Abstract
This study aimed to examine the effect of different water temperatures and light intensities on swim bladder inflation (SBI) and growth of red sea bream Pagrus major larvae to improve rearing techniques for this species. Two sets of experiments were conducted: different rearing temperatures were used in experiment 1 (19, 21, 23, and 25 °C), and different light intensities in experiment 2 (250, 1000, 4000, and 16,000 lx). Water temperature did not affect SBI frequency, but SBI initiation was accelerated at higher temperature, i.e., it was initiated on 3 days post-hatching (dph) at 25 °C and on 6 dph at 19 °C, suggesting that the promotion period for SBI, which needs a surface skimmer to be run, also accelerated with increasing temperature in red sea bream larviculture. A higher temperature also significantly promoted larval growth, although the notochord of larvae at SBI initiation was shorter at higher temperatures. Light intensity had no effect on either the initiation or the frequency of SBI. However, light intensity of 250 lx significantly reduced early larval growth compared to light intensities higher than 1000 lx. These results indicate that light at an intensity greater than 1000 lx at the water surface is suitable for the early larviculture of red sea bream.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Red sea bream (RSB) Pagrus major is one of the oldest cultured fish species in the western Japanese coastal areas. Rearing techniques for this species have been developed in the past five decades (Murata 2000). A form of vertebral deformation, lordosis, a serious problem in seedling production of RSB, is caused by failure of swim bladder inflation (SBI) during early larval stage development (Kitajima et al. 1981). SBI failure results in not only vertebral deformation but also poor growth and low survival in aquacultured fish species belonging to the Scombridae, Serranidae, and Carangidae (Hirata et al. 2009; Imai et al. 2011; Kurata et al. 2014; Honryo et al. 2016), hence, many studies have been conducted to elucidate the mechanism of SBI and find effective physical conditions that will promote SBI (Battaglene and Talbot 1990; Partridge et al. 2011; Wooley et al. 2014). These fish species, including RSB, are physoclists with temporary physostomous larvae. Their larvae must gulp air at the water surface for SBI during a finite window within the temporary physostomous larval period (Kitajima et al. 1981; Friedmann and Shutty 1999; Kurata et al. 2012). However, this gulping of air is inhibited by the presence of films such as an oil film on the rearing water surface. Removal of the surface film using a device such as a surface skimmer during the finite window is essential to promote SBI (Kurata et al. 2012).
Physical conditions, especially rearing water temperature and photo environment, are considered the main factors affecting larval growth, development, and SBI (Komaki 1996; Trotter et al. 2003a, b; Iwasaki et al. 2011). In RSB, SBI is initiated 4–10 days post-hatch (dph) and ends 10–12 dph; however, considerable deviations have been observed in larval body size and time of SBI initiation during the early stages of development (Kitajima et al. 1981; Yamashita 1982; Iseda 1982; Foscarini 1988; Kitajima et al. 1993). Successful development and normal hatching of RSB eggs were observed at rearing water temperatures between 14.0 and 25.6 °C (Mihelakakis and Yoshimatsu 1998), whereas information regarding the optimal rearing environmental conditions for SBI was not reported in these studies. Additionally, the initiation of SBI as well as the window can be affected by the temperature of the rearing water (Bailey and Doroshov 1995). Despite the development of rearing techniques in larviculture of RSB, the effect of water temperature and light intensity on SBI initiation has not yet been investigated. Since the 1980s, rearing techniques and production efficiency in RSB larviculture have largely been advanced by applying the findings of previous studies and improving the control systems for rearing temperature in hatcheries. Despite this, the recent year-round market demand for RSB fingerlings has resulted in an extended fingerling production season and therefore rearing under suboptimal water temperature conditions. Likewise, the rearing light intensity used in hatcheries varies due to insufficient information about suitable light intensities for larviculture. Hence, this study aimed to examine the effects of rearing temperature and light intensity with particular emphasis on SBI initiation and growth of RSB larvae and to contribute new information that will help to advance larviculture techniques.
