Abstract
Probiotics can act as biological control agents against bacterial infection in aquatic animals, and also increase growth and stimulate immunity. In this study, two potential probiotics, Bacillus sp. KUAQ1 and Bacillus sp. KUAQ2, isolated from Nile tilapia Oreochromis niloticus Linn. intestine, were investigated for their biological functions. Species of isolated bacteria were identified by a conventional microbiological assay, biochemical assay and 16S ribosomal RNA polymerase chain reaction analysis. Both Bacillus isolates could survive at a pH ranging from 2 to 9 for 6 h and for 2 h in bile salts. The candidate probiotics were tested for their inhibition activity against the pathogenic bacteria Streptococcus agalactiae and Aeromonas hydrophila, and their specific protease activity. The probiotics had no significant effect (P > 0.05) on the average weight, average daily growth, specific growth rate or feed conversion ratio of tilapia fry after an 8-week feeding trial. Furthermore, supplementation with probiotics did not increase the survival rate of tilapia challenged with S. agalactiae. Several immune parameters including lysozyme, phagocytic activity and respiratory burst activity of juvenile fish treated with probiotics were significantly higher than those of the control (P < 0.05), but levels of the superoxide anion and alternative complement activity did not significantly differ (P > 0.05). Stress tolerance to brackish water at 25 p.p.t. NaCl did not improve significantly in fish treated with probiotics (P > 0.05). These two probiotic Bacillus offer benefits in terms of disease control and stimulation of the immune response of cultured tilapia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nile tilapia Oreochromis niloticus Linn. is the most common freshwater aquaculture fish species in Thailand with an annual domestic production of about 200,000 metric tons. Intensive culture to increase the productivity of these fish can cause stress and weaken their health, leading to disease outbreaks and ultimately economic loss. Many infectious agents have been identified in tilapia culture worldwide, including parasites, bacteria and viruses. The most common pathogenic bacteria in tilapia are Streptococcus agalactiae and Aeromonas hydrophila. Farmers treat bacterial infections with antibiotics, but these can be of high risk to consumers due to residual drug levels and the development of antibiotic resistant strains or strain mutation. To overcome these problems, and to protect fish from bacterial diseases, the safe application of beneficial bacteria that may induce an immune response against pathogenic bacteria has been reported for many fish and shrimp species in recent decades. The benefits of probiotics on growth, the intestinal microbial population and immunity of fish in aquaculture, and in particular in tilapia farming, have been extensively studied and reviewed in recent years (Najeeb et al. 2015; Truong-Giang et al. 2017; Goutam and Arun Kumar. 2017; Narayanan et al. 2018; Emmanuel et al. 2018).
Probiotics can stimulate innate immunity in fish and activate the expression of various cytokines (Nayak 2010). However, the mechanisms by which probiotics stimulate the immune system are not clearly understood. Amongst the many types of probiotics used in aquaculture, Bacillus spp. are amongst the most common, and are applied as single species or in combination with other probiotic species. Bacillus spp. possess many advantages such as high inhibition activity of pathogenic bacteria, immune enhancement, exoenzyme production and the ability to form spores.
In this study, we investigated the effects of dietary supplementation with Bacillus spp. KUAQ1 and KUAQ2, isolated from Nile tilapia intestine, which were characterized as potential probiotics by their inhibition of pathogenic bacteria, their protease activity, pH and bile tolerance. Experimental tilapia were tested for their growth performance, innate immunity, disease resistance and stress tolerance in response to probiotic application.
Materials and methods
Bacterial isolation and characterization
Bacillus spp. were isolated from the intestine of Nile tilapia from cage culture in Thailand using heat shock and cold shock activation. Briefly, approximately 1 cm of Nile tilapia intestine was excised and briefly washed with sterile saline solution. Samples were put into sterile glass test tubes, minced with sterile scissors, and ground with a sterile pestle until homogeneous. The homogenate was heated at 80 °C for 20 min and quickly chilled on ice for 1–2 min. Bacteria were cultured on trypticase soy agar (TSA) and kept at 30 °C overnight. Colonies were then subcultured on TSA to obtain single colonies and bacterial stocks in TSA containing 20% glycerol, then were kept at − 80 °C for further analysis.
For bacterial characterization and species’ identification, isolated bacteria were cultured in trypticase soy broth (TSB) and genomic DNA was extracted by a conventional DNA extraction method (Kannika et al. 2017). Purified DNA was subjected to polymerase chain reaction (PCR) analysis for amplification of the 16S ribosomal RNA (rRNA) gene using primers 20F (5′-GAGTTTGATCCTGGCTCAG-3′) and 1500R (5′-GTTACCTTGTTACGACTT-3′) (Kannika et al. 2017). The PCR master mix contained 1 × Dream Taq buffer, 0.25 mM dNTP, 0.5 µM forward primer and reverse primer, and 0.2 U Dream Taq (Thermo Scientific, USA). The reaction was performed at 94 °C for 3 min followed by 25 cycles of 94 °C for 1 min, 50 °C for 1 min and 72 °C for 2 min. The amplified PCR product was then purified and ligated to pGEMT Easy Vector (Promega, USA) before nucleotide sequencing (Macrogen, Korea). The nucleotide sequence was searched for bacterial identity with data from the National Center for Biotechnology Information (NCBI) database using the BLASTN program (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Eight isolates were selected according to probiotic characteristics and further identified by phylogenetic tree analysis.
Phylogenetic tree analysis
16S rRNA of eight selected Bacillus isolates was subjected to multiple sequence alignment with ClustalW and phylogenetic tree analysis using Mega X (Kumar et al. 2018). Maximum likelihood analysis was performed with 1000 bootstrap replication; 16S rRNA accession numbers of the other Bacillus spp. are listed in Table 1.
