Abstract
The effects of water temperature (15, 20, 25, 30, and 35 °C) on survival, growth performance, digestive enzyme activities, and body composition of Plectropomus leopardus were studied for a period of 6 weeks. One hundred eighty fish with initial body weights of 26.5 ± 1.5 g were randomly arranged into 15 glass aquaria in equal numbers in five recirculating systems to form five groups in triplicate. The results showed that survival of P. leopardus at 35 °C was significantly greater (P < 0.05) than survival at 15 °C. No death was recorded at 20, 25, and 30 °C. Among all treatment groups, the significantly highest average individual harvesting weight, weight gain, feed ingestion rate and protease enzyme activity of P. leopardus were observed in 30 °C group. Similar results were also observed in protein and fat content in this species. Based on the present findings, a culture temperature of 30 °C can be considered to be the optimum temperature for the aquaculture of juvenile P. leopardus. However, more research is still needed to optimize the nutrition and photoperiod of P. leopardus culture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Temperature is one of the most important critical factors that directly affects the growth, food intake, food efficiency, oxygen consumption, survival, and reproduction of all animals, including fish [1, 2]. From an aquaculture perspective, identifying the optimum water temperature is crucial for maximizing growth and nutrient retention, and decreasing feed wastage [3–5]. Optimum temperature in culture systems results in better survival, growth and production of fish [6–8].
Plectropomus leopardus is one of the target fish for all sectors of the fishery because of their high commercial value in China and many other countries in the world [9]. It has been greatly overexploited due to high demand and market price. Presently, it is listed as an endangered species [9]. Depletion of natural stocks, together with the high commercial value has encouraged stock enhancement and aquaculture of this species [10]. Successful hatching of P. leopardus juveniles in 2009 in China facilitated stock enhancement and aquaculture of this species. Although leopard coral grouper is a potential candidate for aquaculture, very little is known about the culture of this species. To date, studies of P. leopardus have mainly focused on their ecology, embryonic development, early life history, molecular classification, and behavior [10–12]. However, to optimize culture conditions it is important to know the optimum water temperature for the culture of this species. Unfortunately, there is no published information regarding optimum water temperature for the culture of this fish. Therefore, this study is designed to understand the effects of different water temperatures on survival, growth performance, digestive enzyme activities, and body composition of the leopard coral grouper.
Materials and methods
Experimental design, fish stocking and management
A 6-week experiment was carried out in 15 glass aquaria (each 80 × 40 × 40 cm) in the Tianjin Fisheries Research Institute, Tianjin, People’s Republic of China. Five water temperatures (15, 20, 25, 30 and 35 °C) were assigned randomly in triplicate. The temperature variation within a treatment group was less than ± 0.5 °C. The P. leopardus were purchased from the Tianjin Shengyi Aquatic Company located in Tianjin City, People’s Republic of China, stocked in experimental tanks, and then allowed to acclimate for 3 days prior to the experiment. The stocking density of each aquarium was 12 leopard coral groupers. All fish were a similar size, with an average initial individual weight of 26.5 ± 1.5 g. The acclimatization temperature, salinity and oxygen concentration of the water were 26 °C, 28–30 g l−1 and 90–95 % saturation, respectively. After acclimation, three tanks were maintained at 25 °C, whereas in the other treatments the temperature was gradually increased or decreased by 2–3 °C day−1 until the test temperature was attained. When all of the temperatures reached the designated level, the initial mass per tank was assessed by weighing all fish after feeding was stopped for 24 h. From then on, constant temperature was maintained until the end of the experiment.
During the experiment, aeration was provided continuously, and mean water quality parameters were maintained as follows: salinity 30 ± 2 g l−1 and dissolved oxygen > 5 mg l−1. All fishes were hand-fed with extruded dry pellet (crude protein ≥ 48 %; crude lipid ≥ 12 %; crude fiber ≤ 2 %; ash ≤ 16 %; Ca ≥ 2.3 %; P ≥ 1.7 %) at 0900 and 1500 h. Uneaten feed and feces were siphoned gently from the aquaria every day 2 h after feed application. The uneaten feed was dried at 60 °C to a constant weight to calculate feed consumption. All aquaria were drained at the end of the experiment and fish were harvested and weighed. Survival rate (SR), weight gain (WG), and ingestion rate (IR) were calculated using the following equations:
where N 1 is the initial number and N 2 is the final number of P. leopardus in each aquarium; W 1 and W 2 are initial and final body weights (g) of the individuals in each aquarium, respectively; T the duration of the experiment (days); C the dry weight of feed consumed (g); and N is the number of this species in each aquarium on a given day.
