Abstract
Production of juvenile sea bass Lateolabrax japonicus cohorts during the period of post-migration into the Ohta River was compared between a drainage channel (DC) and a natural river (NR) in Hiroshima, southwestern Japan. Freshwater discharge during periods of high precipitation through the DC is controlled to minimize discharge into other rivers which run through the urban area. Juveniles in the DC had been expected to be affected by stronger disturbance in physical properties to their habitat due to higher fluctuations of freshwater discharge. In order to test this hypothesis, cohort-specific growth (G, d−1) and mortality (M, d−1) coefficients and the ratio of G to M (G/M as a proxy of juvenile production) were compared between the two rivers. Juvenile vital rates were estimated through (1) repeated sampling at fine time intervals (6–15 days), (2) application of otolith daily increments for cohort identification, and (3) standardization of abundance at age based on the length-dependent catch efficiency of the sampling gear to estimate M more accurately. G (0.012–0.021) did not significantly differ between the DC and NR. M in the DC (0.184–0.239) was significantly higher than in the NR (0.140–0.148) and average ratio of G/M (0.111) in the NR was higher than in the DC (0.082). High mortality due to physical processes (high variability in salinity) was concluded to contribute to the inter-river difference in juvenile production since the differences in prey availability, and vulnerability to predation between the two rivers were minimal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vital rates of fish early life stages such as growth and mortality have been reported to fluctuate under high variability of biotic and abiotic environmental conditions in estuarine habitats within relatively small spatio-temporal scales than in other aquatic ecosystems [1,2,3,4,5,6,7]. Variability in freshwater discharge is considered to be one of the most important determinants for estuarine fish recruitment, as it affects various biotic and abiotic environmental properties [3, 8,9,10]. To date, successful recruitment has been correlated with years with high freshwater discharge in a variety of fish species and estuaries [3], while information on how high flow conditions result in poor fish recruitment is more limited, probably due to difficulty in detecting the mortality process of fish early life stages [1, 3, 11].
The Ohta River, which runs into Hiroshima Bay, western Japan, splits into six rivers at about 10 km upriver from the river mouth (Fig. 1). The Ohta Drainage Channel (DC), the westernmost of the six rivers, was artificially excavated in 1967 in order to alleviate the effects of flood events which prevailed in the urban areas of Hiroshima City in the early 1900s and its downriver areas serve as habitats for young stages of fishes [12]. Freshwater flow through the DC is usually controlled by human operation to be less than 10% of the total flow of the six rivers [13]. Freshwater discharge after periods of high precipitation is channeled more into the DC than into the other rivers, to minimize flooding in urban areas [12, 14]. Salinity in the downriver area of the DC is usually about 20 and abruptly decreases to 0 during high precipitation periods by human manipulation of water flows at the weir. The higher fluctuations of freshwater discharge in the DC within small time scales are expected to more strongly affect survival of organisms than in the other rivers.
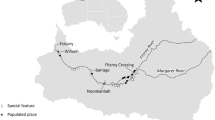
Map of sampling stations in the estuarine area of the Ohta River system and inner part of Hiroshima Bay, western Seto Inland Sea, Japan. Physical and biological surveys were conducted at six sampling stations in both the Ohta Drainage Channel (circles in the drainage channel: DC) and the Temma River (triangles in the natural river: NR) at 6- to 15-day intervals from 19 February to 5 May 2008
The sea bass Lateolabrax japonicus is a euryhaline species widely distributed in temperate coastal waters of the western North Pacific and is commercially and recreationally important in these areas. Adult sea bass spawn from December to January in the sea with a depth of 20–50 m [15,16,17]. Larvae and juveniles are transported to estuaries and then ascend rivers to the brackish water zone (salinity of 0–10) at a standard length (SL) of about 15–17 mm (ca. 50–90 days after hatching) [15, 17]. The larvae and juveniles feed on estuarine copepods and cladocerans and dominate the fish community by number and weight in temperate estuaries of Japan from March through April [12, 19,20,21,22]. In Hiroshima Bay, most of the sea bass larvae distributed along the coastal area are transported to the Ohta River estuaries at 13–15 mm SL and inhabit shallow waters [23]. The seasonal timing of the larval migration into the Ohta River (March to April) and abundance of the larvae and juveniles (50–150 fish 50 m−2) in the DC has been reported to approximate those in the Temma River (Fig. 1), which is next to the DC, their mouths being 900 m apart [23]. The geographical properties of these two estuaries enable us to compare the early dynamics of the production process of sea bass between two habitats with a difference in the temporal fluctuation of freshwater discharge.
