Abstract
Diet-switch experiments with three different species of microalgae were conducted to estimate diet-tissue isotopic fractionation of carbon (C) and nitrogen (N) isotopes in the freshwater clam Corbicula sandai. The δ13C and δ15N values in the soft tissues of C. sandai changed in each experiment, with little inter-individual deviations, thereby reflecting the switch in diet. Isotope values of the clam reached the asymptotic value at around 40 days after the switch. Equilibrium isotopic signatures, as well as turnovers of carbon and nitrogen in the whole soft tissues, were estimated by exponential decay models. Fractionations for C. sandai varied from 0.1 to 0.7 ‰ for carbon, and from 2.1 to 3.6 ‰ for nitrogen; these values fell within or were close to the range of previously accepted fractionation values (0–1 ‰ for carbon and 3–4 ‰ for nitrogen). The half-life values for carbon in the bivalves (12–22 days) were about twofold longer than those for nitrogen (7–9 days). The specific fractionation values estimated in this study contribute towards improving our understanding of inter-specific trophic relationships and aquatic food webs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corbicula sandai is an endemic bivalve which inhabits Lake Biwa and the Yodo River, Japan. Until the middle of the last century it was a dominant member of the benthic community of Lake Biwa, playing a major role in the aquatic ecosystem and water purification. It was also one of the most important fisheries resources in the lake, with annual peak landings of approximately 6000 tons in the 1950s. However, C. sandai landings began to decline in the 1960s and have become less than 100 tons annually (only 1 % of its peak) in recent years. In order to reverse this trend and recover this fisheries resource in Lake Biwa there is an urgent need to identify the cause of the decline.
As a general principle the quality of food affects growth, survival and reproduction in animals. Clams obtain food particles by filtration through their inhalant siphon, which they position above the sediment surface. A wide variety of strategies have been developed to investigate the diet of organisms, but it is difficult to clarify the food source of filter feeding bivalves by conventional methods. Direct observation of their feeding behavior provides little information on the food source in the wild. Gut content analyses are also not suitable since the stomach and guts of bivalves are often full of indistinguishable matter, some or all of which might in addition be unassimilated.
In more recent years stable isotope analysis has received increasing interest in diet studies as it is based on assimilated food sources [1]. Early studies using carbon (C) and nitrogen (N) isotopes showed that the 13C/12C and 15N/14N ratios of an animal directly reflect the contribution of food sources assimilated and incorporated over time, with a slight enrichment of heavier isotopes (13C and 15N) compared to the lighter isotopes (12C and 14N) [2, 3]. Since the isotope composition in each primary source of organic matter has significantly different characteristics, this method has been used successfully in many studies on spatial and temporal variations in potential diets of bivalves [4–7]. In a previous study, we analyzed the carbon and nitrogen isotope ratios of particulate organic matter in the water of the lower reaches of the Kushida River and in the soft tissues of Corbicula japonica, a bivalve that is closely related to C. sandai [8]. The results indicated that the contribution of terrestrial organic matter is significantly important for the diet of C. japonica, although there were gradual changes among sampling sites. Kasai et al. [9] also investigated the diets of C. japonica in three brackish lakes by measuring stable isotope ratios. They showed that the diets of the clam differed among the lakes depending on the water residence time and consequent intensity of primary production.
The analyses performed in both of these studies were based on a fixed isotopic enrichment between animals and their diets, which is referred to as trophic shift or fractionation (hereafter Δ). It has been commonly accepted in many studies that the average values for Δδ13C and Δδ15N are between 0 and 1 ‰ and between 3 and 4 ‰, respectively [2, 3, 10]. However, recent comprehensive investigations have pointed out that the actual degree of fractionation is more variable and that this inconsistency depends on the species and/or tissue analyzed [11–13]. Yokoyama et al. [14] conducted feeding experiments on the bivalves Mactra veneriformis and Ruditapes philippinarum and showed that the Δδ13C and Δδ15N ranged from 0.6 to 0.9 ‰ and from 3.4 to 3.6 ‰, respectively, which fell within the range of previously assumed fractionation values. However, Dubois et al. [15] reported that Crassostrea gigas and Mytilus edulis have Δδ13C and Δδ15N of between 1.9 and 2.2 ‰, and 3.8 and 3.8 ‰, respectively. Their Δδ13C values are nearly twofold higher than those commonly assumed, while the Δδ15N values are comparable to the assumed fractionation value. These results indicate that Δ values vary among species.
