Abstract
Calcein (CAL) and alizarin red S (ARS) at concentrations of 50–200 and 150–300 mg/l, respectively, were used for immersion marking juvenile grass carp Ctenopharyngodon idellus. With the exception of non-lateral line scales from the 150 mg/l ARS treatment and lateral line scales from the 150, 200 mg/l ARS treatments, immersion for 24 h produced detectable marks in sagittae, lateral line and non-lateral line scales, and fin rays (dorsal, pectoral, ventral, anal, and caudal) at 100 days post-marking. Detectable fluorescent marks in sagittae were readily observed at concentrations of 150–200 mg/l CAL or 200–300 mg/l ARS. Marks were poorly visible in all non-lateral line and lateral line scales from ARS-treated groups. Fluorescent marks were readily detected in non-lateral line and lateral line scales at 150–200 mg/l CAL, and in fin rays at 100–200 mg/l CAL or 150–300 mg/l ARS. In particular, optimal marks were observed at comparatively high concentrations investigated in sagittae (300 mg/l ARS) and fin rays (100–200 mg/l CAL or 250–300 mg/l ARS). There was no significant difference on the survival or growth of marked fish compared to controls throughout the experiment (P > 0.05).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Grass carp Ctenopharyngodon idellus, of the belong Cyprinidae family, have been accepted as a principal species in Chinese freshwater fish polyculture systems. In China and many countries worldwide, grass carp have made an important contribution to the animal protein intake of humans [1]. Grass carp are also considered a potential biomanipulation tool for aquatic vegetation control [2, 3]. By contrast, grass carp, together with bighead carp Aristichthys nobilis and silver carp Hypophthalmichthys molitrix, are considered invasive species which compete with native species sharing similar trophic positions for natural resources in the US [4].
The overexploitation of aquatic living resources is common among fisheries worldwide [5]. It has been widely recognized that both inland and marine fishery resources are heavily influenced by intense fishing activities [6]. In China, stocking and ranching programs for indigenous species, including grass carp, bighead carp, silver carp, and black carp Mylopharyngodon piceus, began in early 1950s [7]. Recently, grass carp has become one of the leading cultivated fish species in China with freshwater fisheries gradually shifting from capture to stock-enhanced wild populations [8]. In 2012 alone, Chinese freshwater aquaculture produced approximately 4.78 million tons of grass carp, which accounted for 18.1 % of the total freshwater fishery production [9]. In addition, as stock enhancement of juvenile grass carp in lakes and rivers (e.g., Lake Taihu, Lake Chaohu, and Yangtze River, China) has been intensified [10], a basic challenge for fisheries biologists and managers that has emerged is marking or tagging fish benignly so that they may be identified upon re-capture. An improved marking technique for juvenile grass carp would be beneficial for assessing the effectiveness of the stock enhancement strategies for fisheries management [11].
Various methods have been available for marking populations of juvenile hatchery-reared fish up to now, however, standard methods such as tagging (e.g., T-bar, plate disc and PIT) and fin clipping are often limited by the size and quantity of fish to be marked [12]. Considering the high initial set up costs and slow tag application, the use of micro wire tags (e.g., coded wire tags and anchor tags) can be unnecessarily laborious [13]. Genetic marks may not be economically suitable for the recapture process due to high operating costs and relatively difficult detection [14]. Additionally, unlike fish species such as salmon, which could be thermally marked due to long period of egg incubation [15, 16], grass carp have a relatively short incubation period (around 30 h), with little opportunity for thermal marking. Therefore, developing techniques to mark juvenile grass carp is essential to meet the fish marking needs of fisheries managers and researchers working with this species.
Fluorescent labeling, which uses fluorochrome dyes to produce detectable marks in otoliths, scales, and other bony structures based on differential staining of chemical dyes [17, 18], is a widely used technique for marking cohorts. This technique has been evaluated in both teleosts and elasmobranchs [19, 20]. The choice of a marking technique is dependent upon the life stage, environment (marine or fresh water), and preferred method of application [21], such as injection [22], dietary intake [23], immersion [13], or osmotic induction [24, 25]. Fluorescent marks also have the following advantages: (1) they take less time to apply (i.e., a large number of individuals can be marked in a short time with minimum handling stress), (2) they are often lower in cost, (3) the marks may be retained for several months or even years, (4) they can be applied to fish of all ages (larval to adult), and (5) the marking process and recovery of marks is relatively simple [19, 26]. Therefore, it seems that fluorescent marks as internal-external marks are the most suitable tools for evaluation of large-scale stocking of small juveniles in contrast to other tagging methods [27, 28].
