Abstract
Interleukin-1 receptor-associated kinase 4 (IRAK-4) is an important component of the mammalian innate and adaptive defense system against infectious disease. In this study, ayu Plecoglossus altivelis altivelis IRAK-4 cDNA and genomic DNA were cloned and analyzed. The open reading frame of ayu IRAK-4 cDNA encodes 463 amino acids, including a death domain and a serine/threonine protein kinase domain of the mammalian IRAK-4 motif. The ayu IRAK-4 gene comprises 11 exons, and was found to be very similar to vertebrate IRAK-4 genes described thus far. Ayu IRAK-4 mRNA was expressed in all tissues, and predominantly in the gill, heart, intestine, whole kidney, and spleen. At 12 h after immersion challenge with Flavobacterium psychrophilum, ayu IRAK-4 mRNA expression in the whole blood was up-regulated (21.6-fold) compared to the control. The present study suggests that ayu IRAK-4 may be involved in the immune response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vertebrates are protected against external pathogens by two broad types of immunity: innate and adaptive immunity. The innate immune system of vertebrates is the first line of defense against infections and provides non-specific responses to a wide range of pathogens [1, 2]. It recognizes invading pathogens by their conserved pathogen-associated molecular patterns using pattern recognition receptors, such as those of the Toll-like receptor (TLR) family [3]. The adaptive immune system uses T and B cells to recognize and defend against pathogens by developing effector cells, antibodies, and memory cells [4–6]. Although each system appears to possess distinct activation mechanisms, interleukin-1 receptor (IL-1R)-associated kinase (IRAK)-4 is essential for NF-κB activation in the signaling pathways of Toll-like and T cell receptors [7–9]. As such, IRAK-4 plays a central role in both innate and adaptive immunity.
IRAK-4 is a serine/threonine kinase belonging to the IRAK family of kinases, which comprises IRAK-1, IRAK-2, IRAK-M, and IRAK-4 [10, 11], all containing a death and a kinase domain. IRAK-4 is the only IRAK family member that does not possess a C-terminal extension [11].
Recent studies have reported the complete cDNA sequences of IRAK-4 for mammals [8, 10]. Among teleost fishes, the IRAK-4 cDNA has been cloned and characterized for the zebrafish Danio rerio [12], tongue sole Cynoglossus semilaevis [13], rainbow trout Oncorhynchus mykiss [14], and orange-spotted grouper Epinephelus coioides [15]. However, there is no information available on IRAK-4 in ayu Plecoglossus altivelis altivelis.
Ayu is a freshwater fish that is a popular food and game fish in various Asian countries, and is widely cultured in Japan. Infectious diseases caused by Gram-negative bacteria [16–18], a Gram-positive bacterium [19], a fungus [20], and a parasite [21] have been reported in ayu, which affect both the fish farming industry and natural water ecosystems. Teleost fishes have an innate and adaptive immune system that defends against disease-causing organisms [2, 22–24], and therefore it is important to understand how this immune system in ayu responds to infectious diseases. Innate immunity-regulating genes in ayu, including tumor necrosis factor (TNF), bactericidal permeability-increasing protein/lipopolysaccharide (LPS)-binding protein, and interleukin-1beta (IL-1β), have been cloned and expression analyses performed in various studies [25–28]. Neutrophils of ayu have shown to have unusually high respiratory burst activity (RBA) in comparison to neutrophils of other freshwater teleost fishes [29], and this high RBA present in ayu neutrophils in healthy kidney stock did not change after stimulation with phorbol 12-myristate 13-acetate [30]. In mammals, IRAK-4 is important for controlling reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidation in RBA and neutrophil recruitment [31–33]. In addition, unlike many teleost fishes, which survive for several years, the ayu lives only 1 year, and thus the study of ayu IRAK-4 is valuable for the field of immunology, as the ayu has a unique immune system with respect to neutrophils and life history. The results of passive immunization experiments revealed an adaptive immune system that showed protective efficacy of a specific antibody in ayu against bacterial cold water disease (BCWD) caused by Flavobacterium psychrophilum [34]. Furthermore, vaccination against F. psychrophilum was found to be effective [35]. Although the ayu life span is only 1 year, the adaptive immune system is important for protection against disease. As a contribution to the characterization of the innate and adaptive immune systems of ayu, we cloned the cDNA and gene encoding ayu IRAK-4 and evaluated IRAK-4 mRNA expression in different tissues and its expression after immersion challenge with F. psychrophilum of Gram-negative bacteria.
Materials and methods
Experimental fish
Immature ayu weighing 12–20 g were obtained from a rearing tank in the Gunma Prefectural Fisheries Experimental Station. Fish were maintained in well freshwater at 16 °C with regular feeding.