The Δδ13C and Δδ15N values for C. sandai are currently unknown. Uncertainty in the Δδ13C or Δδ15N of this bivalve could cause errors in estimates of the contribution of various food sources to its diet in the wild. The aim of the study reported here was to determine the Δδ13C and Δδ15N for C. sandai based on laboratory feeding experiments. Early life stages of aquatic animals are generally important to determine biomass because of the high mortality. In addition, C. sandai has a unique early life ecology as it does not have a larval stage but rather settles to the bottom as a plantigrade just after the hatching. Therefore, the focus of our analyses was the isotopic change in this clam during its early life stages.
Materials and methods
Small-sized juveniles of C. sandai were collected shortly after settlement and used for the experiments in order to attain sufficient growth within the relatively short time span of the experiments. As a first step, mature adults of C. sandai were obtained from Lake Biwa and eggs were collected from the adults. Newly hatched plantigrades of C. sandai were cultured in 600-l tanks filled with freshwater and fitted with 25-l up-welling tanks (Tanaka Sanjiro Co., Ltd., Ogori City, Fukuoka, Japan) for 112 days prior to starting the experiments for acclimatization. Freshwater was pumped from Lake Biwa through a sand filter and cartridge filter (mesh size 0.5 μm; Advantec Toyo Kaisha, Ltd., Bunkyo-ku, Tokyo, Japan) to reduce the concentration of particulate organic matter to close to zero. The plantigrades and juvenile stages were reared with the green alga Chlorella homosphaera, which had been cultivated as a diet for C. sandai in the Shiga Prefecture Fisheries Experimental Station [16]. Water temperature was maintained at 28 °C, which is close to the temperature for optimal growth of plantigrade and juvenile stages of C. sandai in terms of seed production.
On 112 day (hereafter referred to as the initial day), 25 clams were randomly sampled to determine the initial values of the studied parameters, and the remaining clams were divided into three groups, each of which contained approximately 130 individuals. The first group (control group) was fed C. homosphaera consistently throughout the experiments, while the diet was switched to other algae in the second and third groups on the initial day. The second group was fed the diatom Chaetoceros calcitrans (YANMAR Co. Ltd., Osaka, Japan), and the third group was fed the green alga Chlorella vulgaris (Chlorella Kogyo, Co. Ltd., Sendai City, Miyagi, Japan). These algae are relatively small in size and often used in bivalve aquaculture for facilitating seed production. All three groups were fed 15 ml of the condensed diets once a day to maintain the concentration of the diets at approximately 100,000 cells l−1 in the tanks. Each group was placed individually in a 15-l plastic vessel filled with freshwater and fitted with a 5-l up-welling tank and covered with a lid. The water temperature was maintained at a constant 28 °C. The water in the vessels was kept still and replaced every 2 days.
Clams with initial shell lengths (SLs) of 2.5–3.2 mm were reared successfully. Feeding experiments were conducted for a total of 71 days for all three groups. Ten clams from each group of those reared successfully were randomly sampled on days 15, 29, 44, 57 and 71 to determine any changes in their isotopic content. A small portion of each alga was extracted on the same days as the clams were sampled to determine the isotope values of the diets.
Sampled clams were kept in filtered water for 1 day to allow the intestine to empty its contents. After the SL had been measured to the nearest 0.1 mm, all of the whole soft tissue was removed under the microscope and rinsed with distilled water. The soft tissue of each individual clam was then dried at 60 °C for more than 1 day and the dry weight (DW) measured to the nearest 1 μg. The dried soft tissue was ground to a fine powder with a mortar and pestle and placed into a tin capsule. If an individual clam was too small for the stable isotope values to be measured, which occurred in particular at the initial stage of the experiments, a sample for measurement was obtained by pooling the powdered dry tissue from several individuals. Other samples consisted of the powdered dry tissue of individual animals.