Bacterial inhibition assay
Bacterial inhibition activity of isolated Bacillus spp. was determined with the pathogenic bacteria S. agalactiae serotype III (Kannika et al. 2017) and A. hydrophila strain KUAQ1. Both bacteria were isolated from Nile tilapia and kept at − 80 °C in the Laboratory of Aquatic Animal Health Management, Department of Aquaculture, Faculty of Fisheries, Kasetsart University, Bangkok, Thailand. A cross streak assay was performed by streaking pathogenic bacteria (1 × 108 CFU/ml) on TSA as the first line. The second line was streaked with Bacillus spp. (the same amount as for the pathogenic bacteria) perpendicular with the first line (cross streak). Plates were then incubated at 30 °C for 72 h and the inhibition area observed.
Specific protease activity analysis
To assess total secreted protease, Bacillus spp. in TSB (1 × 108 CFU/ml) were centrifuged to collect the culture media. Approximately 10 µl of cell-free medium was spotted onto sterile filter paper and placed onto the surface of TSA containing 5% skim milk. The clear zone surrounding the filter paper was measured after incubation at 30 °C for 24 h.
To determine the specific protease activity, selected Bacillus spp. from the skim milk test in TSB culture were mixed with 50 mM Tris-Cl, pH 8.0 and 200 mM NaCl. Culture medium was collected by centrifugation at 15,000 g for 30–60 min and the total protein content determined by Bradford analysis using bovine serum albumin standard. Then, 1 mg/ml of total protein was reacted with 5% azocasein (in 1 M NaOH) and incubated at 30 °C for 15 min. After incubation, 10% TCA was added to the mixture to stop the reaction and the mixture centrifuged at 8000 g for 15 min. The supernatant was mixed with 1 M NaOH (1:1 ratio) and the protease activity determined at 450 nm. The specific protease activity was calculated according to Eq. (1):
Antibiotic sensitivity test
Antibiotic sensitivity tests of the selected probiotics were conducted with 14 antibiotics: novobiocin (5 µg), ampicillin (10 µg), erythromycin (15 µg), ciprofloxacin (5 µg), amoxicillin (25 µg), polymyxin B (300 units), neomycin (30 µg), trimethoprim (5 µg), florfenicol (30 µg), spectinomycin (25 µg), sulfadimethoxazone (25 µg), tetracycline (30 µg), oxytetracycline (30 µg) and enrofloxacin (5 µg) (Oxoid, UK) by using a disk-diffusion method with Mueller Hinton agar with bacteria at 106 colony-forming units (CFU)/ml. The cultures were incubated overnight at 30 °C and the results were interpreted in accordance with the recommendations of the Clinical and Laboratory Standards Institute (Formerly National Committee for Clinical Laboratory Standards 2004).
pH and bile salts tolerance tests
Bacillus spp. were grown in TSB and the cell number adjusted to obtain 108 CFU/ml. For the pH tolerance test, 0.1 ml of culture was adjusted to pH 2, 3, 4, 5, 6, 7, 8 and 9 by HCl or NaOH. For the bile salts tolerance test, 0.1 ml of bacterial culture was mixed with bile salts to obtain final concentrations of 0.5, 1, and 2%. Viability of bacteria was determined by streaking on TSA followed by culture for 1, 2, 4, and 6 h at 30 °C.
Experimental fish
Fry and juvenile Nile tilapia were used for different purposes. For growth performance, including average weight, average daily growth (ADG), specific growth rate (SGR) and feed conversion ratio (FCR) analysis, pathogenic bacterial challenge and stress test, Nile tilapia fry (initial average weight 2 g) from a commercial Good Agricultural Practices farm were used. For immune response, Nile tilapia juveniles (initial average weight 50 g) were used. Fish were acclimatized in 1-ton cement tanks with continuous aeration, and fed three times a day for 2 weeks before the experiment.
Diet preparation and feeding program
Selected Bacillus spp. were separately cultured in 50-ml tubes with 10 ml TSB, at 30 °C for 18–20 h. Then, the bacterial suspension was swabbed onto the sporulation medium (Difco sporulation medium) and incubated at 30 °C for 120 h. Bacillus spores on the surface of the agar were then transferred to 0.85% NaCl in 50-ml tubes and centrifuged twice at 13,000 g for 1 min. The bacterial suspension was heated to 85 °C for 15 min to induce complete spore formation. The concentration of spores was measured at an optical density of 600 nm (OD600, where an OD600 of 1.1 is equivalent to 1012 CFU/ml. The Bacillus spore suspension was sprayed onto commercial feed to obtain 1 × 108, 3 × 108 and 5 × 108 CFU/g diets.
Four experimental diets including the control group (0.85% NaCl) and three probiotic supplement groups of 1 × 108 CFU/g diet, 3 × 108 CFU/g diet, and 5 × 108 CFU/g diet were fed to fish. Fifty tilapia fry were placed in 100-l glass tanks and fed twice daily with the normal diet and once daily with the probiotic supplement diet. The weights of 30% of fish per tank were determined every 2 weeks for 8 weeks to determine the growth parameters including ADG, SGR, total initial and final weights, and FCR. After 4 weeks of feeding, ten fish of each replicate were moved to a 30-l glass tank for the bacterial challenge test and stress test analysis. After 8 weeks of feeding, the total bacterial count in fish intestines was conducted using two fish from each replicate. All experiments were done in triplicate.
Immune parameter analysis
Twenty juvenile tilapia (average weight 50 g) were placed in a cement tank holding about 1 t of water and represented a replicate of each experimental group. The experimental feed and feeding scheme was the same as in the previous trial. After 4 weeks of feeding, fish serum was collected from the caudal vein of ten fish of each experimental diet and analyzed for lysozyme activity, superoxide dismutase (SOD) and hemagglutinin titers (alternative complement activity). White blood cells (WBC) were collected from the head kidney [adapted from Christybapita et al. (2007) and Ortuño et al. (2003)]; 5 × 106 cells/ml were used to measure phagocytosis activity and 6 × 105 cells/ml to measure respiratory burst activity.