In view of previous studies, a variety of fluorochrome labeling dyes, such as oxytetracycline (OTC) [12], tetracycline hydrochloride (TC) [29], calcein (CAL) [30], alizarin red S (ARS) [31], and alizarin complexone (ALC) [32], have proven effective in producing fluorescent or pigmented marks in calcified or bony structures with few negative effects on survival. Beckman and Schulz [33] also observed that much higher concentrations of dyes (e.g., ARS and ALC) can result in excessive mortalities and even reduce mark success. Concerning these studies, the research mainly focused on evaluation of using fluorochrome labeling dyes for marking fish to assess growth, mortality and identification of marks in calcified or bony structures. However, few published studies have evaluated mark retention and survival associated with fluorescent marks in grass carp. Therefore, the objectives of the present study were to: (1) evaluate the effects of immersion marking with different concentrations of CAL and ARS on growth and mortality of juvenile grass carp; and (2) assess mark quality produced by different concentrations of CAL and ARS in otoliths, scales, and fin rays of juvenile grass carp.
Materials and methods
Experimental fish
Juvenile grass carp (93.5 ± 1.8 mm total length, mean ± SE; n = 6) were transported from a commercial hatchery located at Chongqing City in September 2013 and kept in circular polyethylene tanks (D × H = 200 × 100 cm) for approximately 20 days (0.22 individuals per l) under a 12L:12D (light:darkness) photoperiod. Throughout this holding period, fish were fed to satiety with an artificial diet (Tongwei, Sichuan, China) twice per day. Water quality was constantly monitored and maintained as follows: temperature 21.7–23.4 °C, salinity <0.5 PSU, dissolved oxygen (DO) 6.93–7.25 mg/l, and pH 6.6–7.1. Water was exchanged daily at a rate of 20 % of the tank volume.
Immersion marking with CAL and ARS
Stock solutions were prepared by dissolving CAL and ARS in filtered fresh water (salinity, <0.01 PSU) at concentrations of 500 and 4000 mg/l, respectively. A series of five CAL concentrations (0, 50, 100, 150, and 200 mg/l; 30 l each) were prepared in 54-l experimental tanks. All solutions were thoroughly aerated to increase their pH. The juveniles were not fed for 24 h prior to immersion. On September 26, 2013, 300 fish (approximately 120 days old) were randomly allocated to five experimental tanks (60 individuals per tank) at a density of 2.0 individuals per l and immersed in CAL solution for 24 h. Due to the acidifying effects of CAL, the pH of the CAL solutions were monitored hourly during the immersion period, and buffered to the original pH of 6.81–7.25 using sodium carbonate. Fish in the control group were immersed in fresh water without CAL. After the immersion period, fish from each treatment were rinsed in fresh water for 2 h to wash away any residual dye, and were then transferred to five separate tanks and monitored for 72 h to assess the degree of acute mortality caused by CAL.
The same immersion marking procedure was followed for the experiment using ARS. On September 30, 2013, 300 fish were equally allocated to five experiment tanks (60 individuals per tank) at a density of 2.0 individuals per l and immersed in ARS at concentrations of 0, 150, 200, 250, or 300 mg/l (30 l total volume) for 24 h. Fish in the control group were immersed in fresh water without ARS. The degree of acute mortality due to ARS was checked 72 h post-immersion. The range of concentrations of CAL and ARS used were selected based on preliminary experiments conducted at the Fisheries Ecology Laboratory of Southwest University (SWU), and other relevant research into chemical marking techniques in juvenile brown trout Salmo trutta L. [27], Japanese flounder Paralichthys olivaceus [19], and black rockfish Sebastes schlegelii [26].
Growth experiment
To evaluate the effects of CAL and ARS on survival and growth after immersion, 48 fish were randomly selected from each treatment group and used in a subsequent growth experiment. Each treatment group consisted of three replicates, with 16 individuals per replicate, kept in separate polyethylene circular tanks (D × H = 200 × 100 cm) in a water volume of approximately 1256 l (0.012 individuals per l). All fish were fed to satiety with an artificial diet twice daily and were maintained on a 12L:12D photoperiod as described for the holding period. The tanks were aerated continuously and the water quality was monitored throughout the maintenance period (temperature 18.2–25.3 °C, salinity <0.5 PSU, ammonia-N 0.10–0.25 mg/l, dissolved oxygen (DO) 6.53–7.38 mg/l, pH 6.5–7.2). Water was exchanged daily at a rate of 20 % of the aquarium volume.
