table 1). Both smoothing terms, sea-entry date and FL at sea entry, were statistically significant as predictor variables (Table 2). According to the predicted growth rate based on the selected model, growth rates outside the main period of sea entry (i.e., Julian date 144–155) tended to be lower (Fig. 4a). However, this trend was based on a small sample during the corresponding period and should be interpreted with caution. When focusing on FL at sea entry, growth rates of both larger and smaller fish tended to be higher (Fig. 4b). Particularly for juveniles with larger FL at sea entry, i.e., > 65 mm, only one fish (3.1%) exhibited a growth rate less than 0.45 mm/day, while the growth rate of half of these fish exceeded 0.65 mm/day (Fig. 5). Nonetheless, it should be noted that large individual variability was observed in growth rates relative to sea-entry conditions as indicated by the low deviance explained in the selected model (Table 1, Fig. 4).
Relationships between back-calculated post-sea-entry growth rates of juvenile chum salmon captured at Konbumori (n = 364) and their sea-entry dates in 2013 (a) and fork length at sea entry (b). Smoothing curves (solid lines) and 95% confidence intervals (gray shading) fitted by the developed generalized additive model (see Tables 1, 2) are shown
Composition of post-sea-entry growth rates of juvenile chum salmon captured at Konbumori, categorized into < 0.45 mm/day (white), 0.45‒0.65 mm/day (gray), and > 0.65 mm/day (black) for four different fork length groups at the time of sea entry. Growth rates of < 0.45 mm/day and > 0.65 mm/day were suggested as unfavorable and favorable, respectively, for this region (Honda et al. 2017)
The main period of sea entry was immediately after the point at which daily SST at Konbumori exceeded 5 °C (Fig. 6). Compared with SST anomalous data for the period between 1994 and 2012, the SST in 2013 was lower before 8 June (− 0.48 °C on average from 2 May–8 June) and increased after 9 June (+ 0.97 °C on average from 9 June–25 July).
Mean daily sea surface temperature (SST; 3 m below the surface) at Konbumori in 2013 (solid line) and its anomalous values relative to 1994–2012 (open bars). Shaded area indicates the main period during which most (5th–95th percentile) juvenile chum salmon collected at Konbumori were estimated to migrate to the sea. The dotted line indicates the lower limit of target coastal SST (5–13 °C) for the proper timing of Japanese juvenile chum salmon release (Seki 2013)
Discussion
Although many juvenile chum salmon with a FL < 50 mm were sampled at the Kushiro River mouth in early June, they were rare among the Konbumori fish for which back-calculated FL at sea entry was determined for a similar time period. As juvenile chum salmon gradually commence their offshore migration at 70–80 mm FL (Kaeriyama 1986; Irie 1990; Mayama and Ishida 2003), it may be that many smaller river-mouth fish had not yet reached Konbumori by the time the fish were sampled there (on 25 June 2013), and had remained in coastal waters near the Kushiro River mouth. However, FLs at sea entry for Konbumori fish were significantly larger than those of river-mouth fish, not only in early June, but also in late May. Another possibility is that the smaller river-mouth fish passed through closer to the shore, and thus were not captured at Konbumori. Yet all Konbumori fish were sampled at the station nearest to the shore (0.6 km). It seems unlikely that the smaller fish swam exclusively within such a narrow range (i.e., < 0.5 km from shore) (Mayama et al. 1982; Irie 1990; Seki 2005). Considering that substantial mortality of juvenile chum salmon was reported for fish between 45 and 55 mm FL (Healey 1982), our results suggest that many of the fish with < 50 mm FL at the time of sea entry died before reaching Konbumori (i.e., size-selective mortality). Should the freezing of fish decrease their length (Kasugai 2013), the FLs based on back-calculated measurements of freeze–thawed Konbumori fish might have been underestimated; however, our results were not contradictory.