Quantification of digestive enzyme activities
In the experiment, national guidelines for treating laboratory animals in China were followed. Three fishes were selected from each aquarium at the end of the experiment for enzyme activity studies. The selected fishes were anesthetized using clove oil according to Burka et al. [13] and then killed. The intestine of each fish was removed carefully and weighed. Both the intestine and the body wall of P. leopardus were stored at −80 °C for further analysis. The samples from each tank were mixed together as one sample to be analyzed. To analyze digestive enzyme activities, the intestine was homogenized with deionized water 9 times on ice. The samples (homogenate) were centrifuged (4,472g) at 4 °C for 30 min. The supernatant solution was separated from the top lipid layer and divided into subsamples for different enzyme activity tests. All samples were analyzed within 12 h. The total soluble protein content of the crude extract was determined using bovine serum albumin as the standard [14].
Protease activity
The protease activity of each crude extract was determined using casein as a substrate. A mixture of 0.6 ml of crude extract, 0.8 ml of sodium citrate–hydrochloric acid buffer solution (100 mM, pH 7.5), 0.1 ml of 0.04 M EDTA–Na2, and 2 ml of 0.5 % casein was incubated at 37 °C for 30 min. The reaction was stopped with the addition of 1 ml of 30 % trichloroacetic acid, and the mixture was clarified by centrifugation (1,118g for 15 min) [15]. A mixture of l ml of the super solid, 5 ml of 0.55 M Na2CO3, and l ml of Folin reagent was incubated at 37 °C for 15 min. Absorbance was measured using a spectrophotometer (Spectronic 723 N, Shanghai Analytical Instrument Co., Ltd., Shanghai, People’s Republic of China) at 680 nm wavelength. An additional negative control was generated adding 1 ml of trichloroacetic acid (30 %) before adding crude extract. A standard curve of absorbance at 440 nm was established using tyrosine as the standard. One unit of protease activity (U) was defined as the number of micrograms of tyrosine released per minute per gram of protein at 37 °C.
Amylase activity
The amylase activity of each crude extract was determined with 1 ml of 1 % starch solution and 1 ml of phosphate buffer (100 mM, pH 7.5) as the substrate. After adding 0.5 ml of crude extract to 2 ml of substrate, the mixture was incubated at 37 °C for 8 min in a temperature-controlled water bath. Amylase activity was determined by measuring the production of maltose resulting from starch hydrolysis. Maltose production was estimated by reading the color intensity at 550 nm [16]. A negative control was generated by replacing crude extract with boiled crude extract. A standard curve of absorbance at 550 nm was established using a standard maltose solution. One unit of amylase activity (U) was defined as the number of micrograms of maltose released per minute per gram of protein at 37 °C.
Lipase activity
Lipase activity was assayed using a kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, People’s Republic of China). The principle of the assay is as follows: trireactive glyceride and water become an emulsoid, which absorbs and scatters the incident rays as they pass through the mixture. The turbidity decreases when the emulsoid splits as the trireactive glyceride is hydrolyzed with the help of lipase, and the speed is proportional to the activity of lipase in the sample. One unit of lipase activity (U) was defined as the micromole number of substrate released per minute per gram of lipid at 37 °C.
Chemical analysis of feed and animal samples
Chemical analyses of the experimental animal samples were conducted at the end of the experiment. Dry matter was assessed after drying samples at 65 °C until a constant weight, and ash content was determined following combustion at 550 °C for 4 h. Total nitrogen content was estimated following the Dumas method using a Hanon K980 nitrogen/protein determinator (Hanon Group Inc., Jinan City, Shandong Province, People’s Republic of China). Crude protein content was determined indirectly (nitrogen × 6.25) [17]. Crude lipid was measured by the diethyl ether extraction method using a Hanon SOX406 fat analyzer (Hanon Group Inc., Jinan city, Shandong Province, People’s Republic of China).
Statistical analysis
Growth performance, digestive enzyme activities, and body composition data were subjected to one-way analysis of variance (ANOVA) followed by Duncan’s multiple comparison analysis to determine if there were any significant differences (P < 0.05) among treatment means [18–20]. All data were initially examined for normality and homogeneity of variance. Survival data were arcsine transformed before analysis. All statistical analyses were performed using SPSS software 15.0 for Windows.