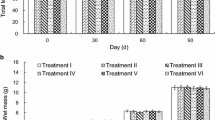
At the beginning of the growth experiment, the total length and wet mass of all fish immersed in CAL and ARS were measured to the nearest 0.1 mm and 0.1 g, respectively. To evaluate the extended effects of CAL and ARS on growth, fish from the different treatment groups were measured again 100 days later at the termination of the growth experiment.
Sampling and mark detection
At 100 days post-marking, two juvenile fish from each replicate of each treatment group (6 individuals per concentration) were chosen randomly and euthanized with tricaine-methanesulfonate (MS-222, 100 mg/l). The otoliths (sagittae), cycloid scales (at and above the lateral line, number 20–40, respectively), and all fin rays (dorsal, pectoral, ventral, anal, and caudal) were then sampled, separated from adherent tissues, and rinsed with freshwater for mark detection. The sagittae were not ground or polished. Fin rays were taken at the base of the pterygiophores. To prevent fading of fluorescent marks, all samples were stored in a dark environment out of direct sunlight and not at high temperature (<26 °C). All samples were observed within 1 week of sampling.
Fluorescent marks produced by CAL and ARS were examined with a Leica DM IL LED fluorescence microscope fitted with a Leica DFC495 high resolution digital camera and equipped with the Leica EL6000 compact light source (Table 1). In addition, a stereoscopic zoom microscope (Nikon SMZ800) fitted with a Nikon DS-Fi1 high resolution digital camera was used to observe the larger samples for visible fluorescent marks as a result of CAL and ARS treatment.
Mark quality was assessed using the 0–5 scale described by Liu et al. [19]: 0 = no mark visible under fluorescence microscopy, 1 = faint mark visible under fluorescence microscopy, 2 = mark easily visible under fluorescence microscopy, 3 = mark shining brightly under fluorescence microscopy, 4 = mark visible in transmitted light, and 5 = mark distinct in transmitted light. Mark quality values ≥2 were deemed acceptable as they could be readily detected in the structure. Mark quality values ≥4 mean that the fluorescent mark could be visible to the naked eye under transmitted light. To evaluate the reliability of mark quality, the sagittae, more than 10 scales, and at least three pieces of fin ray per position from each fish were independently analyzed by two observers. If there was disagreement between the two observers, mark quality was determined by a third observer. All samples were observed directly without resin and polishing.
Statistical analysis
Data of mark quality, total length, and wet mass are presented as mean ± standard error (SE) for all grass carp juveniles. Differences in final fish survival, total length, and wet mass between the treatment groups (CAL and ARS separately) were compared by one-way analysis of variance (ANOVA) (SPSS 19.0). A P value ≤0.05 was considered significant.
Results
Growth and survival
During the 100-day growth experiment, no differences (df = 4, error df = 25, P > 0.05) were found in the total length or wet mass of grass carp in any of the CAL treatment groups (Fig. 1). The same was true for the ARS treatment groups (df = 4, error df = 25, P > 0.05) (Fig. 1).
There was no mortality during the 24 h immersion period or during the first 72 h post immersion in the controls or the CAL- or ARS-treated grass carp. At 100 days post-marking, there was no further mortality in the CAL growth experiment. A mortality rate of 0.42 % was observed in the ARS treatment groups (1 of 240 grass carp died). However, there was no significant difference in mortality between the groups treated with different concentrations of ARS in this experiment (df = 4, P > 0.05).
Mark quality
There were no fluorescent marks detected in the control groups for both CAL and ARS treatments (Fig. 2). When samples from the CAL treatments were exposed to the appropriate wavelengths of blue light (WB) under the fluorescence microscope (Table 1), the marks appeared as a green fluorescent ring (Fig. 3b); when samples from the CAL treatments were exposed to the appropriate wavelengths of ultraviolet light (UV) under the fluorescence microscope, the marks appeared as a light blue fluorescent ring (Fig. 3c) (in fact, the CAL marks produced by UV appeared as a light blue-green fluorescent ring by naked eyes). Marks produced in samples from fish treated with ARS appeared as a red fluorescent ring under WB and green (WG) wavelengths (Fig. 3). The WB and WG wavelengths yielded higher mark intensities for CAL and ARS, respectively, than the other laser wavelengths used (i.e., fluorescent rings produced using a UV laser and a WB laser were fainter for CAL and ARS marks, respectively). In addition, mark intensities of both CAL and ARS fluorescent rings, which were shown on photomicrographs captured by the digital camera, were lower than those actually observed by naked eyes.