Lysozyme activity assay
The lysozyme activity assay was modified from Parry et al. (1965). Briefly, 250 μl of Micrococcus lysodeikticus suspension [0.2 mg/ml in sodium phosphate buffer (pH 6.2)] was added to 10 μl of fish serum in a 96-well plate. The reaction was determined by measuring absorbance at 540 nm (A540) for 0.5–6.5 min at room temperature. The activity of lysozyme (1 unit) in fish serum was calculated as the reduction in A540 of 0.001/min.
Phagocytic activity assay
The phagocytic activity assay was performed by modifying the protocol of Ai et al. (2006). Briefly, 5 × 106 cells/ml WBCs were dropped onto a cover slip and allowed to adhere for 1.5 h. The cells were washed with sterile PBS thrice before adding 2 × 106 latex beads and were then incubated at room temperature for 2 h. The cover slip was then washed thrice with PBS to remove excess beads and the WBC stained with Diff-Quick stain. WBC with engulfed beads were counted for 300 WBCs on each cover slip and the percent phagocytic rate calculated according to Eq. (2).
Respiratory burst activity (superoxide anion assay)
Superoxide anion (O2−) was used to determine respiratory burst activity through nitroblue tetrazolium (NBT) reduction reaction. Approximately 6 × 105 cells of WBC were added to a microtiter plate, mixed with NBT, and incubated at room temperature for 2 h. The supernatant was then decanted and the WBC fixed with 100% methanol for 5 min followed by washing with 75% methanol twice. Potassium hydroxide (2 M) and dimethyl sulfoxide were added to dried WBC on microtiter plates, mixed well and the reaction of the superoxide anion measured at A655.
SOD activity assay
SOD activity was assessed by using the SOD Assay Kit (Sigma) according to the manufacturer’s instructions. The reaction was measured at A450 after 20 min incubation at 37 °C. SOD activity was calculated as the percent inhibition rate.
Alternative complement activity
Alternative complement activity [ACH50; serum dilution at which 50% hemolysis was induced (units/milliliters)] was determined using a modified method of Ortuno et al. (1998). Briefly, a twofold dilution of fish serum was prepared in a microtiter plate. Sheep red blood cells (2 × 106 cells) were added to the diluted serum to obtain final serum concentrations of 50%, 25%, 12.5%, 6.25%, 3.12%, 1.56%, 0.78% and 0.39%. The mixtures were incubated at room temperature for 1 h before centrifugation at 500 g for 5 min. Clear lysate was transferred to a new microtiter plate and measured at A540. For complete (100%) hemolysis, sterile distilled water was used, whereas PBS was used for 0% hemolysis.
Challenge test with S. agalactiae
Ten fish were randomly collected from each replicate and intraperitoneally injected with 100 µl of S. agalactiae (108 CFU/ml). S. agalactiae (serotype III) were freshly prepared by inoculating a single colony of the bacterial strain into BHI broth and culturing at 32 °C for 18 h. S. agalactiae were collected by centrifugation at 3500 r.p.m. at 25 °C for 10 min, followed by washing and resuspension in 0.85% NaCl. The S. agalactiae suspension was adjusted to 108 CFU/ml with 0.85% NaCl before injection. Mortality was recorded for 2 weeks and the results presented as survival rates.
Salinity stress test
Ten fish per replicate were randomly collected from each experimental group and submerged in brackish water with salinity of 25 p.p.t. (NaCl). Mortality was recorded during 24 h.
Total intestinal bacterial counts
At the end of the 8-week feeding trial, tilapia intestines were collected from two fish per replicate, weighed and washed with 0.85% NaCl then homogenized. The samples were treated with heat shock and cold shock as previously described and the total plate count determined. Total bacteria were counted after incubation at 30 °C for 18–24 h, expressed as CFU/grams intestine.
Statistical assay
All experimental data were analyzed by one-way ANOVA and Duncan’s multiple range test using SPSS software (IBM SPSS Statistics 20) with a significance level of P < 0.05.
Results
Bacillus spp. isolation and characterization
The initial step of Bacillus spp. isolation from tilapia intestine was achieved by using a heat shock and cold shock method. A total of 55 bacterial isolates were collected and identified as Gram-positive bacteria by using a conventional Gram-staining method. However, only 52 isolates were identified as Bacillus spp. from 16S rRNA screening and DNA sequencing.
Bacterial inhibition assay
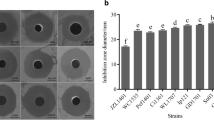
The functional analysis of the Bacillus spp. isolates determined their ability to inhibit and/or colonize pathogenic bacteria. Of 52 isolates, only 22 showed inhibition or colonization of S. agalactiae or A. hydrophila (Fig. 2a). Interestingly, most of the isolates showed colonization of S. agalactiae but not A. hydrophila. Eight isolates were further selected from the 22 isolates based on their degree of colonization and inhibition of the two pathogenic bacteria. Seven of these isolates colonized S. agalactiae; one isolate inhibited S. agalactiae and two isolates inhibited A. hydrophila (Table 2). Two isolates (PL3/2-1 and UD1/3-3) were selected for the feeding trial. The eight isolates were identified by BlastN, which demonstrated that all of them showed more than 90% identity to Bacillus spp. (Table 2). To verify the authenticity of the bacterial species, a phylogenetic tree analysis of all eight strains was conducted by comparing their rRNA with that of 12 other known Bacillus species. Interestingly, several isolated Bacillus in this study could not be exactly identified to species level even though they had high identity with known Bacillus in the database. The phylogenetic tree separated the Bacillus into three groups: group 1—Bacillus vallismortis, Bacillus altitudinis, Bacillus aerius, Bacillus pumilus, Bacillus xiamenensis, Bacillus stratosphericus and Bacillus amyloliquefaciens; group 2—Bacillus licheniformis and Bacillus subtilis; group 3—Bacillus gaemokensis, Bacillus cereus and Bacillus thuringiensis (Fig. 1). Among the isolated Bacillus, only CB1.4-3 was in the same clade as Bacillus amyloliquefaciens, whereas CC1.2-1 was in a sister clade with CB1.4-3 and B. amyloliquefaciens. Therefore, we assume that CB1.4-3 is B. amyloliquefaciens. UD1/3-3 and PL3/2-1 were found to share the same sister clade as B. licheniformis and B. subtilis but differed in divergence (Fig. 1). The two probiotics used for the feeding trial in Nile tilapia were Bacillus KUAQ1 (PL3/2-1) and KUAQ2 (UD1/3-3).