Materials and methods
Experimental design
Experiment 1 was conducted to examine the effect of different rearing water temperatures on SBI and growth of larval RSB, using four different rearing water temperatures, 19, 21, 23, and 25 °C, with three replicate tanks per treatment, from 3 to 7 dph under constant light intensity of 4000 lx; these trials were repeated twice (trial 1 and trial 2). Experiment 2 was conducted to examine the effect of different rearing light intensities on SBI and growth of larval RSB using different light intensity, 250, 1000, 4000, and 16,000 lx, with two replicate tanks (three replicates for the 4000-lx treatment) per treatment, from 3 to 7 dph at 21 °C. Both experiments were conducted under a 12-h light and 12-h dark cycle. Larval SBI frequency, initiation of the SBI, and growth performances were examined in the two experiments.
Eggs and larval rearing
Two cohorts of fertilized eggs spontaneously spawned by cultured RSB broodstock were used for trial 1 and trial 2 in experiment 1 and for the single trial in experiment 2. They were stocked in 1000-l fiberglass reinforced plastic tanks (135-cm internal diameter; MF-1100S; Tanaka Sanjiro, Fukuoka, Japan) at a density of 6000 eggs per tank and the same spawning temperature (19 °C). The mean diameter of the eggs was 0.88 ± 0.02 mm (trial 1 in experiment 1, n = 20) and 0.90 ± 0.03 mm (trial 2 in experiment 1 and experiment 2, n = 20). The hatching rate of the two cohorts was 99.0 and 97.3%. Except for the treatment at 19 °C in experiment 1, the rearing water temperature was increased at a rate of 1–3 °C day−1 from 1 to 2 dph. First feeding was conducted at 2 dph with S-type rotifers Brachionus plicatilis species complex enriched by a commercial product (Marine Glos EX; Marinetech, Aichi, Japan) and synthesized taurine (Chori, Tokyo). The rotifer density in rearing water was maintained at 10–15 rotifers ml−1 throughout the experimental period. An appropriate amount (30–80 ml tank−1) of SV12 (Chlorella Industry, Tokyo) was added to the rearing water for greening. Penetration of natural light into experimental facilities was blocked by 100% shading with curtains, and light intensity at the center of the water surface was adjusted according to the experimental designs using 40-W fluorescent bulbs (FLR40S DM; Toshiba Lighting & Technology, Kanagawa, Japan) hung above the tanks. A surface skimmer (Kurata et al. 2012) installed on the wall nearby the rearing tank was employed to remove the surface film to promote SBI during the photophase (12 h) starting from 3 dph to the end of the experiment. Aeration was provided using an air diffuser (100 mm long, 23-mm diameter) settled in the center at the bottom of the rearing tank. The air flow rate was maintained at 500 ml min−1 from the time of egg stocking until 2 dph, and decreased to 150 ml min−1 from 3 dph to the end of the experiment; the flow was controlled using a flow meter (KOFLOC, Kyoto, Japan). The rearing water was exchanged with bio-filtered and ultraviolet-treated sea water at the rate of 100% day−1 from 2 dph to the end of the experiment using a flow-through system. Rearing water temperature (degrees Celsius), pH, salinity (grams per liter), and dissolved oxygen level (mg l−1) were monitored twice a day during the experimental period; they were similar among the treatments and between the trials (Table 1).
Measurements and observations
To determine the SBI frequency and the initiation period of SBI, more than 20 larvae were sampled from each tank every 3–4 h from 3 to 7 dph. The sampled larvae were immediately anesthetized by ice-cold sea water and the number of larvae with and without gas-inflated swim bladder was determined under a microscope (Olympus SZX-12; Olympus, Tokyo). When the proportion of larvae with an inflated swim bladder in each tank first exceeded 75% during the experiment period, this was considered the time of SBI initiation in order to compare the larval notochord length (NL; millimeters) among the treatments. The NL was inferred by using image analysis software (cellSens; Olympus) from a photograph taken by a camera (DP-73; Olympus) attached to the microscope. To evaluate growth performances of the larvae, the NL was also measured at the end of the experiment on larvae sacrificed at 0900 hours in trial 1 (experiment 1) and at 0800 hours in trial 2 (experiment 1) and experiment 2.