Cloning of full-length ayu IRAK-4 cDNA
Ayu were injected intramuscularly with virulent F. psychrophilum strain GMA0330 derived from ayu at a dose of 3.8 × 106 colony-forming units (CFU) per fish [26, 36], which was cultured with tryptone and yeast extract (TYE broth) (tryptone 0.4 %, yeast extract 0.05 %, CaCl2·2H2O 0.02 %, MgSO4·7H2O 0.05 %, pH 7.2) at 18 °C for 1 day. Twenty-four hours later, total RNA was extracted from the whole kidney using the TRIzol reagent (Invitrogen/Thermo Fisher Scientific, Waltham, MA, USA). First-strand cDNA of ayu whole kidney was synthesized from 7.5 μg of total RNA using the AMV Reverse Transcriptase First-strand cDNA Synthesis Kit (Takara Bio, Shiga, Japan) according to the manufacturer’s protocol. To clone the partial sequence of ayu IRAK-4, degenerate oligonucleotide primers paIRAK-4-DF (5′-GARYTNYTNTTYGAYTGGGGNAC-3′) and paIRAK-4-DR (5′-GCYTCNGGNGCCATRTANGC-3′) were designed for amplification of ayu IRAK-4 cDNA based on conserved amino acid sequences of zebrafish IRAK-4 (GenBank accession no. AAT37635), human Homo sapiens IRAK-4 (NP_057207), and mouse Mus musculus IRAK-4 (NP_084202). Polymerase chain reaction (PCR) amplification was performed in a total reaction volume of 10 µl with a GeneAmp PCR System 9700 (Applied Biosystems/Thermo Fisher Scientific, Waltham, MA, USA). The reaction mixture contained 1 µl of the synthesized ayu whole kidney cDNA as template, 2 nmol of each dNTP, 10 pmol of degenerate oligonucleotide primers, and 0.25 units of Ex Taq DNA polymerase (Takara Bio). The PCR cycling protocol for amplification was carried out as follows: one cycle of 5 min at 94 °C, followed by 35 cycles of 30 s at 94 °C, 30 s at 58 °C, and 60 s at 72 °C, with a final extension step of 5 min at 72 °C. The PCR amplicons from the ayu whole kidney cDNA were ligated with pGEM-T Easy Vector (Promega, Madison, WI, USA). Following transfection into competent Escherichia. coli DH5α cells (TOYOBO, Osaka, Japan), recombinants were screened by PCR. Several independent transformants were subjected to DNA sequencing using M13 universal primers with an ABI PRISM 3730xl DNA sequencer and a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems/Thermo Fisher Scientific) according to the manufacturer’s directions for assessing sequence accuracy. The obtained DNA-sequencing data were analyzed using Chromas Lite software (http://www.technelysium.com.au/chromas_lite.html). We then cloned the full-length ayu IRAK-4 cDNA by 5′- and 3′- rapid amplification of cDNA ends (RACE) PCR using the SMART RACE cDNA Amplification Kit (Clontech Laboratories, Mountain View, CA, USA) according to the manufacturer’s protocol, with gene-specific primers for 5′- and 3′- RACE PCR, paIRAK-4GSP1 (5′RACE) (5′-CACTGGCAGAGATGGCTGGCTGAAC-3′) and paIRAK-4GSP2 (3′RACE) (5′-CTTGTGGCGAAGATCTCCGACTTTGG-3′). The nucleotide sequences of RACE-PCR amplicons with ayu whole kidney cDNA were determined as described above. The ayu whole kidney cDNA with a complete open reading frame (ORF) was generated using primers paIRAK-4-UTRF (5′-TTAGCCTACGGTTGAGATTTTCAG-3′) and paIRAK-4-UTRR (5′-ACATTGCAGGTGGGACTGCACCAG-3′) corresponding to the 5′-untranslated region (UTR) and 3′-UTR, respectively. The PCR cycling protocol for amplification was carried out as follows: one cycle of 5 min at 94 °C, followed by 35 cycles of 30 s at 94 °C, 30 s at 60 °C, and 2 min at 72 °C, with a final extension step of 5 min at 72 °C. The PCR amplicons of the complete ORF with ayu whole kidney cDNA were ligated with pCR-XL-TOPO Vector (Invitrogen/Thermo Fisher Scientific) and used to transform One Shot TOP10-competent E. coli cells (Invitrogen/Thermo Fisher Scientific). The nucleotide sequences of the complete ORF with ayu were determined as described above.
Amino acid alignment and phylogenetic analysis
The deduced amino acid homology search of ayu IRAK-4 was conducted with the NCBI protein BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi/) [37]. Multiple sequence alignment was performed with the ClustalW program (http://www.genome.jp/tools/clustalw/) [38]. The structure of the deduced amino acid sequence of ayu IRAK-4 was predicted using the SMART program (http://smart.embl-heidelberg.de/) [39, 40] and the NCBI Conserved Domain Database [41]. A phylogenetic tree of IRAK amino acid sequences was constructed using the neighbor-joining method with 1000 bootstrap replications [42, 43] in the MEGA5 software program [44]. The published GenBank sequences of known IRAK family members were used for corresponding phylogenetic tree analysis.
Gene structure analysis
To analyze the gene structure of ayu IRAK-4, two specific primers, paIRAK-4-STF (5′-ATGAATCACACGATGACATCGTC-3′) and paIRAK-4-STR (5′-CTAAGCCTCACTCTCCGACATGTG-3′), were designed based on the 5′-end and 3′-end sequences of ayu IRAK-4 complete ORF. Genomic DNA was extracted from ayu muscle using the PureLink DNA Extraction Kit (Invitrogen/Thermo Fisher Scientific). One microliter of genomic DNA was used as template. The PCR amplification was performed in same reaction volume in the above-mentioned. The PCR cycling protocol for amplification was carried out as follows: one cycle of 5 min at 94 °C, followed by 35 cycles of 30 s at 94 °C, 30 s at 60 °C, and 4 min at 72 °C, with a final extension step of 5 min at 72 °C. The PCR amplicons from the ayu muscle genomic DNA were ligated with the pCR-XL-TOPO vector (Invitrogen/Thermo Fisher Scientific) and used to transform One Shot TOP10-competent E. coli cells (Invitrogen/Thermo Fisher Scientific). The nucleotide sequence of the ayu IRAK-4 gene was determined as described above.