Specific protease activity analysis
Secreted exoenzymes are some of the beneficial products of probiotics that can increase nutrient utilization in the host digestive system. In this study, secreted protease was screened by using solid agar containing skim milk. Only 14 of the 52 isolates showed secreted protease activity (Fig. 2b). Only five Bacillus spp. with an expressed high protease activity were chosen to determine specific protease activity. Bacillus sp. KUAQ1 (isolate PL3/2-1) showed the highest specific protease activity (106.112 U/mg protein) for the degradation of azocasein; the remaining isolates showed similar activity ranging from 33.536 to 38.272 U/mg protein (Table 2).
Antibiotic sensitivity test
Bacillus sp. KUAQ1 (isolate PL3/2-1) and Bacillus sp. KUAQ2 (isolate UD1/3-3) were selected for the antibiotic sensitivity test. Both isolates were susceptible to most of the tested antibiotics including ampicillin (10 µg), erythromycin (15 µg), ciprofloxacin (5 µg), amoxycillin (25 µg), polymyxin B (300 units), trimethoprim (5 µg), florfenicol (30 µg), sulfadimethoxazone (25 µg) and enrofloxacin (ENR 5 µg). There were only three antibiotics that both isolates showed intermediate resistance to: novobiocin (5 µg), neomycin (30 µg) and tetracycline (30 µg). Interestingly, Bacillus sp. KUAQ1 showed intermediate resistance to oxytetracycline (30 µg), whereas Bacillus sp. KUAQ2 showed susceptibility to this antibiotic.
pH and bile salts tolerance test
To determine the viability of Bacillus sp. KUAQ1 and Bacillus sp. KUAQ2 under the conditions of the gastrointestinal tract, the viability of bacterial spores was tested in a range of pH and percentages of bile salts. Both Bacillus sp. KUAQ1 and Bacillus sp. KUAQ2 spores could resist a wide range of pH (2–9) for 6 h (Fig. 3); however, Bacillus sp. KUAQ1 appeared to have a much higher tolerance to a higher pH than Bacillus sp. KUAQ2. Both Bacillus spp. could grow for a period of 2 h in bile salts (0.5, 1 and 2%); the viability of bacteria decreased after 4 h (Fig. 4).
Growth performance
To determine the effect of Bacillus sp. KUAQ1 and Bacillus sp. KUAQ2 on growth performance, different concentrations of bacteria were added to commercial feed and fed once a day to fish for 2 months. At the end of the feeding trial, none of the growth performance parameters significantly differed (P > 0.05) between the probiotic supplement diet groups and the control group (Fig. 5). The FCR of the probiotic supplement groups improved when compared with the control but the differences were not significant (P > 0.05). The FCR of the control group and the 1 × 108, 3 × 108 and 5 × 108 CFU/g supplement diet groups were 1.31 ± 0.04, 1.27 ± 0.07, 1.26 ± 0.09 and 1.24 ± 0.01, respectively (Fig. 5).
Immune parameter analysis
After 4 weeks of feeding, the lysozyme activities of the control group (2433.3 ± 928.8) and the probiotic supplement diet groups were not significantly different (P > 0.05), although there was a significant difference between the 1 × 108 and 5 × 108 CFU/g diet groups. The lysozyme activities of the probiotic supplement diet groups were 2908.3 ± 1124.91 (1 × 108 CFU/g), 2416.6 ± 787.19 (3 × 108 CFU/g) and 1500.0 ± 868.33 unit ml−1 (5 × 108 CFU/g), respectively (Fig. 6).
Phagocytic activity of tilapia significantly differed between treatments (P < 0.05). The control group had a phagocytic activity of 16.50 ± 8.00%, which did not significantly differ from those of the probiotic supplement diet groups of 1 × 108 CFU/g (17.78 ± 9.36%) and 3 × 108 CFU/g (26.72 ± 10.9%) (P > 0.05), but the phagocytic activity of tilapia fed with 5 × 108 CFU/g Bacillus spp. (46.17 ± 15.73%) was significantly higher than that of the other groups (Fig. 7).
The respiratory burst activities (superoxide anion) of the control and probiotic supplement diet groups were 0.017 ± 0.009, 0.044 ± 0.017, 0.017 ± 0.007 and 0.033 ± 0.010 U ml−1, respectively and significantly differed (P < 0.05) (Fig. 8). However, neither the SOD nor the ACH50 significantly differed among tested groups (P > 0.05) (Figs. 9, 10). The SOD of the control and probiotic supplement diet groups were 0.30 ± 0.26, 0.53 ± 0.29, 1.12 ± 0.78 and 0.37 ± 0.33 U ml−1, respectively. ACH50 was 248.67 ± 67.36, 228.53 ± 64.80, 212.40 ± 23.43 and 243.32 ± 20.00 U ml−1, respectively.
Challenge test with S. agalactiae
The disease resistance of tilapia against experimental challenge with S. agalactiae (serotype III) was evaluated after fish were fed the probiotic supplement diets for 4 weeks. During the 14-day postchallenge period, mortality was observed on day 2 and steadily increased during the first 7 days (Fig. 11). Percentage survival rate of the control, 1 × 108, 3 × 108 and 5 × 108 CFU/g of probiotic supplement diet were 46.67 ± 15.28%, 43.33 ± 23.09%, 23.33 ± 23.09% and 23.33 ± 15.28%, respectively, after 14 days. However, the average survival rate did not significantly differ between the groups (P > 0.05) (Fig. 12).