Variations in mark quality of the grass carps’ sagittae subjected to CAL and ARS treatments. No marks were detected in the control groups in the CAL and ARS treatments (data = mean ± SE; n = 6). The dotted line indicates acceptable (score ≥2) mark quality. Both blue and UV wavelengths were used to detect CAL marks and both blue and green wavelengths were used to detect ARS marks
Photomicrographs of grass carps’ sagittae after the 100-day growth experiment. Fish were treated with CAL and ARS at concentrations of 150 mg/l (a–c) and 250 mg/l (d–f), respectively. Transmitted light (a, d); blue light (b, f); UV light (c); green light (e). An unmarked sagittae from the control treatment is shown under green light (g), blue light (h) and UV light (i). No fluorescent mark was observed under transmitted light (arrow symbols show the locations of the fluorescent mark)
All concentrations of CAL and ARS produced marks in all sagittae (Fig. 2). Acceptable marks (score ≥2) in sagittae were produced using concentrations of ≥150 mg/l CAL and ≥200 mg/l ARS for 24 h. In this study, no visible marks were detected with the naked eye in sagittae (3≥ score) for any of the CAL or ARS treatments (Fig. 2).
With the exception of non-lateral line scales from the 150 mg/l ARS treatment group and lateral line scales from the 150, 200 mg/l ARS treatment groups, ring marks were observed on all scales (including non-lateral line and lateral line scales) from both CAL- and ARS-treated fish (Fig. 4). However, the marks were poorly visible (score 0–1) in all non-lateral line and lateral line scales from ARS groups (Fig. 4). In contrast, acceptable fluorescent marks (score 2–3) were detected in non-lateral line and lateral line scales at concentrations of 150–200 mg/l CAL (Figs. 4, 5). The mark quality of the scales subjected to CAL treatments was much greater than those treated with ARS at the same concentration (e.g., 150 and 200 mg/l treatments) (Fig. 4). In addition, even the best quality marks in scales were invisible to the naked eye under transmitted light in this study (Fig. 5).
Variation in mark quality of the grass carps’ non-lateral line (a) and lateral line scales (b) subjected to CAL and ARS treatments. No marks were detected in the control groups in the CAL and ARS treatments (data = mean ± SE; n = 6). The dotted lines indicate acceptable (score ≥2) mark quality. Both blue and UV wavelengths were used to detect CAL marks and both blue and green wavelengths were used to detect ARS marks
The mark quality of the fin rays (dorsal, pectoral, ventral, anal, and caudal) was acceptable (score ≥2) at concentrations of 100–200 mg/l CAL and approximately 150–300 mg/l ARS (with the exception of the pectoral and ventral fin rays from the 150 mg/l ARS treatment) (Fig. 6). Visible fluorescent marks (score ≥4) in fin rays were not produced by any of the CAL or ARS treatments (Fig. 7).
Variation in the mark quality in grass carps’ dorsal (a), pectoral (b), ventral (c), anal (d), and caudal (e) fin rays subjected to CAL and ARS treatments. No marks were detected in the control groups in the CAL and ARS treatments (data = mean ± SE; n = 6). The dotted lines indicate acceptable (score ≥2) mark quality. Both blue and UV wavelengths were used to detect CAL marks and both blue and green wavelengths were used to detect ARS marks
Photomicrographs of grass carps’ caudal fin rays after the 100-day growth experiment. Fish were treated with CAL and ARS at concentrations of 100 mg/l (a–c) and 200 mg/l (d–f), respectively. Transmitted light (a, d); blue light (b, f); UV light (c); green light (e). An unmarked caudal fin ray from the control treatment is shown under green light (g), blue light (h) and UV light (i). No fluorescent mark was observed under transmitted light
In the present study, immersion marking of juvenile grass carp was successful in both CAL and ARS treatments, and mark quality approximately increased with increased concentrations of immersion solution (Figs. 2, 4, 6). The sagittae from the 300 mg/l ARS treatment and fin rays (dorsal, pectoral, ventral, anal, and caudal) from the 100–200 mg/l CAL and 250–300 mg/l ARS treatments exhibited optimal visible marks (score = 3). Moreover, fluorescent marks that were visible to the naked eye (score ≥4), were not produced by any of the CAL or ARS treatments in sagittae, scales, or fin rays during this experiment.