Dry body weight (DBW) was measured only in trial 1 of experiment 1 by Ultra Microbalances (XP2U; Mettler Toledo, Tokyo) to determine growth indices with the method described in Margulies et al. (2007). Ten larvae were collected from each tank (n = 30 per treatment) on 0 dph (initial weight) and at 2100 hours on 7 dph (final weight) and their weight measured; the latter were collected 3 h after the photophase. Specific growth rate (SGR; % day−1) was determined from the DBW using the following formula: SGR = 100 × (ln Wf − ln Wi)/time (days), where Wi = initial mean DBW (micrograms), Wf = individual final DBW (micrograms), time = 8 days.
The percentage survival was not evaluated in this study because more than 10% of the fish stocked in experimental tanks were used as samples in later analyses and therefore would have affected significantly the survival evaluation. However, high mortality or significant differences in fish survival among the tanks were not observed.
Statistical analysis
The values of NL, DBW, and SGR were expressed as the mean ± SD. Significant differences among the treatments were tested by one-way ANOVA followed by Tukey’s honest significant difference (HSD) multiple comparison test in experiment 1 and Dunnett’s T3 test in experiment 2. The larval body size after SBI initiation was compared among the treatments by ANOVA followed by HSD multiple comparison tests. All the statistical analyses were carried out in SPSS 16.0j (IBM, Tokyo) at the significance level of P < 0.05.
Results
Experiment 1: effect of water temperature
The mean frequencies of larvae with inflated swim bladder in trial 1 and trial 2 are shown in Figs. 1 and 2, respectively. In trial 1 at 25 °C, the first larvae with inflated swim bladders were detected 3 dph at 0900 hours, and the SBI frequency gradually increased, reaching 32.4% within a day. However, it was not until 2100 hours on 4 dph that the time of SBI initiation and the larval NL were used to compare the treatments. Similarly, in trial 2 at 25 °C, a larva with an inflated swim bladder was first observed on 3 dph at 0400 hours, with the mean frequency reaching 86.3% at 2000 hours on 4 dph. In treatments at 23 °C of both trial 1 and trial 2, the first larvae with inflated swim bladders were detected 3 dph at 1200 hours and the mean SBI frequency exceeded 75% on 4 dph, which coincided with the results obtained in treatments at 25 °C. In contrast, lower temperature delayed the SBI at 19 and 21 °C, the first larvae with inflated swim bladders were detected 5 and 4 dph and the mean frequency of SBI exceeded 75% on 6 and 5 dph, respectively, in both trials. At the end of the experiment, the proportions of SBI were similar among the treatments and reached nearly 100% (Table 2).
Frequency of swim bladder inflation (SBI) in red sea bream Pagrus major larvae reared under different water temperatures (filled circle 19 °C, open circle 21 °C, filled square 23 °C, open square 25 °C) in trial 1. Values indicate means from triplicate tank treatments (n = 3); error bars are not shown to simplify the figure. Gray areas represent the scotophase. Asterisks indicate the time that a larva with an inflated swim bladder was first observed, and black arrows indicate the time when mean SBI frequency exceeded 75%. dph Days post-hatch
Frequency of SBI in red sea bream P. major larvae reared under different water temperatures (filled circle 19 °C, open circle 21 °C, filled square 23 °C, open square 25 °C) in trial 2. Values indicate means from triplicate tank treatments (n = 3); error bars are not shown to simplify the figure. Gray areas represent the scotophase. Asterisks indicate the time that a larva with an inflated swim bladder was first observed, and black arrows indicate the time of mean SBI frequency exceeded 75%
The first fish with inflated swim bladders were mainly detected during the photophase and/or prior to the onset of the scotophase. Additionally, the mean SBI frequency drastically increased during the photophase and this trend was commonly observed at different water temperatures.