Estimated dates for sea entry of most Konbumori fish ranged from 25 May to 5 June (5–95th percentiles). Environmental conditions during this period may have been suitable even for recruitment of the Kushiro 2012 brood-year stock for which adult return was poor (as described previously), simply because of favorable coastal SST (> 5 °C) (Seki 2013). The average biomass of zooplankton at Stn 1 off Konbumori on June 2013 was 0.46 g/m3 (wet weight), and was roughly average that of other years (0.16–0.90 g/m3 from 2004 to 2018) (Sato, unpublished data). Its taxonomic composition also did not differ much from that in other years, suggesting little year-specific variation in terms of prey availability. In early June 2013, SST increased rapidly, and anomalies in SST shifted from negative to positive from 9 June (Fig. 6), indicating a relatively short period of favorable water temperature conditions for coastal residency. In fact, an abrupt increase in SST from winter to summer has often been observed in recent years off Hokkaido, particularly when poor-return stocks of chum salmon migrated to the sea (Morita and Nakashima 2015; Saito and Fukuwaka 2018; Saito and Miyakoshi 2018).
Growth rates estimated in our study (0.57 ± 0.10 mm/day) were similar to those (0.54 ± 0.12 mm/day) of juvenile chum salmon originating from the Kushiro River and sampled at Konbumori from 2005 to 2014 (n = 122, 56.0–97.0 mm FL at capture) reported by Honda et al. (2017); data for 18 specimens were common to both studies. This suggests that the growth rate of our specimens (part of the 2012 brood-year stock) was not remarkably high or low. We reported that some individuals with smaller FL at sea entry exhibited a high growth rate (Fig. 4b). Assuming growth-dependent mortality (Sogard 1997), this suggests that even small fish at sea entry also have a chance of survival, particularly those that maintain a faster growth rate after sea entry. However, considering the possible decrease in survival probability of smaller fish at the river mouth (Fig. 3), and the fact that Konbumori fish assigned to the large FL category (specifically, > 65 mm) at sea entry tended to grow faster (Figs. 4b, 5), increased survival is also likely with the release of larger-sized fish under favorable coastal SST condition in this region. Faster growth rates exhibited by larger-sized fish at sea entry may have been due to their size-based higher swimming performance, which is considered to enhance prey availability through intra-cohort food competition (Ward et al. 2006; Urawa et al. 2018). Moreover, assuming that larger body size at the time of sea entry resulted from the faster growth rate of a fish by the time of sea entry, we suspect that this individual characteristic potentially contributes to faster growth even after sea entry. Saito et al. (2009) reported a positive correlation between back-calculated FL at sea entry and subsequent growth rates of juvenile chum salmon sampled along the southeastern coast of Shiretoko Peninsula, northeastern Hokkaido, except for 1 year during which the zooplankton biomass was relatively high. For poor-return stock of juvenile Chinook salmon Oncorhynchus tshawytscha from California’s Central Valley, estimated FLs at sea entry of fish collected in coastal waters were significantly greater than those of fish collected at an estuary exit point (Woodson et al. 2013). It has also been suggested that release of larger juvenile chum salmon fry/juveniles increases adult return rates (Yatsu and Kaeriyama 2005; Wertheimer and Thrower 2007). Takahashi (2010) compared return rates of adult chum salmon from stocks released at 49- and 53-mm average FL from the Tokushibetsu River, northern Hokkaido on 31 May 2001, under favorable coastal SST, and found the return rate of fish released at the larger size was twice that of fish released at the smaller size.
We identified the sea-entry conditions and subsequent growth rates of fish that survived for about 20–30 days at sea, and traveled about 20 km from the river mouth at which they entered the sea. However, our results were based on stock of a single brood-year originating from a single river, with all the specimens used in the otolith daily increment analysis captured on one date. Moreover, large individual variability in growth rate relative to sea-entry conditions was observed. Thus, to more fully understand the sea-entry conditions that promote survival and growth of juvenile chum salmon in this region, particularly as larger body size at release or sea entry does not always deliver higher Pacific salmon adult return rates (Saito and Nagasawa 2009; Tomaro et al. 2012), further research on environmental conditions during Pacific salmon coastal residency that yield higher return rates is required. To accomplish this, intensive sampling over a wider area along salmon migration routes is advised so that changes in the compositions of sea-entry condition and growth rate of surviving fish can be tracked at larger spatiotemporal scales.