Tissue expression analysis of ayu IRAK-4 cDNA by semiquantitative RT-PCR
The expression patterns of ayu IRAK-4 mRNA in various tissues of healthy ayu were determined using semiquantitative reverse transcription polymerase chain reaction (RT-PCR), as described by Suzuki et al. [26]. Briefly, the various tissues including whole blood, brain, gill, heart, intestine, whole kidney, liver, spleen, muscle, stomach, and skin were dissected from three healthy ayu. Total RNA was extracted using TRIzol reagent (Invitrogen/Thermo Fisher Scientific). Total RNA was treated with RNase-Free DNase (Invitrogen/Thermo Fisher Scientific) to eliminate genomic DNA contamination. First-strand cDNA samples of various ayu tissues were synthesized from 2 μg of total RNA using the PrimeScript 1st Strand cDNA Synthesis Kit (Takara Bio), according to the manufacturer’s protocol, and the samples were diluted threefold with distilled water. Ayu IRAK-4 gene-specific primers, paIRAK-4-RTF (5′-ACACCTGTGCAGGTGCAGGACTC-3′) and paIRAK-4-RTR (5′-AGTCCCGTCCAAGCAAGCCAGTC-3′), were designed for RT-PCR and amplified to give a specific product of 375 base pairs (bp). β-actin was used as a reference gene to normalize gene expression. The ayu β-actin gene-specific primers, paβ-actin-RTF (5′-TGCGTGACATCAAGGAGAAG-3′) and paβ-actin-RTR (5′-CGTGAATACCGCAAGACTCC-3′), were described by Suzuki et al. [26]. One microliter of the reverse-transcribed product was used as template. PCR amplification was performed in the same reaction volume as described above. The PCR cycling protocol for amplification was carried as follows: out one cycle of 5 min at 94 °C, followed by 32 cycles (for IRAK-4) or 23 cycles (for β-actin) of 15 s at 94 °C, 20 s at 68 °C (for IRAK-4) or at 62 °C (for β-actin), and 30 s at 72 °C, with a final extension step of 5 min at 72 °C. To measure transcript abundance, 5 μl of the PCR products was subjected to electrophoresis on 3 % L03 agarose gel (Takara Bio) in Tris acetate ethylenediaminetetraacetic acid (EDTA) buffer. The gel was stained with ethidium bromide and photographed. Electrophoretic images and densitometric analyses of amplified bands were performed using ImageJ software (version 1.40) (http://imagej.nih.gov/ij/) [45]. Data were expressed as mean and standard error of the mean (SEM).
Expression analysis of ayu IRAK-4 cDNA after bacterial challenge
The expression of ayu IRAK-4 mRNA after immersion challenge with F. psychrophilum was analyzed by quantitative RT-PCR (qRT-PCR). The bacterial challenge experiment was performed as described previously [26]. Briefly, the cultured F. psychrophilum was diluted to 2.8 × 107CFU/ml with 5 l of freshwater, and 20 ayu were then placed in the flask for 30 min. After exposure to F. psychrophilum, the fish were transferred to a 70-l fiber-reinforced plastics tank provided with running well freshwater at 16 °C. Control groups were similarly immersed in TYE broth. To extract total RNA from whole blood and whole kidney using TRIzol reagent (Invitrogen/Thermo Fisher Scientific), three individuals per immersion challenge and control groups were routinely sampled at 12, 24, 48, 96, and 168 h after the immersion challenge. After digestion with RNase-Free DNase (Invitrogen/Thermo Fisher Scientific) to eliminate genomic DNA contamination, first-strand cDNA samples of ayu tissues were synthesized from 500 ng of total RNA using PrimeScript RT Master Mix (Takara Bio), according to the manufacturer’s protocol, and these samples were diluted tenfold with distilled water. Ayu IRAK-4 gene-specific primers, paIRAK-4-qRTF (5′-TGAAGGAGGATTTGGGACTG-3′) and paIRAK-4-qRTR (5′-GGAGTTTCTTGACTGCTACCG-3′), were designed for qRT-PCR amplification to give a specific product of 68 bp. The qRT-PCR was performed on an Mx3005P instrument (Stratagene/Agilent Technologies, Santa Clara, CA, USA) using FastStart Universal SYBR Green Master (Rox) (Roche Diagnostics K.K., Tokyo, Japan) in a total volume of 12.5 μl. The qRT-PCR reaction mixture contained 1 µl of the synthesized ayu tissue cDNA as template, 0.375 μl of 10 μM of each gene-specific primer, and 6.25 μl FastStart Universal SYBR Green Master. The qRT-PCR cycling protocol for amplification was carried out as follows: one cycle of 10 min at 95 °C, followed by 40 cycles of 30 s at 95 °C, 1 min at 55 °C, and 30 s at 72 °C. To assess qRT-PCR efficiency, serial dilutions of a standard cDNA, which was inserted into the pGEM-T Easy Vector (Promega), were used to generate a standard curve for determining the expression values for ayu IRAK-4 mRNA. β-actin was used as a reference gene to normalize gene expression. The ayu β-actin gene-specific primers, β-actin-Q-F (5′-CGACCTCACCGACTACCTGATG-3′) and β-actin-Q-R (5′-TGATGTCACGCACGATCTCAC-3′), were described by Ohara et al. [46]. Data were expressed as mean and SEM. Statistical analysis of the data between control (TYE broth) and treatment (immersion challenge) groups was assessed using the t test. Differences were considered significant at P < 0.05.