The stable isotope ratios are described by a per mil (‰) deviation from the respective international standards using the following equation:
where X represents 13C or 15N and R is the 13C/12C or 15N/14N ratio. Peedee Belemnite and atmospheric N2 are the standards for C and N, respectively. DL-Alanine was used as a secondary standard to verify the accuracy of the stable isotope analysis. The standard deviations for the secondary standard were <0.10 ‰ for δ13C and <0.12 ‰ for δ15N.
Negative experimental equations were fitted to the experimental isotope data as
where y is the δ13C or δ15N value of the tissue in question, t is time, a and b are constants and c is an asymptotic value of the tissue on the specific diet. The best-fit curves were optimized by the least squares method. The diet-tissue fractionation, ∆δ13C and ∆δ15N, respectively, was calculated as the difference between the isotopic signatures of the diets and clams after equilibration. The half-life of each element (HL) was also calculated for each diet as
where HL corresponds to the time required to replace 50 % of the initial tissue [17].
The results of the analyses were expressed as the mean ± standard deviation with the number of samples analyzed (n).
Results
The isotopic composition of the diets used in the experiments were fairly constant throughout the experiments. The overall δ13C and δ15N values for Chlorella homosphaera, Chaetoceros calcitrans and Chlorella vulgaris were –15.0 ± 0.2 and –6.3 ± 0.3 ‰, –36.1 ± 2.1 and 0.5 ± 0.1 ‰ and –10.8 ± 0.1 and –3.0 ± 0.1 ‰, respectively (Table 1). The variation in the δ13C of C. calcitrans was comparatively larger than that of the other algae because three different batches were used during the experiment.
On the initial day of the experiments, C. sandai had a mean SL of 2.8 ± 0.2 mm and a mean DW of 0.111 ± 0.020 mg (n = 25). The clams in all groups increased in size and mass over the course of the experiments (Fig. 1). On the last sampling day, C. sandai in the control group had a mean SL of 3.8 ± 0.4 mm and a mean DW of 0.241 ± 0.102 g (n = 10) compared to 5.0 ± 0.2 mm and 0.526 ± 0.109 g (n = 10), respectively, in the second group and 3.9 ± 0.4 mm and 0.242 ± 0.086 g DW (n = 10), respectively, in the third group. During the course of the experiments, C. sandai increased in size by an average of 37, 99 and 26 % in the first (control), second and third group, respectively, and in weight by an average of 131, 656 and 58 %, respectively. The DW of the soft tissue increased relative to the SL to the third power (Fig. 2).
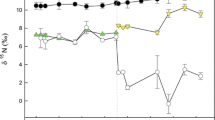
In the control group, δ13C values remained almost unchanged during the course of the experiments (Fig. 3), with a mean value for the entire study period of −14.4 ± 0.3 ‰ (n = 21). Taking into consideration the isotope values of the diet, the fractionation for soft tissue was calculated to be Δδ13C = 0.6 ‰ (Table 2). The δ15N values of the clam decreased slightly during the course of the experiments, reflecting a slight decrease in the δ15N of the diet (Table 1). There was a significant negative relationship (p < 0.001, r 2 = 0.57; n = 21) between δ15N and time elapsed. Since the final δ15N value estimated from the regression line was −2.7 ‰, the fractionation value for soft tissue was calculated to be Δδ15N = 3.6 ‰.