Salinity stress test
Tilapia fed with different concentrations of Bacillus spp. spores were tested for their tolerance to salinity. During the 24-h stress period, mortality due to salinity stress was observed from 6 h onwards. The highest mortality rate was recorded at 24-h post stress induction. The mortality rates did not significantly differ between any of the tested groups (P > 0.05) (Fig. 13).
Total intestinal bacterial count
After the 8-week feeding trial, Bacillus spp. in tilapia fry intestines were isolated by heat shock and cold shock and a total count performed. The total Bacillus counts of all probiotic supplement diet groups were significantly higher than that of the control group (P < 0.05) (Fig. 14).
Discussion
Probiotics are currently used as an alternative biological method for the control of disease and also for the promotion of growth in farmed fish and shrimp worldwide. Several bacterial probiotics have been identified that have a positive effect on fish health, e.g., species of Bacillus, Lactobacillus, Bifidobacterium, Microbacterium, and Staphylococcus (Nayak 2010; Balcazar et al. 2006; Lakshimi et al. 2013; Tuan et al. 2013; Lazado et al. 2015). In this study, we identified Bacillus spp. from the intestine of Nile tilapia and determined their biological potential for use as probiotics. Our isolation method allowed Bacillus spp. to sporulate by simple heat shock and cold shock activation (Pakingking et al. 2015; Molinari et al. 2003). This method should eliminate most contaminating bacteria in fish intestine, and only spores of Bacillus spp. could grow on the culture medium used. The identification of the isolated bacteria as Gram positive was confirmed by conventional Gram staining; the bacteria had a long rod shape and their spore morphology could be observed under a microscope.
Molecular identification in our study indicated that the Bacillus isolates used for our feeding trial (Bacillus sp. KUAQ1 and KUAQ2) are closely related to B. subtilis. Bacillus spp. isolated from the intestines of a few species of freshwater fish for use as probiotics have been recently reported, e.g., from striped catfish (Ho et al. 2017), hybrid catfish (Khotchanalekha et al. 2018), Indian major carp (Kavitha et al. 2018), tilapia and African catfish (Kato et al. 2016) and Nile tilapia (Srisapoome and Areechon 2017). Although 52 different isolates of Bacillus spp. were isolated and identified from tilapia intestine in our preliminary study, only some isolates exhibited probiotic properties. We tested these isolates for several biological activities and related properties to confirm their potential as probiotics.
Secreted protease is an extracellular enzyme produced from probiotics beneficial to a host’s digestive system. In this study, only some of the isolated Bacillus spp. showed secreted protease activity, of which Bacillus isolate PL3/2-1 showed the highest activity (106.112 U/mg protein). Secreted protease or extracellular enzymes are considered common characteristics of Bacillus spp., e.g., several enzymes are secreted by B. amyloliquefaciens such as protease (Cho et al. 2003), gelatinolytic enzymes (Sai-Ut et al. 2013) and xylanase (Saputra et al. 2016). Moreover, protease secreted from Bacillus clausii provided an alternative means of controlling the pathogenesis of other bacteria. Serine protease from B. clausii O/C could neutralize the cytotoxic effects induced by the purified toxins of Clostridium difficile and B. cereus (Ripert et al. 2016), and B. amyloliquefaciens applied as a probiotic in Nile tilapia improved growth and enhanced fish immunity against A. hydrophila (Saputra et al. 2016). Interestingly, Bacillus sp. KUAQ2 (UD1/3-3) isolated in this study also exhibited inhibition activity against A. hydrophila. Therefore, the two isolates, Bacillus spp. as KUAQ1 (PL3/2-1) and KUAQ2 (UD1/3-3), were selected to determine their probiotic properties in a Nile tilapia feeding trial. Moreover, it is interesting that several novel Bacillus spp. were also isolated in this study; their biological functions will be determined in later studies.
The methods used for probiotic application in aquaculture may affect the response of the treated organisms. The oral administration of probiotics is the most suitable method for application to tilapia to enhance and directly activate their immunity and growth. Probiotic supplements in fish feed have been reported to enhance various parameters, especially growth (Reda and Selim 2015; Yan et al. 2015; Lukkana et al. 2015; Chaudhary and Qazi 2014; Krishnan 2014; Ahmed et al. 2014). The results of our study indicated that feeding probiotics to 2-g tilapia for 8 weeks did not affect their growth (i.e., in terms of average weight, ADG and SGR), or FCR, when compared with the control. This agrees with studies on the probiotic Pediococcus acidilactici in Nile tilapia (Standen et al. 2013), where no significant difference in growth or feed utilization was found. Similarly, B. subtilis and L. acidophilus used as probiotics did not promote the growth of adult tilapia (Ridha and Azad 2015), or that of other species, such as red tilapia (Wing-Keong et al. 2015) common carp (Huang et al. 2015), or marine fish (Hauville et al. 2016). This might have been due to the fact that laboratory conditions during these trials, such as temperature, dissolved oxygen etc. and nutrients from feed, were sufficient for the optimal growth of the fish (Pirarat et al. 2006).
However, supplementing feed with probiotics might enhance fish immunity and control disease caused by pathogenic bacteria. The pathogenic bacteria challenge in this study demonstrated that 1 × 108, 3 × 108 and 5 × 108 CFU/g probiotic supplement diets did not prevent S. agalactiae infection. In contrast, B. pumilus used as a probiotic in tilapia induced resistance against various bacterial diseases such as those caused by S. agalactiae (Srisapoome and Areechon 2017) and Edwardsiella ictaluri in striped catfish (Pangasianodon hypophthalmus) (Ho et al. 2017). It should also be noted that a combination of probiotics, e.g., B. licheniformis with B. subtilis, enhanced several parameters of growth and disease resistance in fish (Abarike et al. 2018). Therefore, future trials using a combination of two of more Bacillus species should be conducted to evaluate synergistic effects on the growth and disease resistance of Nile tilapia.