In the present study, processes affecting juvenile sea bass production during the period of post-migration into the Ohta River were compared between artificial (drainage channel: DC) and natural habitats (Temma River: NR) where the temporal pattern of freshwater discharge is different. Fish sampling was conducted at fine time intervals (5–16 days) in order to estimate cohort-specific growth (G, d−1), mortality (M, d−1) and the ratio of G to M (G/M) as a proxy of production, to test how higher fluctuations of freshwater discharge affect fish recruitment. Fish abundance data was standardized according to the length-dependent catch efficiency of juveniles by the sampling gear [24] to estimate the cohort-specific abundance and mortality rate more accurately. The G, M and ratio of G/M obtained for juvenile cohorts were compared between the two rivers in order to see whether differences in physical conditions of habitat during periods of high precipitation affect juvenile growth, survival and production.
Materials and methods
Field survey
Physical and biological surveys were conducted in the estuarine areas of the DC and NR (Fig. 1), at 6- to15-day intervals from 19 February to 7 May 2008. Six sampling stations (located at 0.6- to 1.5-km intervals) were set between the river mouth and weir (ca. 10 km upriver) in each river (Fig. 1). The shape of the DC is much straighter than the other five rivers, which meander with irregular changes in river width and depth. Seawater comes further upriver in the DC so that the salt wedge penetrates further upriver than in the other rivers [13].
The tidal effect dominates in areas downriver of the weir according to the tidal cycle during the normal flow condition (without high precipitation). Since the rivers are so close (<1 km, most of the way), the low salinity zones share a common pattern of fluctuation in the major physical conditions. A seine net (2.3 × 1.0 m, 2-mm mesh with 1-mm mesh cod-end) was towed for 50 m along the shoreline at each sampling station. All sampling processes were completed within 3 h before and after low tide (maximum depth of the estuarine areas, 1.0 m) in the daytime so that the whole estuarine area was accessible. Fish samples were preserved in 90% ethanol.
Surface water temperature and salinity were measured with an environmental monitoring system (HORIBA Ltd., W-20XD) at each sampling station. Invertebrate plankton (copepods and cladocerans), the major prey organisms of sea bass larvae and juveniles in the DC and NR [23], were sampled with a conical plankton net (30-cm mouth diameter, 0.1-mm mesh) equipped with a flow meter was towed horizontally. The plankton samples were preserved in 10% seawater formalin. Concentration of prey organisms (no. m−3) was calculated for each sampling station based on the flow-meter count.
Laboratory procedure
SL of larval and juvenile sea bass was measured to the nearest 0.1 mm. Abundance (number of fish per 100 m2) of the larvae and juveniles was calculated based on the area covered by each tow. Abundance of juveniles at 18–23 mm was standardized according to the length-dependent catch efficiency of the seine net estimated in a previous study [23]:
where C and L are the catch efficiency (%) and SL (mm, 18 < L < 23 mm), respectively. The catch efficiency for fish < 18 mm approximates 100% and data for fish > 23 mm was excluded from further analysis due to small sample size (N = 2). Previous field surveys demonstrated that larval and juvenile distributions are restricted within areas 3–8 km upriver from the river mouth even during periods of low tides on spring tide days, showing that the larvae and juveniles are retained within the estuary throughout the tidal cycle under conditions of usual (non-flood) freshwater discharge [23, 24].