The growth performance of the RSB larvae reared under different water temperatures is shown in Table 2. The results of trial 1 and trial 2 indicate that larval growth (NL) was stimulated by higher temperature. Significant differences were detected among the treatments, although similar growth was observed between treatments at 21 and 25 °C in trial 1 and between treatments at 23 and 25 °C in trial 2 (P < 0.05; ANOVA followed by HSD; n = 45). In contrast to the NL, DBW and SGR were clearly increased by higher temperature, especially the larval DBW in the treatment at 25 °C, which was approximately threefold higher than that of the larvae in the treatment at 19 °C (P < 0.05; ANOVA followed by HSD; n = 30).
Experiment 2: effect of light intensity
The effects of different light intensities on the frequency of SBI in RSB larvae are shown in Fig. 3. The results of the rearing experiments show that in treatments with 250, 1000, 4000, and 16,000 lx, the SBI frequency increased, exceeding 75% at 2000 hours on 5 dph. At the end of the experiment (7 dph, 2000 hours), the proportion of SBI under each treatment was as follows: 100.0 ± 0% (250 lx), 100.0 ± 0% (1000 lx), 100.0 ± 0% (4000 lx), and 98.5 ± 2.1% (16,000 lx). Similar to the results obtained in experiment 1, the majority of larvae with an inflated swim bladder were found and their number increased drastically within the photophase.
Frequency of SBI of red sea bream P. major larvae reared under different light intensities (filled square 250 lx, open square 1000 lx, filled circle 4000 lx, open circle 16,000 lx). Values indicate means from duplicate tank treatments (except for the 4000-lx treatment in which n = 3); error bars are not shown to simplify the figure. Gray areas represent the scotophase. Asterisks indicate the time that a larva with an inflated swim bladder was first observed, and black arrows indicate the time mean SBI frequency exceeded 75%
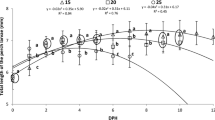
The larval NL in the 250-lx treatment was significantly smaller than that in other treatments at 7 dph (Fig. 4); there was no significant difference between the other treatments (P < 0.05, ANOVA followed by Dunnett’s T3, n = 20).
Larval body size (notochord length; mm) of red sea bream P. major reared under different light intensities and fed a rotifer diet for 5 days. Values indicate means ± SDs from duplicate tank treatments (except for the 4000-lx treatment). Different letters represent significant differences among the treatment (P < 0.05, ANOVA followed by Dunnet’s T3 test, n = 20)
SBI initiation
Table 3 shows the body size (NL) of larval RSB when the proportion of larvae with an inflated swim bladder in each tank exceeded 75% under different water temperatures (experiment 1) and light intensities (experiment 2). The body size of RSB larvae tended to decrease with increasing temperature (P < 0.05; ANOVA followed by HSD test; n = 36). Unlike the body size of larvae in experiment 1, the body size was statistically similar among the treatments in experiment 2.
Discussion
Effect of water temperature
SBI frequency
In the present study, we confirmed that the SBI frequency was similar for different cohorts of eggs even when the rearing water temperature was altered (Table 2). Thus, this study indicates that the SBI frequency in RSB is not affected by the rearing water temperature. Similarly, Hirata et al. (2009) reported that rearing water temperature of 22–28 °C does not affect the SBI frequency in larval greater amberjack Seriola dumerili. However, Trotter et al. (2003a) reported an optimal but narrow temperature range (16–17 °C) for successful SBI in striped trumpeter Latris lineata larvae. The different effects of rearing water temperature on SBI between these species are possibly explained by adaptation to different inhabited temperature ranges. For example, striped trumpeter inhabits cool and rocky bottoms (Furlani and Ruwald 1999), whereas both greater amberjack and RSB inhabit a wide range of temperate areas, with RSB being a particularly suitable cultured marine species in a wide water temperature range (Murata 2000; Foscarini 1988; Miyashita and Kumai 2000). Moreover, the temperature range of 19–25 °C examined in the present study is within the thermal limit of successful development of RSB eggs (Mihelakakis and Yoshimatsu 1998). Therefore, an adaptation to inhabit a wide temperature range may explain why the SBI frequency in RSB was not affected by the rearing water temperature.