Discussion
Immersion marking application
Labeling with fluorochrome dyes is a widespread technique, which can be applied to eggs, larvae, juveniles, and adults [17, 18, 34], that can be used to evaluate stock enhancement or to validate age and growth of fish [26, 35]. Methods of marking include injection, feeding, immersion, and osmotic induction [13, 22–24, 36]. The marking application includes double and triple labeling by a method to regulate a multiple label and double-labeled fish size, even with one or more types of fluorochrome dyes [17, 18, 34]. For example, Tsukamoto [18] already tested a double (with ALC and TC) mark for otlith tagging of Ayu Plecoglossus altivelis. Immersion marking with fluorochrome dyes involves a compromise between cost, concentration, immersion time, salinity, mortality, growing conditions, and mark retention to produce the best mark [19]. In the present study, juvenile grass carp were successfully marked by immersion in 50–200 mg/l CAL or 150–300 mg/l ARS for 24 h. With the exception of non-lateral line scales from the 150 mg/l ARS treatment group and lateral line scales from the 150, 200 mg/l ARS treatment groups, fluorescent marks were observed in all treatments and in all body structures analyzed. Our results further agree with other similar studies demonstrating that CAL and ARS are effective chemical markers for immersion marking which can be used in biological research and for the evaluation of stock enhancement programs (e.g., Baer and Rösch [27]; Leips et al. [30]; Walt and Faragher [32]).
In our experiments, there was no mortality during both the 24-h immersion period and the 72-h post-immersion period. The results of the 100-day growth experiments demonstrated that a 24-h immersion in either 50–200 mg/l CAL or 150–300 mg/l ARS does not affect the growth and survival of marked grass carp. These findings are in accordance with studies involving CAL and ARS marking in other fish species (e.g., Lü et al. [26]; Bashey [37]). Nevertheless, Beckman and Schulz [33] observed that much higher concentrations of dyes (e.g., ARS and ALC) and longer immersion times can result in excessive mortalities during or after immersion and even reduce mark success.
Ever since it was reported that chemicals (e.g., CAL, TC, OTC, ARS, ALC) were available for immersion marking fish, chemical marks have been used successfully in immersion marking experiments with many fish species including turbot Scophthalmus maximus [21], whitefish Coregonus lavaretus [31], mulloway Argyrosomus japonicus [13], and black rockfish [11]. It has also been demonstrated that this method is suitable for application to large numbers of fish, that handling stress is minimized, and that the marking process and recovery are simple procedures [38]. In general, chemical batch marking by immersion has provided a convenient method for large-scale marking of fish [39]. However, there are some general limitations which should not be ignored. The chemical residues from fluorescent dyes in fish tissues may impact food safety [28]. Hence, Liu et al. [19] suggested that it is essential to quantify fluorescent dye residues in tissues for evaluation of chemical marking methods rather than evaluating the mortality and growth potential of marked fish alone. Concerning this issue, no fish was permitted to be released into public waters in the US without prior approval of the Food and Drug Administration (US-FDA) [32]. Up to now, CAL has been approved by the US-FDA as an INADA (Investigative New Animal Drug Application) [40], however, there is no reference on detecting ARS residues. The use of fluorescent dyes for immersion marking fish is often limited by an inability to produce marks for identification of individuals or to distinguish stocks from different hatcheries, even when undertaking double chemical marking [26]. Therefore, the use of the double or triple fluorescent labeling is required in some cases (e.g., [17]). In addition, Purcell et al. [40] noted that fluorescent marks could only show the recapture information rather than any changes in fish behavior, physiology, or ecology in the wild. Therefore, it appears that a combination of chemical marks and other tagging methods (e.g., fin clipping, T-bar, and genetic tags) may be more suitable for some experimental designs.
Mark quality
Immersion marking fish with fluorochrome dyes is widely used to evaluate stock enhancement programs (e.g., Barker and Mckaye, [12]; Lagardère et al. [21]); however, the dye concentrations and immersion times that yield optimal results differs between species [30]. In this study, the methods of mark detection were similar in the sagittae, scales (including non-lateral line and lateral line scales), and fin rays. In addition, as lapillus of juvenile grass carp were too small to visible and difficultly sampled, lapillus was not used to detect mark quality in this study. Compared to the marks with poor visibility (score 0–1) in all non-lateral line and lateral line scales from ARS groups, acceptable marks (score ≥2) were produced by comparatively high concentrations of CAL dyes in sagittae, non-lateral line scales, lateral line scales, and fin rays and by comparatively high concentrations of ARS dyes in sagittae and fin rays. In other words, the mark quality of non-lateral line and lateral line scales subjected to CAL treatments was much better than those treated with ARS at the same concentration. These results may be attributed to much better penetration of CAL than ARS in freshwater environment [37], which allows the fluorochrome dye solution to increase the contact possibility and area between the dye and scale, thereby enhancing mark quality. Our experiments are the first to compare the use of CAL and ARS for immersion marking grass carp juveniles, and the results support the suitability of using CAL and ARS as chemical markers for easy and fast mass-marking of grass carp.