In the sea bass, fin rays develop at about 13 mm SL [32], which enables larvae to swim faster and more effectively catch plankton prey. In addition, high gut fullness of larvae and juveniles indicates that the estuarine habitat of the DC and NR provides plenty of prey for sea bass larvae and juveniles [23]. Therefore, we found no reason for positive emigration of larval and juvenile sea bass out of the estuaries, so that any sampling bias was considered minimal.
Thirty individuals at the most were randomly selected from each river on each sampling day when possible and processed for otolith analysis. The right sagittal otolith was removed under a dissecting microscope. Otolith daily increments were counted using a compound microscope connected to a monitor at 400–1000× magnification. Age of juveniles was estimated by adding four to the increment counts, as the first daily increment is deposited at day 4 [18, 25]. Age of the juveniles that had not been estimated from the otolith daily increments was estimated from an age-length regression constructed for each river on each sampling day. The juveniles were dried for 48 h at 60 °C and weighed on a microbalance scale to the nearest 0.0001 mg.
Hatching dates (5 November 2007–12 February 2008) were used to separate juveniles into specific cohorts, defined as individuals hatched within a 5-day period. Each cohort that had a large enough number of individuals for mortality estimation was designated with an alphabetical character from A (5–9 Dec) to G (4–8 Jan; Table 1). A growth coefficient (G) was estimated for each cohort from the equation:
where W t is the dry weight (mg) at time t (days after reaching 14 mm), 4.488 is the weight at 14 mm (the SL of migration into the Ohta river) [23], and G (day−1) is the weight-specific growth coefficient. Instantaneous mortality coefficients (M, day−1) were estimated for each cohort, applying the exponential model of decline [4, 5, 26, 27]:
where N t is the estimated fish abundance at time t (days after the maximum abundance in each river), N m is the estimated abundance on the day of the maximum abundance of each cohort, and M is the instantaneous daily mortality coefficient. The relative recruitment potential of individual cohorts was assessed for each cohort by examining the ratio G to M, which is commonly used as an index of stage-specific production of fish early life stages [4, 26, 28]. The G, M and ratio of G/M obtained for larval and juvenile bass cohorts were compared between the two rivers.
Mean daily freshwater discharge data at Yaguchi Dai-ichi Observation Station (approximately 5 km upriver from the weir) were used as a measure of freshwater flow through the Ohta River [29]. Effects of sampling date on temperature and prey concentration were examined by the use of Spearman’s correlation coefficient. Differences in G, M and G/M between the habitats (DC and NR) were examined using Mann–Whitney’s U test.
Results
Sea bass larvae and juveniles were collected from 19 February to 7 May 2008 with a maximum mean abundance on March 10 in the DC (162.9 fish 100 m−2) and on March 25 in the NR (171.8 fish 100 m−2: Fig. 2a). Sea bass collected on 7 May were excluded from the analysis because all fish were larger than 23 mm. Sea bass abundance decreased from 89.2 ± 16.7 (mean ± SD) on 25 March to 10.9 ± 2.5 on 2 April and was lower than 10.0 thereafter in the DC. In contrast, in the NR, the sea bass abundance increased on the two latest sampling days (15 and 22 April) following a similar decrease in sea bass abundance from 25 March to 2 April.