References
Armstrong JD, Stewart DC (1997) The effects of initial length and body curvature on shrinkage of juvenile Atlantic salmon during freezing. J Fish Biol 50:903–905
Bartoń K (2018) MuMIn: multi-model inference. In: R package version 1.42.1. The Comprehensive R Archive Network (CRAN). R Foundation for Statistical Computing, Vienna. https://CRAN.R-project.org/package=MuMIn. Accessed 28 Jan 2020
Bax NJ (1983) Early marine mortality of marked juvenile chum salmon (Oncorhynchus keta) released into Hood Canal, Puget Sound, Washington, in 1980. Can J Fish Aquat Sci 40:426–435
Beacham TD, Araujo HA, Tucker S, Trudel M (2018) Validity of inferring size-selective mortality and a critical size limit in Pacific salmon from scale circulus spacing. PLoS One 13:e0199418
Beamish RJ (ed) (2018) Ocean ecology of Pacific salmon and trout. American Fisheries Society, Bethesda
Beamish RJ, Thomson BL, McFarlane GA (1992) Spiny dogfish predation on Chinook and coho salmon and the potential effects on hatchery-produced salmon. Trans Am Fish Soc 121:444–455
Chistyakova AI, Bugaev AV (2016) An assessment of the origin and migration routes of juvenile hatchery pink and chum salmon in the basin of the Okhotsk Sea in 2011–2014. Res Aquat Biol Resour Kamchatka Northwest Part Pac Ocean 40:5–23 (In Russian with English abstract)
Claiborne AM, Miller JA, Weitkamp LA, Teel DJ, Emmett RL (2014) Evidence for selective mortality in marine environments: the role of fish migration size, timing, and production type. Mar Ecol Prog Ser 515:187–202
Fukuwaka M, Suzuki T (2002) Early sea mortality of mark-recaptured juvenile chum salmon in open coastal waters. J Fish Biol 60:3–12
Healey MC (1982) Timing and relative intensity of size-selective mortality of juvenile chum salmon (Oncorhynchus keta) during early sea life. Can J Fish Aquat Sci 39:952–957
Honda K, Kawakami T, Suzuki K, Watanabe K, Saito T (2017) Growth rate characteristics of juvenile chum salmon Oncorhynchus keta originating from the Pacific coast of Japan and reaching Konbumori, eastern Hokkaido. Fish Sci 83:987–996
Honda K, Kawakami T, Saito T, Urawa S (2019) First report of growth rate of juvenile chum salmon Oncorhynchus keta captured in the Sea of Okhotsk offshore. Ichthyol Res 66:155–159
Irie T (1990) Ecological studies on the migration of juvenile chum salmon, Oncorhynchus keta, during early ocean life. Bull Seikai Natl Fish Res Inst 68:1–142 (in Japanese with English abstract)
Kaeriyama M (1986) Ecological study on early life of the chum salmon, Oncorhynchus keta (Walbaum). Sci Rep Hokkaido Salmon Hatch 40:31–92 (In Japanese with English abstract)
Kasugai K (2013) The effects of different methods of preservation and fixation in formalin and ethanol upon body size of juvenile chum salmon. Sci Rep Hokkaido Fish Hatch 84:11–19 (In Japanese with English abstract)
Kitada S (2014) Japanese chum salmon stock enhancement: current perspective and future challenges. Fish Sci 80:237–249
Kobayashi T (1977) Ecology of juvenile salmon in early marine life stage. Bull Jpn Soc Fish Oceanogr 31:39–44 (In Japanese)
Mayama H, Ishida Y (2003) Japanese studies on the early ocean life of juvenile salmon. N Pac Anadr Fish Comm Bull 3:14–67
Mayama H, Kato M, Seki J, Shimizu I (1982) Studies on the chum salmon released in the Ishikari River system. I. On the seaward migration and inshore distribution of liberated fry in 1979. Sci Rep Hokkaido Salmon Hatch 36:1–17 (In Japanese with English abstract)