We found that SBI frequency fluctuated between day and night regardless of different rearing water temperatures (Figs. 1, 2). Similarly, due to the complete deflation of the swim bladder during the photophase, the nominal SBI frequency may become lower than that during the scotophase in Sillago ciliata (Battaglene et al. 1994). Night-inflation of the swim bladder that gives buoyancy to larvae was considered to help to conserve energy during the scotophase and be related to vertical migration for survival (Kitajima et al. 1993; Battaglene et al. 1994). Contrary to a night-inflated swim bladder, the reason why swim bladders deflate during the day has not been examined in detail. We hypothesized that RSB larvae might require the capacity for their internal organs to expand in order for them to feed during the photophase following the period when the swim bladder is deflated. In the case of RSB, diel changes of swim bladder volume have been reported in a previous study (Kitajima et al. 1993); however, complete deflation of the swim bladder has not been reported yet. Accordingly, fluctuations in the frequency of SBI remain to be investigated.
Timing of SBI initiation
In this study, we found that a higher water temperature accelerated the timing of SBI initiation (Figs. 1, 2). These results indicate that the timing of SBI initiation in RSB larvae is also temperature dependent, as in other marine teleosts (Iwasaki et al. 2011; Bailey and Doroshov 1995; Gibson and Johnston 1995). In previous studies, SBI initiation in RSB larvae was observed on 4–8 dph (Kitajima et al. 1993) and on 9 dph (Yamashita 1982). These previous studies may have been conducted under lower and/or uncontrolled rearing water temperature which may explain why the results are partly different from ours. Physostomous RSB need to gulp air at the water surface during the temporary physoclistous period in the early stage of their development; however, during this brief period, the gulping of air may be inhibited by surface film formation (Kitajima et al. 1981). SBI failure has a serious negative impact on hatchery production (Kitajima et al. 1994), thus, it is essential to maintain a clear water surface during this period by surface film removal to effectively induce SBI. Kitajima et al. (1981) have reported that the window for SBI starts at 4 dph and ends at 10–12 dph (total length < 4.72 mm). However, the temperature-dependent timing of SBI initiation elucidated herein implies that the window for SBI is also temperature dependent in RSB. The timing of this window is important to effectively promote SBI and to plan when the surface skimmer should be used in a hatchery, although the relation between the window and rearing temperature has not yet been investigated in any fish species. Further study is required to investigate the effect of rearing water temperature on this window to promote SBI effectively under various rearing temperatures in RSB and other aquaculture fish species.
The period during which larvae gulp air leading to SBI is species specific and related to the photoperiod (Hirata et al. 2009; Battaglene and Talbot 1990; Trotter et al. 2003b; Kurata et al. 2015). In the present study, the surface skimmer was employed only during the photophase; nevertheless, the SBI frequency in RSB larvae reached almost 100%. Additionally, the first larvae with an inflated swim bladder were mainly detected during the photophase and/or just prior to the onset of scotophase. Concurrently with this, SBI frequency increased within a 12-h period, suggesting that RSB larvae gulp air for SBI during the photophase.
Larval growth and development
This study showed that body size (NL) of the RSB larvae at the time of SBI initiation was significantly smaller at a higher water temperature (Table 3). According to the classification of morphological development stages by Moteki et al. (2001), the larval developmental stage at SBI initiation that occurred in this study corresponded to the period of organogenesis and transition from mixed feeding to exogenous feeding, and was completed by the time of intensified feeding. It is known that increasing rearing water temperature accelerates larval development, but decreases larval body size during metamorphosis in some marine teleosts including RSB (Komaki 1996; Trotter et al. 2003a; Seikai et al. 1986). Therefore, it is thought that accelerated SBI at higher water temperatures with decreased larval body size were also caused by accelerated developmental stages of the larvae in RSB.
In the present study, we clearly showed that higher water temperature significantly promoted larval DBWs and SGRs; the effect of water temperature (18, 21, and 24 °C) on larval length and development of RSB were also reported in a preliminary study (Komaki 1996). However, Komaki (1996) did not statistically evaluate differences among treatments during the larval stage. In the present study, significant differences in larval DBWs and SGRs were detected among the treatments, although larval NL was statistically similar among the treatments at 21–25 °C in trial 1 and between treatments at 23–25 °C in trial 2 (Table 2). Thus, our results contribute to our knowledge on the effects of rearing water temperature on RSB growth and indicate that DBW is highly influenced by rearing water temperature. A high growth rate of marine fish larvae is triggered by increased food consumption (Houde 1989). In addition, Takii et al. (1992) reported that somatic growth of RSB larvae (total length < 7.5 mm) is supported by hyperplasia. These results support our conclusion that larval weight is more malleable than larval length, and the combination of larval length and weight bring insights into growth evaluation.