Seasonal changes in a mean abundance of sea bass larvae and juveniles (number of fish per 100 m2), b mean daily freshwater discharge at Yaguchi Dai-ichi Observation Station, c mean water temperature, d salinity, and e concentration of zooplankton as prey for sea bass juveniles in 2008. Circles and triangles indicate the drainage channel (DC) and the natural river (NR), respectively. Vertical bars indicate standard deviations
Mean daily freshwater discharge was mostly higher than 100.0 m3 s−1 from 13 March to 31 March (Fig. 2b). The maximum daily discharge was 219.3 m3 s−1 on 20 March. The mean water temperature was lowest on 4 March both in the DC (8.6 °C) and in the NR (7.7 °C) and highest on 7 May both in the DC (19.7 °C) and in the NR (18.9 °C: Fig. 2c). The effect of sampling date on the temperature was significant (Spearman’s correlation coefficient, n = 9, p < 0.05 for both rivers). Mean salinity was lowest on 25 March in both the DC (1.2) and NR (1.0) and highest on 7 May both in the DC (13.2) and NR (10.2: Fig. 2d). The mean salinity in the DC was higher than that in the NR on all sampling dates except for 10 March. Difference in the mean salinity between the DC and NR was minimal during the period of high freshwater discharge when much of the freshwater ran through the DC. There was no significant effect of habitat and sampling date (within habitat) on the prey concentration. The maximum prey concentration in the DC (583.0 m−3 on 7 May) was close to that in the NR (587.4 m−3 on 10 March).
Hatch dates ranged from 5 November 2007 to 12 February 2008 (Fig. 3). The relationship between sea bass dry body weight and age was well expressed by the exponential model (Table 1). The G estimated for each cohort ranged between 0.014 (cohorts C and D) and 0.021 (cohort B) in the DC and between 0.012 (cohort D) and 0.017 (cohort E) in the NR (Table 1; Fig. 4). There was no significant effect of either habitat (river) or cohort within the habitats on G.
Weight-specific growth coefficient (G), mortality coefficient (M) and the ratio of G/M estimated for sea bass 5-day cohorts (A–G) in the drainage channel (DC: circles) and the natural river (NR: triangles). There was a significant difference in the M between the DC and NR (Mann–Whitney’s U test, p < 0.05)
The M for each cohort ranged between 0.184 (cohort B) and 0.239 (cohort G) in the DC and 0.140 (cohorts B and F) and 0.148 (cohort C) in the NR (Table 1; Fig. 4). There was a significant effect of habitat on M (Mann–Whitney’s U test, p < 0.05), although the effect of cohort (within habitat) was not significant (p > 0.05). The ratio of G/M was estimated for four cohorts (B, C, E and F) of the seven cohorts both in the DC and NR because of lack of either G or M values in the other cohorts (Table 1; Fig. 4). The ratio of G/M estimated for each cohort ranged between 0.064 (cohort C) and 0.114 (cohort B) in the DC, and between 0.100 (cohort B) and 0.120 (cohort E) in the NR. The average G/M in the NR (0.111) was higher than that in the DC (0.082) although there was no significant effect of habitat on the ratio of G/M.
Discussion
Predation, starvation and physical processes are recognized as the major sources of mortality during fish early life stages [30]. However, it has been difficult to evaluate the relative contributions of these three sources for recruitment of each fish species. In the present study, surveys in the two estuarine habitats located within a short distance of each other enabled us to compare the larval and juvenile sea bass cohort mortalities at a fine spatial scale. Differences in the prey availability and predation risk of the sea bass cohort were expected to be minimal between the two habitats. Therefore, physical processes are suggested to affect cohort survival more than the other two sources do. In addition, (1) repeated sampling at fine time intervals, (2) application of otolith daily increments for cohort identification, and (3) standardization of abundance at age, based on the length-dependent catch efficiency of the sampling gear improved the accuracy of estimation of the vital rates (G, M and the ratio of G/M) of juvenile sea bass cohorts. These values estimated in the two habitats where different variability in the freshwater flow was expected showed that juvenile sea bass production in the NR was higher than that in the DC. The significantly higher M was attributed to the lower ratio of G/M of the sea bass cohort in the DC while G was not significantly different between the two habitats.