Results
Growth performance
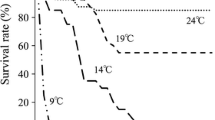
Temperature significantly (P < 0.05) affected the average individual harvesting weight, average individual WG, survival and IR of leopard coral grouper (Table 1). Average individual harvesting weight of P. leopardus was highest at 30 °C, followed by 25, 20, 15 and 35 °C. The weight of fish at the end of the experiment decreased in the 15 and 35 °C groups. Highest average individual WG of leopard coral grouper was observed at 30 °C, followed by 20 and 15 °C. The species ingested the highest quantity of feed at 30 °C, followed by 25, 35, 20 and 15 °C. Survival of the individuals at 35 °C was significantly greater (P < 0.05) than the survival at 15 °C. No death was recorded at 20, 25, and 30 °C.
Digestive enzyme activities
Table 2 presents the digestive enzyme activities of P. leopardus reared at different temperatures. Culture temperature had significant effects (P < 0.05) on protease and amylase activities of P. leopardus. Protease activity of this fish at 30 °C was greater than that at 15 °C (P < 0.05). Amylase activity of P. leopardus was observed to be highest at 20 °C, and lowest at 35 °C. Lipase activity was statistically the same (P > 0.05) among fish cultured at different temperatures. A significant positive correlation (r = 0.667; P < 0.01) was observed between protease activity and feed IR in leopard coral grouper (Fig. 1).
Body composition
Culture temperature significantly affected the body composition of P. leopardus (Table 3). The effects of different temperatures on protein and fat content showed a similar trend in the leopard coral grouper at the end of the experiment. Protein and fat contents of P. leopardus were highest at 30 °C among all temperatures tested for culturing this fish. An almost opposite result was observed in the case of water and ash content of the leopard coral grouper. At the end of the experiment, water content in the leopard coral grouper was highest at 15 °C, followed by 35, 20, 25 and 30 °C. Ash content in P. leopardus was highest at 35 °C, followed by 15, 20, 25 and 30 °C.
Discussion
Water temperature is one of the most important environmental factors affecting the life cycle of fish [3]. Temperature can affect enzyme activity, metabolism, feed intake, growth, production, and survival [8]. This study provides experimental evidence of changing protease activity, feed IR, WG and survival of leopard coral grouper with temperature. In the current study, some fish died at 15 and 35 °C. This indicates that 15 and 35 °C are not suitable for the aquaculture of this species. These results in a way agree with Zhang et al. [21] who observed that P. leopardus could not hatch when the temperature was below 18 °C. Their hatching and larval survival decreased significantly when the temperature was above 32 °C. Fish show an increase in growth and feeding, metabolic rate, and food digestion under an optimal culture temperature, but these functions decline if the temperature exceeds the optimal temperature. In the present study, harvesting weight, WG, survival and feed IR of leopard coral grouper were highest at 30 °C [4]. These results clearly indicated that the optimum temperature for the aquaculture of P. leopardus was 30 °C for 27.76 ± 0.44 ~ 40.85 ± 0.91 g fish. However, 30 °C might not be suitable for early stages of the fish, especially for larval fish. More research is needed to optimize the temperature for early stages of P. leopardus. There is no previous study comparing the effects of temperature on WG, survival and feed IR of P. leopardus. However, Zhang et al. [22] studied the effects of temperature on a different species of grouper Epinephelus coioides and observed that the optimal temperature ranged from 24 to 26 °C. In another study, Qu et al. [23] found that the optimal temperature for the grouper Epinephelus lanceolatus ranged from 26 to 30 °C.
In our study, the highest feed IR and WG of P. leopardus at 30 °C was further supported by the protease activity, which was also highest at 30 °C. According to Rungruangsak-Torrissen et al. [24] and Sunde [25], temperature is one of the most important factors that affect protease activity in fish, which is generally highest at the optimum temperature. Protease activity is the measure of protein digestion, which is a very important limiting factor of growth rate as well as feed utilization in fish [23, 26, 27]. In the present study, the fact that protein digestion can be measured by protease activity was further supported by the significant positive correlation between protease activity and feed IR in this species. Therefore, highest protease activity resulted in highest protein digestion, metabolism and assimilation, which in turn increased feed IR and WG in P. leopardus. However, feed ingestion was decreased at 35 °C because fish needed to use more energy to adapt to the environment. This resulted in lower nutrient retention and growth in P. leopardus, which also occurred in the 15 °C group. This concurs with Li et al. [28], who reported that the protease activity in the stomach of turbot increased when temperature increased from 15 to 18 °C and then decreased significantly in fish reared at 21 °C. Temperature accelerates the metabolic rate of the body to increase protein synthesis by accelerating the secretion of protease. However, this positive correlation is not infinite. When water temperature exceeds the optimum value, the activity of gastric mucosa and protease secretion are significantly reduced, which in turn reduces the protein metabolism and food IR. High temperature also alters the ion concentration and pH, which inhibit digestive enzyme activity and normal metabolism in fish [28].