Changes in the δ13C and δ15N of the diets (open circles) and soft tissue of Corbicula sandai fed on the three different algae (Chlorella homosphaera, Chaetoceros calcitrans and Chlorella vulgaris; solid circles) during the experiments. Solid lines and equations Best-fit model with coefficients of determination (r 2), respectively
Both the δ13C and δ15N values of clams in groups 2 and 3 changed following the switch in diet from their initial condition, converging on asymptotic values that reflected the new diets during the experiments (Fig. 3). It is worth noting that there was very little deviation in isotope values for individuals sampled on the same dates, independent of size. The exponential model provided a good fit for changes in both δ13C and δ15N for both diets (r 2 > 0.95, p < 0.001). The theoretical diet-tissue fractionations were calculated as Δδ13C = 0.7 and Δδ15N = 2.1 ‰ for Chaetoceros calcitrans and as Δδ13C = 0.1 and Δδ15N = 3.3 ‰ for Chlorella vulgaris (Table 2). The HL values for the soft tissue were calculated as 22.2 and 6.7 days for δ13C and δ15N, respectively, for C. calcitrans, and as 12.3 and 8.9 days for δ13C and δ15N, respectively, for C. vulgaris. The HL values of carbon are longer than those of nitrogen. The relation between isotope values and the DW of the soft tissue shows that δ15N values for both diets and δ13C values for C. vulgaris almost reached the asymptotic value when the DW of soft tissue increased to 0.2 mg (nearly twofold increase; Fig. 4). The δ13C changes for C. calcitrans took longer.
Discussion
During the course of our experiments, the DW of the soft tissue samples of Corbicula sandai increased in proportion to SL to the third power, with a significant correlation (r 2 = 0.92, Fig. 2), indicating that the C. sandai grew normally under the study conditions. The growth of these C. sandai during the experiments was sufficient to reflect the new diets in terms of isotope values of the clam body (Figs. 1, 4). A number of individual clams were both smaller and lighter on days 15 and 29 of the study relative to the respective values on the initial day (Figs. 1, 2). However, this observation does not necessarily mean that these clams experience poor growth as the reared clams were randomly sampled and may have been very small on the initial day.
There was a tendency for the second group of clams (fed the diatom Chaetoceros calcitrans) to gain more growth than the other groups. The dietary conditions may have influenced growth since all groups were cultured under the same conditions except for diet. C. calcitrans is one of the most common diets used to cultivate bivalves in aquaculture systems worldwide [18] due to its good nutritional properties, including high levels of polyunsaturated fatty acids [19]. As such, a diet of C. calcitrans would have enhanced the growth of the clams in this experimental group. To the contrary, it has been reported that Chaetoceros homosphaera and Chlorella vulgaris are the most suitable diets for the newly hatched plantigrade stage of Corbicula sandai [16]. This difference in growth rate could come from their size difference. Cells of Chlorella spp. are several micrometers in diameter and thus considerably smaller than those of diatoms. As such, the size of Chlorella cells would be suitable for plantigrade stage clams, but they would not be a sufficient diet for juveniles. Newly hatched clams would not be able to ingest diatoms due to their relatively large size. These observations suggest that C. sandai can change its diet depending on developmental stage.
Clams in each diet group differed only slightly in terms of isotope values and all converged on the asymptotic values that reflected each diet (Fig. 3). This finding indicates that individual differences in isotope ratios are negligible among clams ingesting the same diet. To the contrary, animals sampled from natural waters usually show a variety of isotope values. Our results thus suggest that the variation in isotope values found in wild populations is not caused by inter-individual differences or inter-species differences, but rather by differences in ingested diets. The variation in isotope values of animals from natural waters should reflect the variety of food sources that animals consume in the wild.
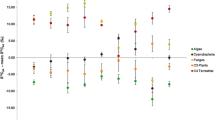
The results of our experiments indicate that the diet–animal isotopic fractionations for Corbicula sandai are almost within the range of the commonly accepted δ13C and δ15N fractionations [2, 3], although the Δδ15N value for Chaetoceros calcitrans was slightly lower. Previous studies have reported a large variation in nitrogen fractionation for bivalves (Fig. 5) [14, 15, 20] and, therefore, the Δδ15N value for C. calcitrans reported in this study is not particularly outside the commonly accepted range. The fractionation in 15N for benthic animals is influenced by the quality of diet, such as protein contents and C:N ratios [21] and metabolic condition [14, 20]. However, reliable data cannot be obtained from field studies due to the general availability of a variety of potential food sources. Our results indicate that the use of one specific value of isotope fractionation could lead to errors in estimating the contributions of various food sources to the diet of organisms in the field.