Results
Cloning of full-length ayu IRAK-4 cDNA
The ayu IRAK-4 cDNA fragment was amplified by degenerate oligonucleotide primers (paIRAK-4-DF and paIRAK-4-DR) according to regions of high identity between teleost fish and mammalian IRAK-4 amino acids. Subsequently, the 5′- and 3′-regions of the cDNA and ORF regions were amplified using the 5′- and 3′-RACE-specific primers (paIRAK-4GSP1 and paIRAK-4GSP2) and ORF primers (paIRAK-4-UTRF and paIRAK-4-UTRR). The ORF of ayu IRAK-4 cDNA was 1392 bp and encoded a protein of 463 amino acids (Fig. S1). It contained a 5′-UTR of 154 bp and a 3′-UTR of 822 bp. The 3′-UTR contained a probable polyadenylation (ATTAAA) and three ATTTA sequence motifs. A conventional polyadenylation signal sequence was located at 13 bp upstream of the poly(A) tail. Ayu IRAK-4 cDNA was sequenced and the sequence was deposited in the DNA Data Bank of Japan (DDBJ) with accession number AB469846.
Amino acid alignment and phylogenetic analysis
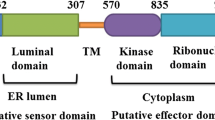
Amino acid analysis of ayu IRAK-4 indicated the existence of a death domain (DD) between amino acid residues 9 and 108 and a serine/threonine protein kinase domain (S_TKc) between amino acid residues 179 and 445, using the NCBI protein BLAST and SMART programs to search for homologous sequences and conserved domains (Fig. S1). The multiple sequence alignment of deduced amino acid sequences ayu IRAK-4 and other known IRAK-4 using the ClustalW program revealed areas of amino acid conservation throughout the vertebrates (Fig. S2). Alignment analysis of ayu IRAK-4 amino acid residues showed 52–70 % identity with other vertebrate IRAK-4 and 32–35 % identity with invertebrate IRAK-4 (Table 1). Furthermore, ayu IRAK-4 DD and S_TKc were found to share higher amino acid identity with the homologous domains in other vertebrates (Table 1). To further examine the relationship between the ayu IRAK-4 and IRAK families (IRAK-1, 2, M, 4) and those of other species, a phylogenetic tree was constructed from the amino acid sequences using the neighbor-joining method (Fig. 1). In a phylogenetic tree of vertebrate IRAK proteins, ayu IRAK-4 grouped with IRAK-4 of other species.
Phylogenetic tree of IRAK family members produced using the neighbor-joining method. Numbers on the line indicate percentage bootstrap values after 1000 replications. Scale bar = 0.1. GenBank accession numbers used: ayu IRAK-4, AB469846; zebrafish IRAK-4, AAT37635; rainbow trout IRAK-4, CBI63173; Atlantic salmon IRAK-4, CDG49297; orange-spotted grouper IRAK-4, AGQ48127; tongue sole IRAK-4, ACU80549; medaka IRAK-4, XP_004069560; Japanese pufferfish, XP_003967681; Western clawed frog IRAK-4, NP_001116877; human IRAK-4, NP_057207; rhesus monkey IRAK-4, XP_001091707; cattle IRAK-4, NP_001069466; mouse IRAK-4, NP_084202; acorn worm IRAK-4, XP_002739502; small abalone IRAK-4, ADC53123; red abalone, AGZ03661; zebrafish IRAK-1, XP_697688; human IRAK-1, NP_001560; cattle IRAK-1, NP_001035645; mouse IRAK-1, NP_032389; human IRAK-2, NP_001561; cattle IRAK-2, NP_001069164; mouse IRAK-2, NP_751893; zebrafish IRAK-M, CAQ13227; human IRAK-M, AAD40879; cattle IRAK-M, XP_587469; mouse IRAK-M, NP_82955. UniProt accession number used: chicken IRAK-4, E1C1A7
Gene structure analysis
The ayu IRAK-4 gene structure was determined using gene structure-specific primers (paIRAK-4-STF and paIRAK-4-STR). The size of the ayu IRAK-4 gene, not including the 5′- and 3′-flanking regions, is 4948 bp (GenBank accession no. AB469845). Alignment of cDNA and gene sequences showed that the ayu IRAK-4 comprised 11 exons, all with intron/exon boundaries following the GT/AG rule. The gene structure of ayu IRAK-4 was found to be very similar to that of vertebrate IRAK-4 genes described thus far, whereas the rainbow trout and whitefish Coregonus maraena sequence was slightly different, with 9 exons (Fig. 2).