In the current study, no visible marks were observed under normal light in any of the body structures analyzed from any of the treatment groups. In contrast to these results, Liu et al. [19] and Lü et al. [26] have reported that ARS marks were visible to the naked eye under normal light in the sagittae of Japanese flounder and black rockfish treated with 200–400 and 200–500 mg/l ARS, respectively. Liu et al. [19] suggested that high dosage and 24 h immersion times were essential to form visible otolith mark. Lü et al. [26] also suggested that visible otolith marks under normal light may have been due to the saline water environment and stable pH during ARS immersion marking, resulting in better penetration of the dye and increased mark quality. Therefore, the lack of visible marks under normal light in the current study may be a result of species differences or differences between the freshwater and saltwater environments.
According to earlier studies, there are two major limitations to detecting marks in sagittae compared with marks in scales and fin rays [27, 40]. In our study, fish were sampled 100 days after marking at a TL < 100 mm, thus, it was not necessary to grind the sagittae. Hence, all samples were analyzed in a relatively short time. However, Baer and Rösch [27] reported that when sagittae of marked fish larger than a certain size (i.e., when sagittae marks are not externally visible) are to be analyzed, they should be ground and polished to expose the nucleus. In addition, the major disadvantage of the analysis of sagittae marks is that marked fish must be sacrificed [40]. For these reasons, the analysis of marks in scales and fin rays may be more suitable methods of mark detection in grass carp as the sampling is less time-consuming and is non-lethal.
Mark retention
In general, the successful use of marking methods for assessing stock enhancement depends on high retention of the mark and low mortality during marking procedures and after release [41]. Retention of fluorescent marks in the present investigation exceeded a period of 100 days after marking. Similar results have been reported by Crook et al. [24], Lü et al. [26], and Bashey [37], and these studies suggested that this retention period may be even longer. For example, it has been reported that CAL and ARS marks in sagittae can persist for up to 12 months and at least 8 months, respectively [31, 42]. Therefore, it appears that mark retention will not be an issue in grass carp marked with CAL or ARS. In addition, the observed differences in retention time may be attributed to individual metabolic differences or mark detection techniques (e.g., grinding and polishing methods) [19]. Bashey [37] also suggested that the differences in mark retention may be due to different methods of mark detection or related to differences among taxa or environments (e.g., high water temperature and direct sunshine).
In conclusion, the results of the present study suggest that both CAL and ARS immersion are suitable for mass-marking juvenile grass carp. Acceptable marks in sagittae were observed at concentrations of 150–200 mg/l CAL or 200–300 mg/l ARS. Fluorescent marks were poorly visible (score 0–1) in all non-lateral line and lateral line scales from ARS-treated groups. Acceptable fluorescent marks were detected in non-lateral line and lateral line scales at 150–200 mg/l CAL, and in fin rays at 100–200 mg/l CAL or approximately 150–300 mg/l ARS. In particular, optimal marks (score = 3) were observed at comparatively high concentrations investigated in sagittae (300 mg/l ARS) and fin rays (100–200 mg/l CAL or 250–300 mg/l ARS). In addition, marks in lateral line scales, non-lateral line scales, and fin rays allow non-lethal detection of mark information. We encourage the use of CAL and ARS as an economical method of immersion-marking large numbers of hatchery-produced grass carp juveniles. In further research, it is essential to quantify CAL and ARS residues in grass carp tissues for evaluation of chemical marking methods. Moreover, a combination of chemical marks (e.g., CAL and ARS marks) and other tagging methods (e.g., fin clipping, T-bar, and genetic tags) could be experimentally conducted to assess the success of stock enhancement programs of grass carp in the field.