The effects of probiotics in stimulating host innate immunity was assessed to explain possible immune responses and to optimize the dose of probiotics in aquaculture feed. Lysozyme produced by WBC (leucocytes), especially neutrophils and macrophages, can hydrolyze the peptidoglycan layer in the cell walls of bacteria when fish have been infected by them (Schmekel et al. 2013). In this study, the group fed with the low dose of probiotics (1 × 108 CFU/g feed) showed the highest lysozyme level. The group receiving the highest dose of probiotics was likely to produce less lysozyme, which would explain why there was no significant difference in the lysozyme level between this group and the control. The role of lysozyme is well recognized in the non-specific immunity of animals and might be activated by foreign substances. However, in the common carp (Cyprinus carpio) (Kazuń et al. 2018), brown trout (Salmo trutta) (Balcázar et al. 2007) and rainbow trout (Oncorhynchus mykiss) (Panigrahi et al. 2005) probiotics did not influence the activation of lysozyme.
The hemagglutinin titer is used to analyze the alternative complement activity based on the ability to break down sheep red blood cells. The alternative complement is an element of non-specific immunity, which is mostly induced at the cell wall of microorganisms. The alternative complement did not significantly differ between the treatments, which contrasts with most other reports (Pirat et al. 2006; Liu et al. 2012; Giri et al. 2012; Wang et al. 2008; Safari and Atash 2013), although Ridha and Azad (2015) found a similar result to ours. Thus, adding probiotics to feed in this study had no impact on the alternative complement pathway.
The superoxide anion level an indicator of respiratory burst, a reaction that helps destroy microorganisms after phagocytosis through the creation of O2− which is converted to H2O2 by SOD (Gottfredsen et al. 2013). In our study, the superoxide anion volume of fish fed with probiotics was significantly higher than that of the control group, with the exception of the group fed a dose of 3 × 108 CFU/g feed. The SOD level did not significantly differ between any experimental group, i.e., when probiotics were applied at 3 × 108 CFU/g feed, SOD was not significantly higher than in the other treatments. It is possible that when SOD is secreted at a high level the superoxide anion is converted to H2O2, which would have resulted in a lower superoxide anion level than in the other treatment groups at the same stage.
After fish had been fed with probiotics for 4 weeks their phagocytic activity was higher than that of the control group, especially in the group fed 5 × 108 CFU/g feed (P < 0.05). This process is a result of the increased action of phagocytic cells, including neutrophils, monocytes and macrophages, that engulf foreign cells. Peptidoglycan in the cell wall of Bacillus can stimulate specific proteins called pattern recognition molecules, which can subsequently stimulate the immune response of the host animal (Lee et al. 2012; Richard et al. 2014). Moreover, Alexandre et al. (2014) also reported that some probiotics can also stimulate the secretion of cytokines from macrophages.
In conclusion, among the Bacillus isolates from Nile tilapia intestine examined in this study, Bacillus sp. KUAQ1 and Bacillus sp. KUAQ2 showed the highest probiotic properties. They exhibited a positive effect in vitro including antimicrobial activity against S. agalactiae, and tolerance to a wide range of pH and to bile salts. However, neither of these probiotics showed a positive effect on the growth of Nile tilapia. Innate immunity tended to increase when the diet of Nile tilapia was supplemented with the probiotics. However, probiotic supplementation may not be economically viable according to the preliminary results of our study. Therefore, more studies need to carried out on the mechanisms of immune activation and fish excretion in response to probiotic supplementation in this species.
References
Abarike ED, Cai J, Lu Y, Yu H, Chen L, Jian J, Tang J, Jun L, Kuebutornye FKA (2018) Effects of a commercial probiotic BS containing Bacillus subtilis and Bacillus licheniformis on growth, immune response and disease resistance in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 82:229–238
Ahmed T, Hasan SJ, Hassain Md RA, Haidar I, Rubel AKMSA, Pramanik MH (2014) Assessment on impact of dietary probiotic supplementation on growth indices of mono-sex tilapia (Oreochromis niloticus) cage culture at Dakatia River, Chandpur, Bangladesh. World J Fish Mar Sci 6(5):441–446
Ai Q, Mai K, Xu W, Tan B, Zhang W (2006) Effects of dietary vitamin C on survival, growth, and immunity of large yellow croaker, Pseudosciaena crocea. Aquaculture 261:327–336
Alexandre Y, Blay GL, Gastrin SB, Gall FL, Arnaud GH, Gouriou S, Vallet S, Berre RL (2014) Probiotics: a new way to fight bacterial pulmonary infections? Med Mal Infect 44:9–17
Balcázar JL, de Blas I, Rui-Zarzuela I, Cunningham D, Vendrell D, Muzquiz JL (2006) The role of probiotics in aquaculture. Vet Microbiol 114(3–4):173–186
Balcázar JL, de Blas I, Ruiz-Zarzuela I, Vendell D, Calvo AC, Marquez I, Gironés O, Muzquiz JL (2007) Changes in intestinal microbiota and humoral immune response following probiotic administration in brown trout Salmo trutta. Br J Nutr 97:522–527
Chaudhary A, Qazi JI (2014) Probiotic antagonism of Sphingomonas sp. against Vibrio anguillarum exposed Labeo rohita fingerlings. Adv Life Sci 4(3):156–165