Genomic structure of IRAK-4 in fish and other vertebrates. Open boxes indicate exons for each gene, and numbers indicate the length (bp) of exons and introns. GenBank accession numbers of IRAK-4 used: ayu IRAK-4, AB469845; human IRAK-4, AY186092; rhesus monkey IRAK-4, NC_007868; cattle IRAK-4, NC_007303; Western clawed frog IRAK-4, NW_004668234; medaka IRAK-4, NC_019864; Japanese pufferfish IRAK-4, NC_018898; rainbow trout IRAK-4, FN598578; whitefish IRAK-4, FN598581. Ensembl accession numbers of IRAK-4 used: mouse IRAK-4, ENSMUST00000074936; chicken IRAK-4, ENSGALT00000015616
Tissue expression analysis of ayu IRAK-4 cDNA by semiquantitative RT-PCR
Semiquantitative RT-PCR analysis showed that ayu IRAK-4 mRNA was expressed in all tissues (Fig. 3), with transcripts predominantly expressed in the gill, heart, intestine, whole kidney, and spleen, and moderately expressed in whole blood, brain, liver, stomach, and skin. In contrast, expression was weak in muscle tissue. β-actin amplicon as a positive control was detected in all tissues (Fig. 3).
Analysis of ayu IRAK-4 mRNA semiquantitative expression in various tissues of three individual healthy ayu by RT-PCR. a Representative electrophoretic analysis of the mRNA expression of ayu IRAK-4. Lane assignments: M 100 bp ladder marker, WB whole blood, Brn brain, Gill, Hrt heart, Int intestine, Kid whole kidney, Liv liver, Spln spleen, Mus muscle, Stom stomach, Skin, NC negative control without the template. b Ayu IRAK-4 mRNA band density relative to β-Actin mRNA band density. Error bars indicate SEM of three individual experiments
Expression analysis of ayu IRAK-4 cDNA after bacterial challenge
We used the causative agent of BCWD—F. psychrophilum—for the bacterial challenge. The expression level of ayu IRAK-4 mRNA in whole blood using qRT-PCR analysis was dramatically up-regulated, approximately 21.6-fold, in comparison to the control (no challenge) at 12 h after immersion challenge (Fig. 4, significantly different from control, P < 0.05; t test, n = 3), after which levels decreased sharply. The expression level of ayu IRAK-4 mRNA in whole kidney was up-regulated approximately 2.4-fold in comparison with the control at 24 h after immersion challenge, followed by a gradual decrease. The expression level of ayu IRAK-4 mRNA then began increasing again, to approximately 2.1-fold in comparison with the control, until 168 h after immersion challenge (Fig. 4, significantly different from control, P < 0.05; t test, n = 3).
Expression analysis of three individual ayu IRAK-4 mRNA by quantitative RT-PCR in response to immersion challenge of Flavobacterium psychrophilum strain GMA0330. β-actin is used as a reference gene to normalize gene expression. a Whole blood expression. b Whole kidney expression. Statistical analysis of data between control (no challenge) and treatment (12, 24, 48, 96, and 168 h after immersion challenge) groups was performed using the t test. Error bars indicate SEM of three individual experiments. Differences were considered significant at P < 0.05, and are indicated by an asterisk
Discussion
In this work, we sequenced the full ayu IRAK-4 cDNA, which comprised 463 amino acids containing an N-terminal death domain and a C-terminal serine/threonine protein kinase domain, both found in human and mouse IRAK-4 [7–11]. This conservation of the observed domain suggests that the function of mammalian and fish IRAK-4 is similar. In mammals, IRAK-4 and MyD88 use their death domains for binding interactions and are critical signaling mediators of the TLR/IL1-R superfamily [47, 48]. A serine/threonine protein kinase domain belonging to the protein kinase family is related to many cellular processes, including division, proliferation, apoptosis, and differentiation [49].
The amino acid sequence of ayu IRAK-4 showed higher shared sequence identity with the IRAK-4 proteins of vertebrates (52–70 %) than with those of invertebrates such as the acorn worm of the deuterostomes (35 %) and abalone of the protostomes (32 %). Specifically, the peptide of ayu IRAK-4 amino acids shared high identity with teleost fishes, such as the rainbow trout IRAK-4 (70 %) and Atlantic salmon IRAK-4 (69 %) (Table 1). In a phylogenetic tree of vertebrate IRAK proteins, ayu IRAK-4 was grouped with the IRAK-4 of other species, confirming that it is an IRAK-4 homologue (Fig. 1), which suggests that ayu IRAK-4 may share many common functions of IRAK-4 with its family members. In addition, because two conserved domains, DD and S_TKc, among fish and/or vertebrate IRAK-4 is not conserved in IRAK-4, it could be speculated that these domains are important for adaptive immune response.
The isolated ayu IRAK-4 cDNA contains three ATTTA sequence motifs in its 3′-UTR. The ATTTA consensus sequence is typically found in cytokine genes, such as TNF and IL-1β, as well as in chemokines, proto-oncogenes, and vertebrate transcription factors [27, 50–54]. This motif mediates the rapid turnover of mRNAs encoding proteins regulating cellular growth and immune response to exogenous agents such as microbes and inflammatory and environmental stimuli [50].
We also determined the ayu IRAK-4 gene structure, and found that the sizes of ayu IRAK-4 coding exons matched well with the corresponding vertebrate exons, except for those of rainbow trout and whitefish (Fig. 2). Rainbow trout IRAK-4 exons 3, 4, 8, and 9 and whitefish IRAK-4 exons 8, 9, and 10 have been subjected to rearrangement through an exon fusion event [14]. Japanese pufferfish Takifugu rubripes genes are compressed severalfold relative to human homologues due to small introns [55]. The sizes of ayu IRAK-4 introns are markedly smaller than those of human IRAK-4 genes.