It is interesting to note that body composition of P. leopardus was significantly affected by culture temperature. The protein and lipid content in the leopard coral grouper body increased when the temperature increased from 15 to 30 °C, but they decreased at 35 °C. This result concurs with Cui and Wootton [29], who observed that the protein content of the minnow Phoxinus phoxinus increased first and then decreased as water temperature increased. This might be because temperature within an appropriate range can increase the feed intake, allowing more protein and lipid to be retained in the body. When the temperature was too high, however, the IR was reduced and more nutrients were used for increased metabolism, resulting in a decline in lipid and protein content.
Based on the present findings, a culture temperature of 30 °C can be proposed for the aquaculture of P. leopardus. More research is still needed to optimize the nutrition (especially protein and lipid contents in feed) and photoperiod of the species.
References
Qu M, Ding S, Xu X, Shen M, You Y, Su Y (2012) Ontogenetic development of the digestive system and growth in coral trout (Plectropomus leopardus). Aquaculture 334–337:132–141
Masuma S, Tezuka N, Teruya K (1993) Embryonic and morphological development of larval and juvenile coral trout, Plectropomus leopardus. Jpn J Ichthyol 40:333–342
Martinez-Palacios CA, Tovar EB, Taylor JF, Duran GR, Ross LG (2002) Effect of temperature on growth and survival of Chirostoma estor estor, Jordan 1879, monitored using a simple video technique for remote measurement of length and mass of larval and juvenile fishes. Aquaculture 209:369–377
Ye L, Yang S, Zhu X, Liu M, Lin J, Wu K (2011) Effects of temperature on survival, development, growth and feeding of larvae of yellowtail clownfish Amphiprion clarkii (Pisces: Perciformes). Acta Ecol Sin 31:241–245 (in Chinese with English abstract)
Handeland SO, Imsland AK, Stefansson SO (2008) The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 283:36–42
Choa BY, Carter CG, Battaglene SC (2010) Effects of temperature regime on growth and development of post-larval striped trumpeter (Latris lineata). Aquaculture 305:95–101
Rahman MM, Verdegem MJ (2010) Effects of intra- and interspecific competition on diet, growth and behaviour of Labeo calbasu (Hamilton) and Cirrhinus cirrhosus (Bloch). Appl Anim Behav Sci 128:103–108
Tsuji M, Abe H, Hanyuu K, Kuriyama I, Tsuchihashi Y, Tsumoto K, Nigou T, Kasuya T, Katou T, Kawamura T, Okada K, Uji S, Sawada Y (2014) Effect of temperature on survival, growth and malformation of cultured larvae and juveniles of the seven-band grouper Epinephelus septemfasciatus. Fish Sci 80:69–81
Ayling RD, Baker SE, Peek ML, Simon AJ, Nicholas RJ (2000) Comparison of in vitro activity of danofloxacin, florfenicol, oxytetracycline, spectinomycin and tilmicosin against recent field isolates of Mycoplasma bovis. Vet Rec 146:745–747
Yang M, Wang Y, Fu S, Shen M, Zheng F, Wang G, Yin S, Li X (2012) Effects of different temperatures and salinities and pH values on the early development of Plectropomus leopardus Lacépède. J Trop Org 3:104–108 (in Chinese with English abstract)
Zhu ZY, Yue GH (2008) The complete mitochondrial genome of red grouper Plectropomus leopardus and its applications in identification of grouper species. Aquaculture 276:44–49
Kenzo Y, Kazuhisa Y, Kimio A, Masayuki C, Koji H, Shinichi K (2008) Influence of light intensity on feeding, growth, and early survival of leopard coral grouper (Plectropomus leopardus) larvae under mass-scale rearing conditions. Aquaculture 279:55–62
Burka JF, Hammell KL, Horsberg TE, Johnson GR, Rainnie DJ, Speare DJ (1997) Drugs in salmonid aquaculture—a review. J Vet Pharmacol Ther 20(5):333–349
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Kunitz M (1947) Crystalline soybean trypsin inhibitor: II. General properties. J Gen Physiol 30:291–310