Cho S-J, Oh S-H, David PR, Marcel AJ, Lee C-H (2003) Purification and characterization of proteases from Bacillus amyloliquefaciens isolated from traditional soybean fermentation starter. J Agric Food Chem 51(26):7664–7670
Christybapita D, Divyagnaneswari M, Michael RD (2007) Oral administration of Eclipta alba leaf aqueous extract enhances the non-specific immune responses and disease resistance of Oreochromis mossambicus. Fish Shellfish Immun 23:840–852
Emmanuel DA, Jia C, Yishan L, Huang Y, Lihua C, Jichang J, Jufen T, Liang J, Felix KAK (2018) Effects of a commercial probiotic BS containing Bacillus subtilis and Bacillus licheniformis on growth, immune response and disease resistance in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 82:229–238
Giri SS, Sen SS, Sukumaran V (2012) Effects of dietary supplementation of potential probiotic Pseudomonas aeruginosa VSG-2 on the innate immunity and disease resistance of tropical freshwater fish Labeo rohita. Fish Shellfish Immunol 32(6):1135–1140
Gottfredsen RH, Larsen UG, Enghild JJ, Petersen SV (2013) Hydrogen peroxide induces modifications of human extracellular superoxide dismutase that results in enzyme inhibition. Redox Biol 1:24–31
Goutam B, Arun Kumar R (2017) The advancement of probiotics research and its application in fish farming industries. Res Vet Sci 115:66–77
Hauville MR, Zambonino-Infante JL, Gordon Bell J, Migaud H, Main KL (2016) Effects of a mix of Bacillus spp. as a potential probiotic for Florida pompano, common snook and red drum larvae performances and digestive enzyme activity. Aquacult Nutr 22(1):51–60
Ho TTT, Nguyen NT, Ong MQ, Kannika K, Unajak S, Areechon N (2017) Effects of the dietary supplementation of mixed probiotic spores of Bacillus amyloliquefaciens 54A, and Bacillus pumilus 47B on growth, innate immunity and stress responses of striped catfish (Pangasianodon hypophthalmus). Fish Shellfish Immunol 60:391–399
Huang L, Ran C, He S, Ren P, Hu J, Zhao X, Zhou Z (2015) Effects of dietary Saccharomyces cerevisiae culture or live cells with Bacillus amyloliquefaciens spores on growth performance, gut mucosal morphology, hsp70 gene expression, and disease resistance of juvenile common carp (Cyprinus carpio). Aquaculture 438:33–38
Kannika K, Pisuttharachai D, Srisapoome P, Wongtavatchai J, Kondo H, Hirono I, Unajak S, Areechon N (2017) Molecular serotyping, virulence gene profiling and pathogenicity of Streptococcus agalactiae isolated from tilapia farms in Thailand by multiplex PCR. J Appl Microbiol 122:1497–1507
Kato DC, Mugaanyi MB, Majalija S, Tamale A, Lubowa NM, Sengooba A (2016) Isolation and identification of potential probiotics bacteria from the gut of Oreochromis niloticus and Clarias gariepinus in Uganda. Br Microbiol Res J 17(5):1–8
Kavitha M, Raja M, Perumal P (2018) Evaluation of probiotic potential of Bacillus spp. isolated from the digestive tract of freshwater fish Labeo calbasu (Hamilton, 1822). Aquacult Rep 11:59–69
Kazuń B, Małaczewska J, Kazuń K, Żylińska-Urban J, Siwicki AK (2018) Immune-enhancing activity of potential probiotic strains of Lactobacillus plantarum in the common carp (Cyprinus carpio) fingerling. J Vet Res 62(4):485–492
Khotchanalekha K, Doolgindachbaporn S, Nagasawa T, Nakao M, Sakai K, Tongpim S (2018) Evaluation of probiotic Bacillus aerius B81e isolated from healthy hybrid catfish on growth, disease resistance and innate immunity of Pla-mong Pangasius bocourti. Fish Shellfish Immunol 73:1–10
Krishnan R (2014) Probiotic potential of Bacillus species isolated from freshwater fish Anabas testudineus in Labeo rohita. Int J Multidiscip Res Dev 1(1):46–50
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Lakshmi PJ, Chitturi Kumari ChM, Lakshmi VV (2013) Efficient degradation of feather by keratinase producing Bacillus sp. Int J Microbiol. https://doi.org/10.1155/2013/608321
Lazado CC, Caipang CM, Estante EG (2015) Prospects of host-associated microorganisms in fish and penaeids as probiotics with immunomodulatory functions. Fish Shellfish Immunol 45(1):2–12
Lee J, Geddeds K, Streutker C, Philpott DJ, Girardin SE (2012) Role of mouse peptidoglycan recognition protein PGLYRP2 in the innate immune response to Salmonella enteric serovar Typhimurium infection in vivo. Infect Immun 80(8):2645–2654
Liu C-H, Chiu C-H, Wang S-W, Cheng W (2012) Dietary administration of the probiotic, Bacillus subtilis E20, enhances the growth, innate immune responses, and disease resistance of the grouper, Epinephelus coioides. Fish Shellfish Immunol 33:699–706
Lukkana M, Jantrakajorn S, Wongtavatchai J (2015) In vivo suppression against streptococcal bacteria and health promoting effects of probiotic Bacillus polyfermenticus in tilapia (Oreochromis niloticus). Thai J Vet Med 45(1):121–129
Molinari LM, de Scoaris O, Pedroso RB, Bittencourtde LR, Nakamura CV, Ueda-Nakamura T, de Filho BA, Filho BPD (2003) Bacterial microflora in the gastrointestinal tract of Nile tilapia, Oreochromis niloticus, cultured in a semi-intensive system. Maringá 25(2):267–271
Najeeb A, Bin W, Aamir MM, Muhammad M (2015) Probiotics and prebiotics associated with aquaculture: a review. Fish Shellfish Immunol 45:733–741
Narayanan G, Baskaralingam V, Jiann-Chu C, Ravichandran R, Sekar V, Mahalingam A, Arokiadhas I (2018) Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish Shellfish Immunol 74:501–508