Miyakoshi Y (2018) Current status of chum salmon stocks in Hokkaido. Aquabiology 40:330–334 (In Japanese with English abstract)
Miyakoshi Y, Urabe H, Saneyoshi H, Aoyama T, Sakamoto H, Ando D, Kasugai K, Mishima Y, Takada M, Nagata M (2012a) The occurrence and run timing of naturally spawning chum salmon in northern Japan. Environ Biol Fishes 94:197–206
Miyakoshi Y, Ando D, Fujiwara M, Hayano H, Nagata M (2012b) Downstream migration of chum salmon released in the Abashiri River. Sci Rep Hokkaido Fish Hatch 82:19–26 (In Japanese with English abstract)
Miyakoshi Y, Nagata M, Kitada S, Kaeriyama M (2013) Historical and current hatchery programs and management of chum salmon in Hokkaido, northern Japan. Rev Fish Sci 21:469–479
Morita K, Nakashima A (2015) Temperature seasonality during fry out-migration influences the survival of hatchery-reared chum salmon Oncorhynchus keta. J Fish Biol 87:1111–1117
Morita K, Saito T, Miyakoshi Y, Fukuwaka M, Nagasawa T, Kaeriyama M (2006) A review of Pacific salmon hatchery programmes on Hokkaido Island, Japan. ICES J Mar Sci 63:1353–1363
Morita K, Takahashi S, Ohkuma K, Nagasawa T (2013) Estimation of the proportion of wild chum salmon Oncorhynchus keta in Japanese hatchery rivers. Nippon Suisan Gakkaishi 79:206–213 (in Japanese with English abstract)
Morita K, Fukuzawa H, Suzuki K (2019) Comparison of fry-to-adult survival rates between wild and hatchery chum salmon in the Chitose River, Hokkaido, Japan. J Fish Tech 11:9–14 (In Japanese with English abstract)
Okado J, Koshino Y, Kudo H, Watanuki Y (2020) Consumption of juvenile chum salmon by a seabird species during early sea life. Fish Res 222:105415
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.r-project.org/. Accessed 28 Jan 2020
Saito T, Fukuwaka M (2018) Status of Pacific salmon production in the North Pacific Ocean. Aquabiology 40:319–329 (In Japanese with English abstract)
Saito T, Miyakoshi Y (2018) Current status of chum and pink salmon: what is reducing adult returns in Japan? N Pac Anadr Fish Comm Tech Rep 11:8–9
Saito T, Nagasawa K (2009) Regional synchrony in return rates of chum salmon (Oncorhynchus keta) in Japan in relation to coastal temperature and size at release. Fish Res 95:14–27
Saito T, Kaga T, Seki J, Otake T (2007) Otolith microstructure of chum salmon Oncorhynchus keta: formation of sea entry check and daily deposition of otolith increments in seawater conditions. Fish Sci 73:27–37
Saito T, Shimizu I, Seki J, Nagasawa K (2009) Relationship between zooplankton abundance and the early marine life history of juvenile chum salmon Oncorhynchus keta in eastern Hokkaido, Japan. Fish Sci 75:303–316
Salo EO (1991) Life history of chum salmon (Oncorhynchus keta). In: Groot C, Margolis L (eds) Pacific salmon life histories. UBC, Vancouver, pp 233–309
Seki J (2005) Study of characteristics of feeding habitats of juvenile chum salmon and their food environment in the Pacific coastal waters, central part of Hokkaido. Bull Natl Salmon Resour Center 7:1–104 (In Japanese with English abstract)
Seki J (2013) Development of hatchery techniques for releasing juvenile chum salmon in Japan. J Fish Technol 6:69–82 (In Japanese with English abstract)
Seki J, Shimizu I (1996) Effect time of larval release on return rate in chum salmon (Oncorhynchus keta) in the Hiroo River, Hokkaido, Japan. Bull Jpn Soc Fish Oceanogr 60:339–347 (In Japanese with English abstract)
Sogard SM (1997) Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull Mar Sci 60:1129–1157