We confirmed the expression of healthy ayu IRAK-4 mRNA in various tissues using semiquantitative RT-PCR analysis. Ayu IRAK-4 mRNA is predominantly expressed in the gill, heart, intestine, whole kidney, and spleen, and weakly in muscle (Fig. 3). A similar IRAK-4 mRNA expression profile was found in teleost fishes: transcripts were detected in all examined tissues, with highest expression in the kidney and spleen and lowest in the muscle [12–15]. The highest expression of ayu IRAK-4 mRNA in whole kidney and spleen supported the role of these organs in governing immunity in fish.
Because BCWD causes the greatest damage to farmed and wild ayu [17, 56], we used the causative agent of this disease—F. psychrophilum—for the bacterial challenge to induce immune response. During the infection experiment, the expression level of ayu IRAK-4 mRNA was up-regulated markedly in whole blood and gradually in whole kidney (Fig. 4); this result is in agreement with previous reports of bacterial infection [12, 13]. In teleost fishes, large numbers of neutrophils are released from the kidney into the peripheral blood in response to the initial phase of bacterial stimulus, and neutrophil release decreases rapidly thereafter [57]. In addition, a small number of leucocytes containing the F. psychrophilum antigen in their cytoplasm were first observed in ayu 1 day after immersion challenge with F. psychrophilum. On day 2, many of these cells appeared in the kidney and spleen. These cells were continually observed on days 3 and 5 in most fish [58]. The RBA of ayu kidney neutrophils was high in healthy kidney stock even prior to infection [30]. In addition, IRAK-4 is a central mediator of the TLR/IL-1R signaling pathway and operates during regulation of the NADPH oxidase activation with neutrophils in response to LPS [7, 31]. These results suggest that a large number of IRAK-4-expressing, persistently activated neutrophils migrated toward peripheral blood from the kidney in the early stage of infection of F. psychrophilum with LPS. Moreover, we speculate that the gradual upregulation of ayu IRAK-4 in the whole kidney after bacterial infection may be due to the accumulation of neutrophils and other leukocytes as a result of the high level of IRAK-4 expression in the kidney. In addition, the expression level of ayu IRAK-4 in whole kidney again began to increase until 168 h after the F. psychrophilum immersion challenge, which may be related to the adaptive immune response.
After the of F. psychrophilum immersion challenge, ayu IRAK-4 mRNA copy numbers increased in the whole blood and whole kidney of immunity-related organs. The expression of IRAK-4 mRNA in the spleen and head kidney of tongue sole were similarly up-regulated by a challenge with Vibrio anguillarum [13]. The response of ayu IRAK-4 mRNA in whole blood immediately began to increase upon exposure to F. psychrophilum, suggesting that the innate immune system in blood was activated by the immersion challenge of F. psychrophilum, and that ayu IRAK-4 gene transcripts could thus be a useful marker of ayu response to infectious Gram-negative bacteria. In contrast, the level of mouse IRAK-4 mRNA in a macrophage-like cell line, RAW 264, was not significantly affected by bacterial lipopeptide Pam3CSK4 stimulation [59]. Furthermore, the IRAK-4 expression level in zebrafish and rainbow trout was down-regulated after infection with snakehead rhabdovirus and Aeromonas salmonicida, respectively [12, 14]. This difference in ayu IRAK-4 mRNA expression pattern from that of mouse, zebrafish, and rainbow trout may be due to differences in the method of analysis, ligand, sampling intervals, or infectious reagents (virus, bacteria, and their species). The ayu neutrophils exhibit much higher RBA activity than those of other animals [29]. In addition, NADPH activity is regulated with IRAK-4 in neutrophils [31], implying that the IRAK-4-neutrophil system of the ayu differs from that of other animals. As such, ayu could be an interesting model for investigating neutrophil regulation and function. A thorough understanding of ayu IRAK-4 function in innate and acquired immunity, and the effect of IRAK-4 on NF-κB activation, will make it possible to design novel strategies for therapeutic intervention in infections.