The G of fish in early life stages has been reported to vary under fluctuations in physical and biological conditions of their habitat [31]. Recent field surveys with repeated sampling at fine time intervals revealed that cohort-specific G of larval and juvenile estuarine-dependent fishes fluctuates depending on variability in ambient temperature [4, 5, 26, 27], prey availability [26] and larval and juvenile density [19]. In the present study, comparison of cohort-specific G of juvenile sea bass between the artificial and natural estuarine habitats showed that there was no inter-habitat difference in G. Analyses of spatio-temporal variability in the concentration of prey (cladocerans and copepods) and gut contents of the sea bass collected in the DC and NR found high gut fullness (gut content weight/body weight) values due to high prey concentrations in the two rivers [23]. In addition, difference in temperature between the two rivers was small throughout the sampling period in the present study. Therefore, the larval and juvenile sea bass cohorts that hatched in the same period in Hiroshima Bay seemed to have shared common experiences of exposure to similar temperature and prey availability so that the inter-habitat difference in G was not significant although the original cohorts ascended and inhabited different rivers after transportation from their spawning ground to the Ohta River estuaries.
The cohort-specific M of larval and juvenile sea bass in the DC (0.184–0.239) was significantly higher than that in the NR (0.140–0.148). The M of fish in early life stages fluctuates depending on starvation, physical processes and predation [30]. Previous surveys found that there is no possible source of starvation because of high prey concentration and gut fullness in the DC and NR [23]. Sea bass larvae > 13 mm, at which length their fins develop, can swim fast enough to catch zooplankton prey [32]. In addition, a recent study on seasonal change in fish community structures in the DC and NR showed that there are few piscivorous fishes in the tidal reaches of the two rivers [12], indicating the difference in vulnerability to predation between the two rivers is minimal. Therefore, it seems that the variability in mortality due to starvation and predation was not the important determinant for the inter-river difference in sea bass mortality.
The longitudinal distribution of the sea bass larvae and juveniles showed that they are distributed in the low salinity regions (3–8 km upriver from the river mouth) [23] in each river even during the low tide on the days of spring tide in both the DC and NR, without being passively transported downriver by ebb tides throughout the survey period. However, the sea bass juveniles were subject to higher variability in the physical conditions of their habitat in the DC. The temporal fluctuation of salinity was higher in the DC than in the NR (Fig. 2c). Change in salinity in ambient water has been shown to be stressful for juvenile sea bass in the Chikugo River Estuary, Ariake Sea, southern Japan [33]. In addition, exposure to low salinity conditions, due to abrupt fluctuation in salinity, increased mortality of juvenile sea bass in laboratory experiments [34]. Therefore, it is plausible that variability in mortality due to a physical process (temporal variability in salinity of the habitat), but not starvation or predation, induced the difference in the cohort-specific M between the DC and the NR.
In conclusion, production of juvenile sea bass cohorts was estimated to be lower in the DC (artificially made habitat) than in the NR (natural river) due to the higher cohort mortality rates in the DC, although the differences in prey availability and growth rate of the juveniles were not significant between the two habitats. The physical process of temporal change in salinity of the habitat was considered to be an important determinant for the difference in the mortality rates of juvenile sea bass cohorts between the DC and NR.