Effect of light intensity
SBI frequency
In this study, light intensity between 250 and 16,000 lx had no effect on either SBI frequency or SBI initiation in RSB larvae. Lower light intensity had a more significant positive effect on SBI in striped trumpeter and Australian bass Macquaria novemaculeata larvae due to a negative phototactic response exhibited by the larvae (Battaglene and Talbot 1990; Trotter et al. 2003b). In contrast, higher light intensity did not affect SBI in positively phototactic larvae such as striped bass Morone saxatilis and red porgy Pagrus pagrus (Martin-Robichaud and Peterson 1998; Suzer and Kamaci 2005). RSB in their early life stage are natant and neustonic, and wild RSB larvae migrate inshore (Murata 2000; Tanaka et al. 1983). In addition, the results of experiment 1 suggested that RSB larvae need to gulp air during the photophase. Considering these characters of RSB, we conclude that light intensity between 250 and 16,000 lx had no effects on SBI.
Growth
In experiment 2, growth of the larvae reared under 250 lx was significantly reduced compared to that in treatments with light intensities of 1000, 4000, and 16,000 lx (Fig. 4). Similarly, reduced growth under lower light intensity was reported in Pagrus pagrus, another species from the same genus as RSB (Suzer and Kamaci 2005). Light intensity is considered to affect growth because of its effect on food visibility (Strand et al. 2007; Jirsa et al. 2009). Yano and Ogawa (1982) suggested that the preferable photo environment for RSB was greater than 1100 lx, which is possibly related to larval viability. In addition, larval RSB smaller than 10.6 mm exhibit lower visual acuity (Kawamura et al. 1984), which may result in poorer growth under 250-lx light conditions during 3–7 dph. Therefore, it is considered that light at an intensity greater than 1000 lx improved food visibility for RSB larvae, resulting in increased food intake and growth.
References
Bailey HC, Doroshov IS (1995) The duration of the interval associated with successful inflation of the swimbladder in larval striped bass (Morone saxatilis). Aquaculture 131:135–143
Battaglene CS, Talbot RB (1990) Initial swim bladder inflation in intensively reared Australian bass larvae, Macquaria novemaculeata (Steindanchner) (Perciformes: Percichthyidae). Aquaculture 86:431–442
Battaglene CS, McBride S, Talbot RB (1994) Swim bladder inflation in larvae of cultured sand whiting, Sillago ciliata Cuvier (Sillaginidae). Aquaculture 128:177–192
Foscarini R (1988) A review: intensive farming procedure for red sea bream (Pagrus major) in Japan. Aquaculture 72:191–246
Friedmann BR, Shutty KM (1999) Effect of timing of oil film removal and first feeding on swim bladder inflation success among intensively cultured striped bass larvae. N Am J Aquac 61:43–46
Furlani DM, Ruwald FP (1999) Egg and larval development of laboratory-reared striped trumpeter Latris lineata (Forster in Bloch and Schneider 1801) (Percoidei: Latridiidae) from Tasmanian waters. N Z J Mar Freshwater Res 33:153–162
Gibson S, Johnston IA (1995) Temperature and development in larvae of the turbot Scophthalmus maximus. Mar Biol 124:17–25
Hirata Y, Hamasaki K, Imai A, Teruya K, Iwasaki T, Hamada K, Mushiake K (2009) Effects of different photoperiods and water temperature on survival, growth, feeding and initial swim bladder inflation of greater amberjack Seriola dumerili larvae. Nippon Suisan Gakkaishi 75:995–1003 (in Japanese with English abstract)