Rick W, Stegbauer HP (1984) Alfa-amylase: measurement of reducing groups. In: Bergermeyer HU, Grab M (eds) Methods of enzymatic analysis. Enzymes, vol 5, 3rd edn. Chemie Verlag, Weinheim, pp 885–889
Ebling ME (1968) The Dumas method for nitrogen in feeds. J AOAC Int 51:766–770
Rahman MM, Jo Q, Gong YG, Miller SA, Hossain MY (2008) A comparative study of common carp (Cyprinus carpio L.) and calbasu (Labeo calbasu Hamilton) on bottom soil resuspension, water quality, nutrient accumulations, food intake and growth of fish in simulated rohu (Labeo rohita Hamilton) ponds. Aquaculture 285:78–83
Rahman MM, Meyer CG (2009) Effects of food type on diel behaviours of common carp Cyprinus carpio L. in simulated aquaculture pond conditions. J Fish Biol 74:2269–2278
Khatune-Jannat M, Rahman MM, Bashar MA, Hasan MD, Ahamed F, Hossain MY (2012) Effects of stocking density on survival, growth and production of Thai climbing perch (Anabas testudineus) under fed ponds. Sains Malays 41:1205–1210
Zhang Y, Yu D, Huang G (2011) Impacts of ecological factors on hatching of fertilized eggs and survival of larvae of coral grouper Plectropomus leopardus. Guangdong Agric Sci 10:102–105 (in Chinese with English abstract)
Zhang H, Liu X, Wang Y, Liufu Y, Huang G, Luo G, Wang H, Lin H (2006) Effects of temperature, salinity and pH on hatch and larval activity of Epinephelus coioides. J Trop Oceanogr 25:31–36 (in Chinese with English abstract)
Qu H, Li X, He Q, Li Z (2009) Effects of temperature and salinity on hatching rates and larval survival of Epinephelus lanceol. Hebei Fish 8:6–9 (in Chinese with English abstract)
Rungruangsak-Torrissen K, Moss R, Andresen LH, Berg A, Waagbø R (2006) Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon (Salmo salar L.). Fish Physiol Biochem 32:7–23
Sunde J (2006) Digestive protease activities, growth and feed utilisation in Atlantic salmon (Salmo salar L.). PhD thesis, University of Bergen, Norway
Rungruangsak-Torrissen K, Sundby A (2000) Protease activities, plasma free amino acids and insulin at different ages of Atlantic salmon (Salmo salar L.) with genetically different trypsin isozymes. Fish Physiol Biochem 22:337–347
Sunde J, Taranger GL, Rungruangsak-Torrissen K (2001) Digestive protease activities and free amino acids in white muscle as indicators for feed conversion efficiency and growth rate in Atlantic salmon (Salmo salar L.). Fish Physiol Biochem 25:335–345
Li Y, Sun G, Liu Y, Gao T, Yu K, Liu J (2011) Effects of temperature on feed intake, growth and digestive enzyme activity of turbot Scophthalmus maximus L. in high stocking density of closed recirculation aquaculture system. Prog Fish Sci 32:17–24 (in Chinese with English abstract)
Cui Y, Wootton RJ (1988) Effects of ration, temperature and body size on the body composition, energy content and condition of the minnow, Phoxinus phoxinus (L.). J Fish Biol 32:749–764
Acknowledgments
We would like to thank Yanguang Yu, Hongzhen You, and Xueliang Yao for their help in the experiment. In particular, we would also like to thank two anonymous reviewers for professional revision of the manuscript. This research was supported by Tianjin Agricultural Science and Technology Achievement Transformation and Promotion project (No. 201103040), Natural Science Fund of Tianjin (Nos. 14JCQNJC15200, 14ZXNZNC00045, and 11JCYBJC08800), Tianjin Science and Technology Key Program (No. 12ZCZDNC01100), China Spark Program (No. 2011GA610002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Z., Xia, S., Feng, S. et al. Effects of water temperature on survival, growth, digestive enzyme activities, and body composition of the leopard coral grouper Plectropomus leopardus . Fish Sci 81, 107–112 (2015). https://doi.org/10.1007/s12562-014-0832-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-014-0832-9