Carbon and nitrogen fractionation values (Δδ13C and Δδ15N, respectively) for various bivalves based on published data from feeding experiments (open symbols) and estimated from the present experiments (closed symbols). Lipids were not removed from the samples prior to measurements. Shaded square Range of the previously accepted δ13C and δ15N fractionations. Data sources: 1 Yokoyama et al. [14], 2 Yokoyama et al. [20], 3 Dubois et al. [15]
McClelland and Montoya recently compared the δ15N values of two amino acids (phenylalanine and glutamic acids) in planktonic consumers and their food sources with the aim to estimate the trophic level of organisms in food webs [24]. The advantage of this powerful method is that the δ15N values of these two amino acids from a single organism can be used to elucidate the trophic level of the organism [25]. However, δ15N values of individual amino acids do not reflect any variation in environmental and/or physiological conditions of the organisms, although they can be used to provide accurate trophic levels without any information on primary producers. To the contrary, the bulk method, which is applied in the present study, reveals predator–prey relationships directly, although it does require the analyses of isotope values of multiple organisms. A combination of the amino acid method and bulk method would be the most suitable tool for understanding complicated ecosystems.
Our experiments show that the HL of carbon in the soft tissue of Corbicula sandai in the second group (fed the diatom Chaetoceros calcitrans) was larger than that for nitrogen in this group, as well as for the HL of carbon and nitrogen in the third group (Table 2; Fig. 3), even though the growth of clams in the second group was greater than that in the third group (Fig. 2). This difference may be caused by the large difference between the δ13C values of the initial C. sandai and the diet (C. calcitrans). As the δ13C value of C. calcitrans (average of −36.1 ‰) is considerably lower than that of natural phytoplankton in freshwater lakes (normal level −20 ‰) [22] and therefore considered to be unrealistic, the HL value for carbon in the second group could not be applicable to field studies. The HL value for carbon in the third group was larger than that for nitrogen. This tendency was also reported for Lateolabrax japonicus from feeding experiments [23]. It is normal that the turnover rates of carbon and nitrogen differ to a certain degree [15]. Hobson and Bairlein suggested that the difference may correspond to a decoupling of the nitrogen and carbon metabolic pathways [17]. Overall, HL values depend on the turnover rate of tissues. Immature clams store a very low quantity of energy, with almost all assimilated food being used for growth. Therefore, wild clams would have longer HL values than those estimated in this study.
In conclusion, our data on the isotopic fractionation of C. sandai fills a gap in our knowledge on the contribution of various food sources to its diet and provides Δ values for this freshwater clam, thereby contributing to the library of Δ values for invertebrate species. Stable isotope values for whole soft tissues of C. sandai satisfactorily converged on the asymptotic values with little inter-individual deviations, thereby consistently reflecting their diets, and the Δδ13C and Δδ15N values were 0.1–0.7 and 2.1–3.6 ‰, respectively. These values can be used to better interpret the food source of C. sandai in freshwater ecosystems. Phytoplankton, benthic microalgae and terrestrial organic matter have significantly different isotope values [5, 8, 22]. By comparing the isotope values of these potential diets and those of C. sandai, it will be possible to estimate the contribution of each organism to this clam’s diet. If such isotope analyses could demonstrate a preferred diet in Lake Biwa, increased availability of this food source(s) may increase future production of C. sandai.