Honryo T, Tanaka T, Guillen A, Wexler JB, Cano A, Margulies D, Scholey VP, Stein MS, Sawada Y (2016) Effect of water surface condition on survival, growth and swim bladder inflation of yellowfin tuna, Thunnus albacares (Temminck and Schlegel), larvae. Aquac Res 47:1832–1840
Houde ED (1989) Comparative growth, mortality, and energetics of marine fish larvae: temperature and implied latitudinal effects. Fish Bull 87:471–495
Imai A, Iwasaki T, Hashimoto H, Hirata Y, Hamasaki K, Teruya K, Hamada K, Mushiake K (2011) Mechanism for initial swim bladder inflation in larvae of greater amberjack Seriola dumerili inferred from larval rearing experiments and ontogenetic development of a swim bladder. Nippon Suisan Gakkaishi 77:845–852 (in Japanese with English abstract)
Iseda H (1982) Prevention of demonstration in the juveniles of red sea bream, Pagrus major reared in ponds. III. Relationship between the initial conditions of rearing environment and gas content in air bladder. Report of Kumamoto Prefecture Fisheries Research Center, vol 2, pp 25–45 (in Japanese)
Iwasaki T, Imai A, Hashimoto H, Hamasaki K, Teruya K, Hamada K, Mushiake K (2011) Timing of initial swim bladder inflation in larvae of greater amberjack Seriola Dumerili reared under different temperature and light condition. Aquac Sci 59:637–640 (in Japanese with English abstract)
Jirsa D, Drawbridge M, Stuart K (2009) The effects of tank color and light intensity on growth, survival, and stress tolerance of white seabass, Atractoscion nobilis, larvae. J World Aquac Soc 40:702–709
Kawamura G, Tsuda R, Kumai H, Ohashi S (1984) The visual cell morphology of Pagrus major and its adaptive changes with shift from palagic to benthic habitats. Nippon Suisan Gakkaishi 50:1975–1980
Kitajima C, Tsukashima Y, Fujita S, Watanabe T, Yone Y (1981) Relationship between uninflated swim bladders and lordotoic deformity in hatchery-reared red sea bream Pagrus major. Nippon Suisan Gakkaishi 47:1289–1294 (in Japanese with English abstract)
Kitajima C, Yamane Y, Matsui S, Kihara Y, Furuichi M (1993) Ontogenetic change in buoyancy in the early stage of red sea bream. Nippon Suisan Gakkaishi 59:209–216
Kitajima C, Watanabe T, Tsukashima Y, Fujita S (1994) Lordotic deformation and abnormal development of swim bladders in some hatchery-bred marine physoclistous fish in Japan. J World Aquac Soc 25:64–77
Komaki H (1996) Temperature effects on larval growth and development of red sea bream, Pagrus major under laboratory conditions. Aquac Sci 44:99–104 (in Japanese with English abstract)
Kurata M, Seoka M, Nakagawa Y, Ishibashi Y, Kumai H, Sawada Y (2012) Promotion of initial swimbladder inflation in Pacific bluefin tuna, Thunnus orientalis (Temminck and Schlegel), larvae. Aquac Res 43:1296–1305
Kurata M, Ishibashi Y, Takii K, Kumai H, Miyashita S, Sawada Y (2014) Influence of initial swimbladder inflation failure on survival of Pacific bluefin tuna, Thunnus orientalis (Temminck and Schlegel), larvae. Aquac Res 45:882–892
Kurata M, Seoka M, Ishibashi Y, Honryo T, Katayama S, Takii K, Kumai H, Miyashita S, Sawada Y (2015) Timing to promote initial swimbladder inflation by surface film removal in Pacific bluefin tuna, Thunnus orientalis (Temminck and Schlegel), larvae. Aquac Res 46:1222–1232
Margulies D, Suter JM, Hunt SL, Olson RJ, Scholey VP, Wexler JB, Nakazawa A (2007) Spawning and early development of captive yellowfin tuna (Thunnus albacares). Fish Bull 105:249–265
Martin-Robichaud DJ, Peterson RH (1998) Effects of light intensity, tank colour and photoperiod on swimbladder inflation success in larval striped bass, Morone saxatilis (Walbaum). Aquac Res 29:539–547
Mihelakakis A, Yoshimatsu T (1998) Effects of salinity and temperature on incubation period, hatching rate and morphogenesis of the rea sea bream. Aquac Int 6:171–177