References
Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA (1999) Phylogenetic perspectives in innate immunity. Science 284:1313–1318
Magnadóttir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20:137–151
Kawai T, Akira S (2009) The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol 21:317–337
Davis MM, Bjorkman PJ (1988) T-cell antigen receptor genes and T-cell recognition. Nature 334:395–402
Richards MH, Nelson JL (2000) The evolution of vertebrate antigen receptors: a phylogenetic approach. Mol Biol Evol 17:146–155
Cooper MD, Alder MN (2006) The evolution of adaptive immune systems. Cell 124:815–822
Suzuki N, Suzuki S, Duncan GS, Millar DG, Wada T, Mirtsos C, Takada H, Wakeham A, Itie A, Li S, Penninger JM, Wesche H, Ohashi PS, Mak TW, Yeh WC (2002) Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature 416:750–756
Suzuki N, Saito T (2006) IRAK-4-a shared NF-kappaB activator in innate and acquired immunity. Trends Immunol 27:566–572
Suzuki N, Suzuki S, Millar DG, Unno M, Hara H, Calzascia T, Yamasaki S, Yokosuka T, Chen NJ, Elford AR, Suzuki J, Takeuchi A, Mirtsos C, Bouchard D, Ohashi PS, Yeh WC, Saito T (2006) A critical role for the innate immune signaling molecule IRAK-4 in T cell activation. Science 311:1927–1932
Li S, Strelow A, Fontana EJ, Wesche H (2002) IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc Natl Acad Sci USA 99:5567–5572
Janssens S, Beyaert R (2003) Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell 11:293–302
Phelan PE, Mellon MT, Kim CH (2005) Functional characterization of full-length TLR3, IRAK-4, and TRAF6 in zebrafish (Danio rerio). Mol Immunol 42:1057–1071
Yu Y, Zhong Q, Li C, Jiang L, Wang Y, Sun Y, Wang X, Wang Z, Zhang Q (2012) Identification and characterization of IL-1 receptor-associated kinase-4 (IRAK-4) in half-smooth tongue sole Cynoglossus semilaevis. Fish Shellfish Immunol 32:609–615
Brietzke A, Goldammer T, Rebl H, Korytář T, Köllner B, Yang W, Rebl A, Seyfert HM (2014) Characterization of the interleukin 1 receptor-associated kinase 4 (IRAK4)-encoding gene in salmonid fish: the functional copy is rearranged in Oncorhynchus mykiss and that factor can impair TLR signaling in mammalian cells. Fish Shellfish Immunol 36:206–214
Li YW, Mo XB, Zhou L, Li X, Dan XM, Luo XC, Li AX (2014) Identification of IRAK-4 in grouper (Epinephelus coioides) that impairs MyD88-dependent NF-κB activation. Dev Comp Immunol 45:190–197
Muroga K, Egusa S (1970) Vibrio anguillarum isolated from ayu in fresh-water farm-ponds. Fish Pathol 5:16–20 (in Japanese with English abstract)
Iida Y, Mizokami A (1996) Outbreaks of coldwater disease in wild ayu and pale chub. Fish Pathol 31:157–164
Nishimori E, Kita-Tsukamoto K, Wakabayashi H (2000) Pseudomonas plecoglossicida sp. nov., the causative agent of bacterial haemorrhagic ascites of ayu, Plecoglossus altivelis. Int J Syst Evol Microbiol 50:83–89
Kitao T, Aoki T, Sakoh R (1981) Epizootic caused by ß-haemolytic Streptococcus species in cultured freshwater fish. Fish Pathol 15:301–307
Hatai K, Takahashi S, Egusa S (1984) Studies on the pathogenic fungus mycotic granulomatosis-IV. Changes of blood constituents in both ayu, Plecoglossus altivelis experimentally inoculated and naturally infected with Aphanomyces piscicida. Fish Pathol 19:17–23 (in Japanese with English abstract)
Takahashi S, Egusa S (1977) Studies on Glugea infection of the ayu, Plecoglossus altivelis-I. Description of the Glugea and a proposal of a new species, Glugea plecoglossi. Fish Pathol 11:175–182 (in Japanese with English abstract)
Scapigliati G, Romano N, Abelli L (1999) Monoclonal antibodies in fish immunology: identification, ontogeny and activity of T- and B-lymphocytes. Aquaculture 172:3–28
Nakanishi T, Fischer U, Dijkstra JM, Hasegawa S, Somamoto T, Okamoto N, Ototake M (2002) Cytotoxic T cell function in fish. Dev Comp Immunol 26:131–139
Magnadottir B, Lange S, Gudmundsdottir S, Bøgwald J, Dalmo RA (2005) Ontogeny of humoral immune parameters in fish. Fish Shellfish Immunol 19:429–439
Uenobe M, Kohchi C, Yoshioka N, Yuasa A, Inagawa H, Morii K, Nishizawa T, Takahashi Y, Soma G (2007) Cloning and characterization of a TNF-like protein of Plecoglossus altivelis (ayu fish). Mol Immunol 44:1115–1122
Suzuki K, Izumi S, Tanaka H, Katagiri T (2009) Molecular cloning and expression analysis of the BPI/LBP cDNA and its gene from ayu Plecoglossus altivelis altivelis. Fish Sci 75:673–681
Lu XJ, Chen J, He YQ, Shi YH (2013) Molecular characterization of an IL-1β gene from ayu, Plecoglossus altivelis. Fish Shellfish Immunol 34:1253–1259
Lu XJ, Chu CQ, Chen Q, Chen J (2014) A novel lipopolysaccharide-binding protein (LBP) gene from sweetfish Plecoglossus altivelis: molecular characterization and its role in the immune response of monocytes/macrophages. Fish Shellfish Immunol 38:111–118
Moritomo T, Serata K, Teshirogi K, Aikawa H, Inoue Y, Itou T, Nakanishi T (2003) Flow cytometric analysis of the neutrophil respiratory burst of ayu, Plecoglossus altivelis: comparison with other fresh water fish. Fish Shellfish Immunol 15:29–38