National Committee for Clinical Laboratory Standards (NCCLS) (2004) Performance standards for antimicrobial disk susceptibility testing. In: Fourteenth information supplement. NCCLS document M100-514
Nayak SK (2010) Review probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29:2–14
Ortuño J, Esteban MA, Meseguer V, Mulero V (1998) Methods for studying the haemolytic, chemoattractant and opsonic activities of seabream (Sparus aurata L.) serum. In: Barnes AC, Davidson GA, Mclntosh MPD (eds) Methodology in fish diseases research. Fisheries Research Services, Aberdeen, pp 97–100
Ortuño J, Esteban MA, Meseguer J (2003) The effect of dietary intake of vitamins C and E on the stress response of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 14(2):145–156
Pakingking R Jr, Palma P, Usero R (2015) Quantitative and qualitative analyses of the bacterial microbiota of tilapia (Oreochromis niloticus) cultured in earthen ponds in the Philippines. World J Microbiol Biotechnol 31:265–275
Panigrahi A, Kiron V, Puangkaew J, Kobayashi T, Satoh S, Sugita H (2005) The viability of probiotic bacteria as a factor influencing the immune response in rainbow trout Oncorhynchus mykiss. Aquaculture 243:241–254
Parry RM Jr, Chandan RC, Shahani KM (1965) A rapid and sensitive assay of muramidase. Proc Soc Exp Biol Med 119:384–386
Pirarat N, Kobayashi T, Katagiri T, Maita M, Endo M (2006) Protective effects and mechanisms of a probiotic bacterium Lactobacillus rhamnosus against experimental Edwardsiella tarda infection in tilapia (Oreochromis niloticus). Vet Immunol Immunopathol 113(3–4):339–347
Reda RM, Selim KM (2015) Evaluation of Bacillus amyloliquefaciens on the growth performance, intestinal morphology, hematology and body composition of Nile tilapia, Oreochromis niloticus. Aquacult Int 23:203–217
Richard W, Grégoire C, Gérard E, Ivo GB (2014) The biology of bacterial peptidoglycans and their impact on host immunity and physiology. Cell Microbiol 16(7):1014–1023
Ridha MT, Azad IS (2015) Effect of autochthonous and commercial probiotic bacteria on growth, persistence, immunity and disease resistance in juvenile and adult Nile tilapia Oreochromis niloticus. Aquacult Res 47(9):2757. https://doi.org/10.1111/are.12726
Ripert G, Racedo SM, Elie AM, Jacquot C, Bressollier P, Urdaci MC (2016) Secreted compounds of the probiotic Bacillus clausii strain O/C inhibit the cytotoxic effects induced by Clostridium difficile and Bacillus cereus toxins. Antimicrob Agents Chemother 60(6):3445–3454
Safari O, Atash MS (2013) Study on the effects of probiotic, Pediococcus acidilactici in the diet on some biological indices of Oscar Astronauts ocellatus. Int Res J Appl Basic Sci 4(11):3458–3464
Sai-Ut S, Benjakul S, Sumpavapol P (2013) Gelatinolytic enzymes from Bacillus amyloliquefaciens isolated from fish docks: characteristics and hydrolytic activity. Food Sci Biotechnol 22(4):1015–1021
Saputra F, Shiu YL, Chen YC, Puspitasari AW, Danata RH, Liu CH, Hu SY (2016) Dietary supplementation with xylanase-expressing B. amyloliquefaciens R8 improves growth performance and enhances immunity against Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immonol 58:397–405
Schmekel B, Blomstrand P, Venge P (2013) Serum lysozyme—a surrogate marker of pulmonary microvascular injury in smokers? Clin Physiol Funct Imaging 33:307–312
Srisapoome P, Areechon N (2017) Efficacy of viable Bacillus pumilus isolated from farmed fish on immune responses and increased disease resistance in Nile tilapia (Oreochromis niloticus): laboratory and on-farm trials. Fish Shellfish Immunol 67:199–210
Standen BT, Rawling MD, Davies SJ, Castex M, Foey A, Gioacchini G, Carnevali O, Merrifield DL (2013) Probiotic Pediococcus acidilactici modulates both localized intestinal and peripheral-immunity in tilapia (Oreochromis niloticus). Fish Shellfish Immunol 35:1097–1104
Truong-Giang H, Ya-Li S, Thanh-Phuong N, Quoc-Phu T, Jiann-Chu C, Chun-Hung L (2017) Current applications, selection, and possible mechanisms of actions of synbiotics in improving the growth and health status in aquaculture: a review. Fish Shellfish Immunol 64:367–382
Tuan TN, Duc PM, Hatai K (2013) Overview of the use of probiotics in aquaculture. Int J Res Fish Aquac 3:89–97
Wang Y-B, Tian Z-Q, Yao J-T, Li W-F (2008) Effect of probiotics, Enteroccus faecium, on tilapia (Oreochromis niloticus) growth performance and immune response. Aquaculture 277:203–207
Wing-Keong N, Kim Y-C, Koh C-B, Yang S-Y (2015) Effects of dietary probiotics on the growth and feeding efficiency of red hybrid tilapia, Oreochromis sp., and subsequent resistance to Streptococcus agalactiae. J Appl Aquac 26:22–31
Yan Y-Y, Xia H-Q, Yang H-L, Hoseinifar SH, Sun Y-Z (2015) Effects of dietary live or heat-inactivated autochthonous Bacillus pumilus SE5 on growth performance, immune responses and immune gene expression in grouper Epinephelus coioides. Aquac Nutr 22(3):698–707
Acknowledgements
This work was partially supported by the Center for Advanced Studies for Agriculture and Food, Institute for Advanced Studies, Kasetsart University under the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, Ministry of Education, Thailand (grant no. CASAF164).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sookchaiyaporn, N., Srisapoome, P., Unajak, S. et al. Efficacy of Bacillus spp. isolated from Nile tilapia Oreochromis niloticus Linn. on its growth and immunity, and control of pathogenic bacteria. Fish Sci 86, 353–365 (2020). https://doi.org/10.1007/s12562-019-01394-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-019-01394-0