References
Zhong Y, Power G (1997) Fisheries in China: progress, problems, and prospects. Can J Fish Aquat Sci 54(1):224–238
Jagdish M, Rana SVS, Agarwal VP (1995) Efficacy of grass carp (Ctenopharyngodon idella) in weed control and its growth in Karna Lake (Haryana). J Inland Fish Soc India 27:49–55
Leslie AJJ, Van Dyke JM, Hestand RS III, Thompson BZ (1987) Management of aquatic plants in multi-use lakes with grass carp (Ctenopharyngodon idella). Lake Reserv Manag 3:266–276
Irons KS, Sass GG, McClelland MA, Stafford JD (2007) Reduced condition factor of two nativefish species coincident with invasion of non-native Asian carps in the Illinois River, USA. Is this evidence for competition and reduced fitness? J Fish Biol 71(sd):258–273
Li YK, Chen Y, Song B, Olson D, Yu N, Chen LQ (2009) Ecosystem structure and functioning of Lake Taihu (China) and the impacts of fishing. Fish Res 95(2):309–324
Mchich R, Charouki N, Auger P, Raïssi N, Ettahiri O (2006) Optimal spatial distribution of the fishing effort in a multi fishing zone model. Ecol Model 197(3–4):274–280
Zhang G, Cao W, Chen Y (1997) Effects of fish stocking on lake ecosystems in China. Acta Hydrobiol Sin 21(3):271–280
Jia PQ, Zhang WB, Liu QG (2013) Lake fisheries in China: challenges and opportunities. Fish Res 140:66–72
MAFBC M.o.A.a.F.B.o.C. (2013) 2012 China Fishery Statistical Yearbook. China Agriculture Press, Peking (in Chinese)
Zhang M, Xie CX, Hansson LA, Hu WM, Che JP (2012) Trophic level changes of fishery catches in Lake Chaohu, Anhui Province, China: trends and causes. Fish Res 131–133:15–20
Lü HJ, Zhang XM, Fu M, Xi D, Gao TX (2014) Use of tetracycline hydrochloride and alizarin complexone for immersion marking black rockfish, Sebastes schlegelii. Chin J Oceanol Limnol 32(4):810–820
Barker JM, McKaye KR (2004) Immersion marking of juvenile midas Cichlids with oxytetracycline. N Am J Fish Manag 24(1):262–269
Taylor MD, Fielder DS, Suthers IM (2005) Batch marking of otoliths and fin spines to assess the stock enhancement of Argyrosomus japonicus. J Fish Biol 66(4):1149–1162
Lv HJ, Zhang XM, Zhang PD, Li WT, Miao ZQ (2011) The implement of plastic oval tags for mark-recapture in juvenile Japanese flounder, Paralichthys olivaceus, on the northeast coast of Shandong Province, China. Afr J Biotechnol 10(61):13263–13277
Hagen P, Munk K, Van Alen B, White B (1995) Thermal mark technology for inseason fisheries management: a case study. Alaska Fish Res Bull 2(2):143–155
Volk EC, Schroder SL, Grimm JJ (1999) Otolith thermal marking. Fish Res 43(1):205–219
Tsukamoto K (1985) Mass-marking of ayu eggs and larvae by tetracycline-tagging of otoliths. Nippon Suisan Gakkaishi 51(6):903–911
Tsukamoto K (1988) Otlith tagging of ayu embryo with fluorescent substances. Nippon Suisan Gakkaishi 54(8):1289–1295
Liu Q, Zhang XM, Zhang PD, Nwafili SA (2009) The use of alizarin red S and alizarin complexone for immersion marking Japanese flounder Paralichthys olivaceus (T.). Fish Res 98(1):67–74
Gelsleichter J, Cortés E, Manire CA, Hueter RE, Musick JA (1997) Use of calcein as a fluorescent marker for elasmobranch vertebral cartilage. Trans Am Fish Soc 126(5):862–865
Lagardère F, Thibaudeau K, Anras MLB (2000) Feasibility of otolith markings in large juvenile turbot, Scophthalmus maximus, using immersion in alizarin-red S solutions. ICES J Mar Sci 57(4):1175–1181
Monaghan JP Jr (1993) Comparison of calcein and tetracycline as chemical markers in summer flounder. Trans Am Fish Soc 122(2):298–301
Honeyfield DC, Ostrowski CS, Fletcher JW, Mohler JW (2006) Dietary calcein marking of brook trout, Atlantic salmon, yellow perch, and coho salmon scales. N Am J Fish Manag 26(2):431–437
Crook DA, O’Mahony D, Gillanders BM, Munro AR, Sanger AC (2007) Production of external fluorescent marks on Golden Perch fingerlings through osmotic induction marking with Alizarin Red S. N Am J Fish Manag 27:670–675