Miyashita S, Kumai H (2000) Greater amberjack. In: Kumai H (ed) Aquaculture of marine teleost. SOBUNSHA, Tokyo, pp 78–87 (in Japanese)
Moteki M, Ishikawa T, Teraoka N, Fushimi H (2001) Transition from endogenous to exogenous nutritional sources in larval red sea bream, Pagrus major. Suisanzoshoku 49:323–328 (in Japanese with English abstract)
Murata O (2000) Red sea bream. In: Kumai H (ed) Aquaculture of marine teleost. Sobunsha, Tokyo, pp 89–107 (in Japanese)
Partridge GJ, Benetti DD, Stieglitz JD, Hutapea J, McIntyre A, Chen B, Hutchinson W, Scholey VP (2011) The effect of a 24-hour photoperiod on the survival, growth and swim bladder inflation of pre-flexion yellowfin tuna (Thunnus albacares) larvae. Aquaculture 318:471–474
Seikai T, Tanagonan JB, Tanaka M (1986) Temperature influence on larval growth and metamorphosis of the Japanese flounder Palalichtys olivaceus in the laboratory. Nippon Suisan Gakkaishi 52:977–982
Strand Å, Alanärä A, Staffan F, Magnhagen C (2007) Effects of tank colour and light intensity on feed intake, growth rate and energy expenditure of juvenile Eurasian perch, Perca fluviatilis L. Aquaculture 272:312–318
Suzer C, Kamaci HO (2005) Effects of different light intensities on the larval development of the red porgy (Pagrus pagrus, L. 1758) larvae. Sci Eng J Firat Univ 17:613–620 (in Turkish and English abstract)
Takii K, Nakamura G, Takaoka O, Furuta S, Kumai H (1992) Nucleic acid content and chemical composition of red sea bream, from larvae just after hatching to juveniles. Suisanzoshoku 40:285–290
Tanaka M, Sugiyama M, Tamai K, Miyaji K (1983) The ecological studies of the larvae and juvenile of the red seabream in Shijiki Bay. II. An observation of the vertical distribution of pelagic larvae and juvenile. Bull Seikai Reg Fish Res Lab 59:33–45 (in Japanese with English abstract)
Trotter AJ, Pankhurst PM, Morehead DT, Battaglene SC (2003a) Effects of temperature on initial swim bladder inflation and related development in cultured striped trumpeter (Latris lineata) larvae. Aquaculture 221:141–156
Trotter AJ, Battaglene SC, Punkhurst PM (2003b) Effects of photoperiod and light intensity on initial swim bladder inflation, growth and post-inflation viability in cultured striped trumpeter (Latris lineata). Aquaculture 224:141–158
Wooley LD, Fielder DS, Qin JG (2014) Swimbladder inflation, growth and survival of yellowtail kingfish Seriola lalandi (Valenciennes, 1833) larvae under different temperature, light and oxygen conditions. Aquac Res 45:1489–1498
Yamashita K (1982) Differentiation of the swimbladder structure in larvae of the red seabream, Pagrus major. Jpn J Ichthyol 29:193–202 (in Japanese with English abstract)
Yano I, Ogawa Y (1982) Effects of intensity of underwater illumination on vertical movements of larvae and juveniles of red sea bream, Chrysophrys major. Bull Natl Res Inst Aquac 3:45–49 (in Japanese with English abstract)
Acknowledgments
This study was partly supported by the SATREPS program Comparative Studies of the Reproductive Biology and Early Life History of Two Tuna Species for the Sustainable Use of these Resources, of the Japan Science and Technology Agency and Japan International Cooperation Agency.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Honryo, T., Kurata, M., Sandval, D. et al. Effect of water temperature and light intensity on swim bladder inflation and growth of red sea bream Pagrus major larvae. Fish Sci 84, 553–562 (2018). https://doi.org/10.1007/s12562-018-1194-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-018-1194-5