Serada K, Moritomo T, Teshirogi K, Itou T, Shibashi T, Inoue Y, Nakanishi T (2005) Comparison of respiratory burst activity of inflammatory neutrophils in ayu (Plecoglossus altivelis) and carp (Cyprinus carpio). Fish Shellfish Immunol 19:363–373
Pacquelet S, Johnson JL, Ellis BA, Brzezinska AA, Lane WS, Munafo DB, Catz SD (2007) Cross-talk between IRAK-4 and the NADPH oxidase. Biochem J 403:451–461
Bouma G, Doffinger R, Patel SY, Peskett E, Sinclair JC, Barcenas-Morales G, Cerron-Gutierrez L, Kumararatne DS, Davies EG, Thrasher AJ, Burns SO (2009) Impaired neutrophil migration and phagocytosis in IRAK-4 deficiency. Br J Haematol 147:153–156
Singh A, Zarember KA, Kuhns DB, Gallin JI (2009) Impaired priming and activation of the neutrophil NADPH oxidase in patients with IRAK4 or NEMO deficiency. J Immunol 182:6410–6417
Kato G, Suzuki K, Sakai T, Kawakami M, Takano T, Matsuyama T, Nakayasu C (2014) The role of a specific antibody against Flavobacterium psychrophilum infection in ayu sweetfish, Plecoglossus altivelis altivelis (Temminck & Schlegel, 1846). J Fish Dis 38:107–112
Kondo M, Kawai K, Okabe M, Nakano N, Oshima S (2005) Efficacy of oral vaccine against bacterial coldwater disease in ayu Plecoglossus altivelis. Dis Aquat Organ 55:261–264
Arai H, Morita Y, Izumi S, Katagiri T, Kimura H (2007) Molecular typing by pulsed-field gel electrophoresis of Flavobacterium psychrophilum isolates derived from Japanese fish. J Fish Dis 30:345–355
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Schultz J, Milpetz F, Bork P, Ponting CP (1998) SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci USA 95:5857–5864
Letunic I, Copley RR, Pils B, Pinkert S, Schultz J, Bork P (2006) SMART 5: domains in the context of genomes and networks. Nucleic Acids Res 34:D257–D260
Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY, Geer RC, He J, Gwadz M, Hurwitz DI, Lanczycki CJ, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Bryant SH (2015) CDD: NCBI’s conserved domain database. Nucleic Acids Res 43:D222–D226
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evol Int J Org Evol 39:783–791
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Abramoff MD, Magalhaes PJ, Ram SJ (2004) Image Processing with ImageJ. Biophotonics Int 11:36–42
Ohara K, Kageyama T, Kuwada T, Umino T, Furusawa S, Yoshiura Y (2009) Quantitative estimation of Flavobacterium psychrophilum infected ayu Plecoglossus altivelis altivelis by real-time PCR. Nippon Suisan Gakkaishi 75:258–260 (in Japanese)
Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z (1997) MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7:837–847
Kawai T, Akira S (2007) TLR signaling. Semin Immunol 19:24–32
Manning G, Plowman GD, Hunter T, Sudarsanam S (2002) Evolution of protein kinase signaling from yeast to man. Trends Biochem Sci 27:514–520
Bakheet T, Frevel M, Williams BR, Greer W, Khabar KS (2001) ARED: human AU-rich element-containing mRNA database reveals an unexpectedly diverse functional repertoire of encoded proteins. Nucleic Acids Res 29:246–254
Laing KJ, Wang T, Zou J, Holland J, Hong S, Bols N, Hirono I, Aoki T, Secombes CJ (2001) Cloning and expression analysis of rainbow trout Oncorhynchus mykiss tumour necrosis factor-alpha. Eur J Biochem 268:1315–1322
Laing KJ, Zou JJ, Wang T, Bols N, Hirono I, Aoki T, Secombes CJ (2002) Identification and analysis of an interleukin 8-like molecule in rainbow trout Oncorhynchus mykiss. Dev Comp Immunol 26:433–444
Savan R, Kono T, Aman A, Sakai M (2003) Isolation and characterization of a novel CXC chemokine in common carp (Cyprinus carpio L.). Mol Immunol 39:829–834
Kono T, Zou J, Bird S, Savan R, Sakai M, Secombes CJ (2006) Identification and expression analysis of lymphotoxin-beta like homologues in rainbow trout Oncorhynchus mykiss. Mol Immunol 43:1390–1401
Venkatesh B, Gilligan P, Brenner S (2000) Fugu: a compact vertebrate reference genome. FEBS Lett 476:3–7
Wakabayashi H, Toyama T, Iida T (1994) A study on serotyping of Cytophaga psychrophila isolated from fishes in Japan. Fish Pathol 29:101–104
Park SW, Wakabayashi H (1989) Kinetics of neutrophils in the kidney of eel, Anguilla japonica, intraperitoneally injected with formalin-killed Escherichia coli as a irritant. Fish Pathol 24:233–239 (in Japanese with English abstract)
Miwa S, Nakayasu C (2005) Pathogenesis of experimentally induced bacterial cold water disease in ayu Plecoglossus altivelis. Dis Aquat Organ 67:93–104
Hatao F, Muroi M, Hiki N, Ogawa T, Mimura Y, Kaminishi M, Tanamoto K (2004) Prolonged Toll-like receptor stimulation leads to down-regulation of IRAK-4 protein. J Leukoc Biol 76:904–908
Acknowledgments
We thank the staff of the Gunma Prefectural Fisheries Experimental Station for their assistance during the experiment. We are also grateful to Dr. T. Takano, Dr. T. Matsuyama, Dr. T. Kamaishi, and Dr. C. Nakayasu of the National Research Institute of Aquaculture, Fisheries Research Agency, for valuable advice on DNA analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suzuki, K., Izumi, S., Tanaka, H. et al. Identification and expression analysis of IRAK-4 cDNA and its gene from the ayu Plecoglossus altivelis altivelis . Fish Sci 82, 47–57 (2016). https://doi.org/10.1007/s12562-015-0942-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-015-0942-z