References
Crecco VA, Savoy TF (1984) Effects of fluctuations in hydrographic conditions on year-class strength of American shad (Alosa sapidissima) in the Connecticut River. Can J Fish Aquat Sci 41:1216–1223
Limburg KE, Pace ML, Arend KK (1999) Growth, mortality, and recruitment of larval Morone spp. in relation to food availability and temperature in the Hudson River. Fish Bull 97:80–91
North EW, Houde ED (2003) Linking ETM physics, zooplankton prey, and fish early-life histories to striped bass Morone saxatilis and white perch M. americana recruitment. Mar Ecol Prog Ser 260:219–236
Rooker JR, Holt GJ, Holt SA, Fuiman LA (1999) Spatial and temporal variability in growth, mortality, and recruitment potential of postsettlement red drum, Sciaenops ocellatus, in a subtropical estuary. Fish Bull 97:581–590
Secor DH, Houde ED (1995) Temperature effects on the timing of striped bass egg production, larval viability, and recruitment potential in the Patuxent River (Chesapeake Bay). Estuaries 18:527–544
Yamashita Y, Tominaga O, Takami H, Yamada H (2003) Comparison of growth, feeding and cortisol level in Platichthys bicoloratus juveniles between estuarine and nearshore nursery grounds. J Fish Biol 63:617–630
Shoji J, North EW, Houde ED (2005) The feeding ecology of white perch Morone americana (Pisces) larvae in the Chesapeake Bay estuarine turbidity maximum: the influence of physical conditions and prey concentrations. J Fish Biol 66:1328–1341
Turner JL, Harold KC (1972) Distribution and abundance of young-of-the-year striped bass, Morone saxatilis, in relation to river flow in the Sacramento-San Joaquin estuary. T Am Fish Soc 101:442–452
Rulifson RA, Charles SM III (1990) Recruitment of juvenile striped bass in the Roanoke River, North Carolina, as related to reservoir discharge. N Am J Fish Manag 10:397–407
Shoji J, Masuda R, Yamashita Y, Tanaka M (2005) Effect of low dissolved oxygen concentrations on behavior and predation rates on Red Sea bream Pagrus major larvae by the jellyfish Aurelia aurita and by juvenile Spanish mackerel Scomberomorus niphonius. Mar Biol 147:863–868
Hayman RA, Albert VT (1980) Environment and cohort strength of Dover sole and English sole. T Am Fish Soc 109:54–70
Mishiro K, Iwamoto Y, Inoue S, Morita T, Mizuno K, Kamimura Y, Hirai K, Shoji J (2014) Fish community in shallow waters of tidal reach of the Ohta River, southwestern Japan: comparison between a drainage channel and a natural river. Bull Jpn Soc Fish Oceanogr 78:169–175 (in Japanese with English abstract)
Sunaga T, Endoh T (1985) Variability in biotic and abiotic conditions of estuaries with special emphasis on the Ohta River system. In: Kosaka A (ed) Environmental features of Seto Inland Sea. Koseisha-koseikaku, Tokyo, pp 165–197 (in Japanese)
Kawanishi K, Kurumida T, Razaz M, Mizuno M, Fukuoka S (2008) Transport characteristics of salt water and SPM in the Ohtagawa Diversion Channel. Annu J Hydraul Eng 52:1321–1326 (in Japanese with English abstract)
Matsumiya Y, Masumoto H, Tanaka M (1985) Ecology of ascending larval and early juvenile Japanese sea bass in the Chikugo River Estuary. Nippon Suisan Gakkaishi 51:1955–1961
Islam MDS, Ueno M, Yamashita Y (2010) Growth-dependent survival mechanisms during the early life of a temperate seabass (Lateolabrax japonicus): field test of the ‘growth–mortality’ hypothesis. Fish Oceanogr 19:230–242
Hibino M, Ohta T, Isoda T, Nakayama K, Tanaka M (2007) Distribution of Japanese temperate bass, Lateolabrax japonicus, eggs and pelagic larvae in Ariake Bay. Ichthyol Res 54:367–373
Shoji J, Ohta T, Tanaka M (2006) Effects of river flow on larval growth and survival of Japanese seaperch Lateolabrax japonicus (Pisces) in the Chikugo River estuary, upper Ariake Bay. J Fish Biol 69:1662–1674