Crook DA, O’Mahony D, Sanger AC, Munro AR, Gillanders BM (2009) Development and evaluation of methods for osmotic induction marking of Golden Perch Macquaria ambigua with Calcein and Alizarin Red S. N Am J Fish Manag 29:279–287
Lü HJ, Zhang XM, Xi D, Gao TX (2014) Use of calcein and alizarin red S for immersion marking of black rockfish Sebastes schlegelii juveniles. Chin J Oceanol Limnol 32(1):88–98
Baer J, Rösch R (2008) Mass-marking of brown trout (Salmo trutta L.) larvae by alizarin: method and evaluation of stocking. J Appl Ichthyol 24(1):44–49
Brown ML, Powell JL, Lucchesi DO (2002) In-transit oxytetracycline marking, nonlethal mark detection, and tissue residue depletion in yellow perch. N Am J Fish Manag 22(1):236–242
Oliveira K (1996) Field validation of annular growth rings in the American eel, Anguilla rostrata, using tetracycline-marked otoliths. Fish Bull 94(1):186–189
Leips J, Baril CT, Rodd FH, Reznick DN, Bashey F, Visser GJ, Travis J (2001) The suitability of calcein to mark poeciliid fish and a new method of detection. Trans Am Fish Soc 130(3):501–507
Eckmann R (2003) Alizarin marking of whitefish, Coregonus lavaretus otoliths during egg incubation. Fish Manag Ecol 10(4):233–239
Walt BVD, Faragher RA (2003) Otolith marking of rainbow trout fry by immersion in low concentrations of alizarin complexone. N Am J Fish Manag 23(1):141–148
Beckman DW, Schulz RG (1996) A simple method for marking fish otoliths with alizarin compounds. Trans Am Fish Soc 125(1):146–149
Tsukamoto K, Kuwada H, Hirokawa J, Oya M, Sekiya S, Fujimoto H, Imaizumi K (1989) Size-dependent mortality of red sea bream, Pagrus major, juveniles released with fluorescent otolith-tags in News Bay, Japan. J Fish Biol 35(Supplement A):59–69
Yamashita Y, Nagahora S, Yamada H, Kitagawa D (1994) Effects of release size on survival and growth of Japanese flounder Paralichtys olivaceus in coastal waters off Iwate Prefecture, northeastern Japan. Mar Ecol Prog Ser 105:269–276
Ibáñez AL, Rodríguez-Canto A, Cortés-Martínez J, García-Calderón JL (2013) Evaluation of marking efficiency of different alizarin red S concentrations on body fish structures in Oreochromis niloticus (Perciformes: Cichlidae) juveniles. Int J Trop Biol Conserv 61(1):193–201
Bashey F (2004) A comparison of the suitability of alizarin red S and calcein for inducing a nonlethally detectable mark in juvenile guppies. Trans Am Fish Soc 133(6):1516–1523
Skov C, Grønkjær P, Nielsen C (2001) Marking pike fry otoliths with alizarin complexone and strontium: an evaluation of methods. J Fish Biol 59(3):745–750
Mohler JW (2003) Producing fluorescent marks on Atlantic salmon fin rays and scales with calcein via osmotic induction. N Am J Fish Manag 23(4):1108–1113
Purcell SW, Blockmans BF, Nash WJ (2006) Efficacy of chemical markers and physical tags for large-scale release of an exploited holothurians. J Exp Mar Biol Ecol 334(2):283–293
Simon J, Dörner H (2005) Marking the European eel with oxytetracycline, alizarin red and coded wire tags: an evaluation of methods. J Fish Biol 67(5):1486–1491
Frenkel V, Kindschi G, Zohar Y (2002) Noninvasive, mass marking of fish by immersion in calcein: evaluation of fish size and ultrasound exposure on mark endurance. Aquaculture 214(1–4):169–183
Acknowledgments
This work was supported by ther National Natural Science Foundation of China (No. 31400396) and the Fundamental Research Funds for the Central Universities (XDJK2014C019 and SWU113056). We wish to express our thanks to Prof. Weizhi Yao, Shengqi Su, and Zhengli Wu for their suggestions on the manuscript and participation in many aspects of this study. We also thank the three anonymous reviewers for their valuable and constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lü, H., Chen, H., Fu, M. et al. Experimental evaluation of calcein and alizarin red S for immersion marking grass carp Ctenopharyngodon idellus . Fish Sci 81, 653–662 (2015). https://doi.org/10.1007/s12562-015-0884-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-015-0884-5