References
Fry B, Sherr EB (1984) δ13C measurements as indicators of carbon flow in marine and freshwater ecosystem. Contrib Mar Sci 27:13–47
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 42:495–506
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 48:1135–1140
Riera P, Richard P (1996) Isotopic determination of food sources of Crassostrea gigas along a trophic gradient in the estuarine bay of Marennes-Oleron. Estuar Coast Shelf Sci 42:347–360
Kasai A, Horie H, Sakamoto W (2004) Selection of food sources by Ruditapes philippinarum and Mactra veneriformis (Bivalva: Mollusca) determined from stable isotope analysis. Fish Sci 70:11–20
Yokoyama H, Ishihi Y (2003) Feeding of the bivalve Theora lubrica on benthic microalgae: isotopic evidence. Mar Ecol Prog Ser 255:303–309
Kang CK, Lee YW, Choy EJ, Shin JK, Seo IS, Hong JS (2006) Microphytobenthos seasonality determines growth and reproduction in intertidal bivalves. Mar Ecol Prog Ser 315:113–127
Kasai A, Nakata A (2005) Utilization of terrestrial organic matter by the bivalve Corbicula japonica estimated from stable isotope analysis. Fish Sci 71:151–158
Kasai A, Toyohara H, Nakata A, Miura T, Azuma N (2006) Food sources for the bivalve Corbicula japonica in the foremost fishing lakes estimated from stable isotope analysis. Fish Sci 72:105–114
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320
Vander Zanden MJ, Rasmussen JB (2001) Variation in delta N-15 and delta C-13 trophic fractionation: implications for aquatic food web studies. Limnol Oceanogr 46:2061–2066
Vanderklift MA, Ponsard S (2003) Sources of variation in consumer-diet delta N-15 enrichment: a meta-analysis. Oecologia 136:169–182
McCutchan JH, Lewis WM, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102:378–390
Yokoyama H, Tamaki A, Harada K, Shimoda K, Koyama K, Ishihi Y (2005) Variability of diet-tissue isotopic fractionation in estuarine macrobenthos. Mar Ecol Prog Ser 296:115–128
Dubois S, Jean-Louis B, Bertrand B, Lefebvre S (2007) Isotope trophic-step fractionation of suspension-feeding species: implications for food partitioning in coastal ecosystems. J Exp Mar Biol Ecol 351:121–128
Hatano M, Ishizaki D, Kume H, Sakiyama K (2014) Initial food of freshwater clam Corbicula sandai and suitable diet size. Nippon Suisan Gakkaishi 81:62–67 (in Japanese with English abstract)
Hobson KA, Bairlein F (2003) Isotopic fractionation and turnover in captive Garden Warblers (Sylvia borin): implications for delineating dietary and migratory associations in wild passerines. Can J Zool 81:1630–1635
Brown RM (2002) Nutritional value and use of microalgae in aquaculture. Avances en Nutrición Acuícola VI. Memorias del VI Simposium Internacional de Nutrición Acuícola 3:281–292
Volkman JK, Jeffrey SW, Nichols PD, Rogers GI, Garland CD (1989) Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J Exp Mar Biol Ecol 128:219–240
Yokoyama H, Ishihi Y, Yamamoto S (2008) Diet-tissue isotopic fractionation of the Pacific oyster Crassostrea gigas. Mar Ecol Prog Ser 358:173–179
Fantle MS, Dittel AI, Schwalm SM, Epifanio CE, Fogel ML (1999) A food web analysis of the juvenile blue crab, Callinectes sapidus, using stable isotopes in whole animals and individual amino acids. Oecologia 120:416–426
Kim C, Miyajima T, Nagata T (2008) Production and decompose of organic matter (I)—evaluation by carbon isotope ratio of dissolved inorganic carbon. In: Nagata T, Miyajima T (eds) Stable isotopes in environmental assessment of watersheds—progress towards an integrated approach. Kyoto University Press, Kyoto, pp 133–152
Suzuki W, Kasai A, Nakayama K, Tanaka M (2005) Differential isotopic enrichment and half-life among tissues in Japanese temperate bass (Lateolabrax japonicus) juveniles: implications for analyzing migration. Can J Fish Aquat Sci 62:671–678
McClelland JW, Montoya JP (2002) Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology 83:2173–2180
Chikaraishi Y, Ogawa NO, Kashiyama Y, Takano Y, Suga H, Tomitani A, Miyashita H, Kitazato H, Ohkouchi N (2009) Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol Oceanogr Methods 7:740–750
Acknowledgments
This study was supported by a project commissioned by Shiga Prefecture and conducted using cooperative research facilities (Isotope Ratio Mass Spectrometer) of the Center for Ecological Research, Kyoto University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasai, A., Ishizaki, D. & Isoda, T. Isotopic trophic-step fractionation of the freshwater clam Corbicula sandai . Fish Sci 82, 491–498 (2016). https://doi.org/10.1007/s12562-016-0970-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-016-0970-3