Hibino M, Ueda H, Tanaka M (1999) Feeding habits of Japanese temperate bass and copepod community in the Chikugo River Estuary, Ariake Sea, Japan. Nippon Suisan Gakkaishi 65:1062–1068 (in Japanese with English abstract)
Shoji J, Tanaka M (2007) Density-dependence in post-recruit Japanese seaperch Lateolabrax japonicus in the Chikugo River, Japan. Mar Ecol Prog Ser 334:255–262
Iwamoto Y, Mishiro K, Morita T, Kamimura Y, Mizuno K, Umino T, Shoji J (2009) Larval and juvenile fishes collected at sandy beaches of upper Hiroshima Bay, Seto Inland Sea. Aquac Sci 57:639–643 (in Japanese with English abstract)
Fuji T, Kasai A, Suzuki KW, Ueno M, Yamashita Y (2010) Freshwater migration and feeding habits of juvenile temperate seabass, Lateolabrax japonicus, in the stratified Yura River estuary, the Sea of Japan. Fish Sci 76:643–652
Iwamoto Y, Morita T, Shoji J (2010) Occurrence and feeding habits of Japanese sea bass Lateolabrax japonicus larvae and juveniles around the Ohta River estuary, upper Hiroshima Bay, Seto Inland Sea. Nippon Suisan Gakkaishi 76:841–848 (in Japanese with English abstract)
Iwamoto Y, Morita T, Kamimura Y, Hirai K, Shoji J (2008) Estimation of catch efficiency of a small seine for larval and juvenile Japanese sea bass, in the Ohta estuary. J Grad Sch Biosp Sci, Hiroshima Univ 47:1–5 (in Japanese with English abstract)
Ohta T (2004) Ecological studies on the river ascending migration of Japanese sea bass Lateolabrax japonicus in Ariake Bay, on the basis of otolith information. Ph. D. dissertation, Kyoto University, Kyoto
Shoji J, Tanaka M (2007) Growth and mortality of larval and juvenile Japanese seaperch Lateolabrax japonicus in relation to seasonal changes in temperature and prey abundance in the Chikugo Estuary. Estuar Coast Shelf Sci 73:423–430
Kamimura Y, Shoji J (2013) Does macroalgal vegetation cover influence post-settlement survival and recruitment potential of juvenile black rockfish Sebastes cheni? Estuar Coast Shelf Sci 129:86–93
Houde ED (1996) Evaluating stage-specific survival during the early life of fish. In: Watanabe Y, Yamashita Y, Oozeki Y, Balkema AA (eds) Survival strategies in early life stages of marine resources: proceedings of an international workshop. Japan, Yokohama, pp 51–66
River Bureau, Ministry of Land, Infrastructure and Transport (2010) Ohta River Basin. River discharge year book of Japan 61:310–314 (in Japanese)
Houde ED (1987) Fish early life dynamics and recruitment variability. Am Fish Soc Sym Ser 2:17–29
Houde ED, Zastrow CE (1993) Ecosystem- and taxon-specific dynamic and energetic properties of larval fish assemblages. Bull Mar Sci 53:290–335
Kinoshita I (1988) Lateolabrax japonicus. In: Okiyama M (ed) An atlas of early stage fishes in Japan. Tokai University, Tokyo, pp 402–403 (in Japanese)
Pérez R, Tagawa M, Seikai T, Hirai N, Takahashi Y, Tanaka M (1999) Developmental changes in tissue thyroid hormones and cortisol in Japanese sea bass Lateolabrax japonicus larvae and juveniles. Fish Sci 65:91–97
Hirai N, Tagawa M, Kaneko T, Seikai T, Tanaka M (1999) Distributional changes in branchial chloride cells during freshwater adaptation in Japanese sea bass Lateolabrax japonicus. Zool Sci 16:43–49
Acknowledgements
We express our thanks to Yasuhiro Kamimura, Takuma Morita, Shintaro Inoue, Kazuki Mishiro, and Ken-ichiro Mizuno for their assistance in the field survey. Constructive comments on the manuscript from Takaya Kudoh, Osamu Kawaguchi and anonymous reviewers were much appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwamoto, Y., Shoji, J. Natural habitat contributes more to estuarine fish production than artificial habitat: an example from inter-river comparison in the Ohta River estuaries. Fish Sci 83, 795–801 (2017). https://doi.org/10.1007/s12562-017-1112-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-017-1112-2