Takahashi F (2010) Findings obtained from otolith-thermal marked chum salmon. Part 2: comparison of release timing and size. FRA Salmon Res Rep 4:12–14 (In Japanese)
Tomaro LM, Teel DJ, Peterson WT, Miller JA (2012) When is bigger better? Early marine residence of middle and upper Columbia River spring Chinook salmon. Mar Ecol Prog Ser 452:237–252
Torao M, Takeuchi K, Sasaki Y, Kasugai K, Murakami Y, Nagata M (2010) Seasonal timing of downstream migration and migrating speed of the hatchery and wild pink salmon, Oncorhynchus gorbuscha fry in the Tohoro River, eastern Hokkaido, Japan. Sci Rep Hokkaido Fish Hatch 64:7–15 (In Japanese with English abstract)
Tucker S, Hipfner JM, Trudel M (2016) Size- and condition-dependent predation: a seabird disproportionately targets substandard individual juvenile salmon. Ecology 97:461–471
Urawa S, Hagen P, Meerburg D, Rogatnykh A, Volk E (2001) Compiling and coordinating salmon otolith marks in the North Pacific. N Pac Anadr Fish Comm Tech Rep 3:13–15
Urawa S, Beacham TD, Fukuwaka M, Kaeriyama M (2018) Ocean ecology of chum salmon. In: Beamish RJ (ed) Ocean ecology of Pacific salmon and trout. American Fisheries Society, Bethesda, pp 161–317
Vega SL, Sutton TM, Murphy JM (2017) Marine-entry timing and growth rates of juvenile chum Salmon in Alaskan waters of the Chukchi and northern Bering seas. Deep Sea Res Part II 135:137–144
Ward AJW, Webster MM, Hart PJB (2006) Intraspecific food competition in fishes. Fish Fish 7:231–261
Wertheimer AC, Thrower EP (2007) Mortality rates of chum salmon during their initial marine residency. In: Grimes PE, et al. (eds) Ecology of juvenile salmon in the Northeast Pacific Ocean: regional comparisons. American Fisheries Society Symposium, vol. 57. American Fisheries Society, Bethesda, pp 233–247
Wood S (2019) mgcv: mixed GAM computation vehicle with automatic smoothness estimation. In R package version 1.8-28. The Comprehensive R Archive Network (CRAN). R Foundation for Statistical Computing, Vienna https://cran.r-project.org/web/packages/mgcv/mgcv.pdf. Accessed 28 Jan 2020
Woodson LE, Wells BK, Weber PK, Macfarlane RB, Whitman GE, Johnson RC (2013) Size, growth, and origin-dependent mortality of juvenile Chinook salmon Oncorhynchus tshawytscha during early ocean residence. Mar Ecol Prog Ser 487:163–175
Yatsu A, Kaeriyama M (2005) Linkages between coastal and open-ocean habitats and dynamics of Japanese stocks of chum salmon and Japanese sardine. Deep Sea Res Part II 52:727–737
Acknowledgments
We thank staff of the Japan Fisheries Research and Education Agency, and in particular S. Toda and F. Ito, for their cooperation in collecting field data over the successive years of the study. We also thank T. Kitagawa, T. Kawakami, and Y. Kusaba at the University of Tokyo for their support and help with the analysis using the electron probe micro-analyzer. This study was supported by the Fisheries Agency of Japan as part of the Salmon Resources along the Pacific Coast of Japan Restoration Project, and by the Cooperative Program of Atmosphere and Ocean Research Institute, The University of Tokyo (no. 145, 2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Honda, K., Shirai, K., Komatsu, S. et al. Sea-entry conditions of juvenile chum salmon Oncorhynchus keta that improve post-sea-entry survival: a case study of the 2012 brood-year stock released from the Kushiro River, eastern Hokkaido, Japan. Fish Sci 86, 783–792 (2020). https://doi.org/10.1007/s12562-020-01442-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-020-01442-0