Abstract
Lipoprotein lipase (LPL) plays a critical role in the metabolism of circulating triacylglycerols (TAGs) packaged in lipoproteins. Fish have two LPL genes, encoding the LPL1 and LPL2 enzymes, but their functions are not yet clearly understood. To provide a basis for further investigation, we cloned a cDNA encoding LPL from the liver of adult medaka Oryzias latipes. The cloned LPL gene was found to share a high identity with LPL1 genes from several fish species and was denoted medaka LPL1. Screening against publicly available medaka expressed sequence tag (EST) databases revealed that LPL1 transcripts are abundant in ESTs from various developmental stages and tissues. The EST screening also identified a second LPL-like gene, denoted medaka LPL2, the transcripts of which were found exclusively in EST libraries from embryonic stages. The LPL1 cDNA obtained by a combination of 3′ and 5′ rapid amplification of cDNA ends was 2,259 bp, with an open reading frame of 1,551 bp encoding 516 amino acids. Quantitative real-time PCR further confirmed the ubiquitous distribution of LPL1 transcripts, with high levels in the liver and visceral adipose tissue, and the lowest level in the intestine. A relatively high expression of the LPL1 gene was also found in the brain, suggesting the potential function of LPL1 in this organ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triacylglycerol (TAG) is the major lipid providing the energy required for metabolic processes in organisms. It is composed of one glycerol molecule esterified with three fatty acids. Due to its poor water solubility, TAG transport in the bloodstream can only occur through the packaging of circulating TAGs in lipoproteins, such as chylomicrons and very low-density lipoproteins [1, 2]. The outer layer of these lipoprotein particles is polar, containing free cholesterols, phospholipids and apolipoproteins, which enables these particles to be transported in the bloodstream [1, 3, 4]. However, due to the large size and water solubility of the lipoprotein particles, they cannot diffuse through plasma membranes. Consequently, partial degradation or receptor-mediated endocytosis is needed for the passage of these lipoproteins and, therefore, TAGs into cells.
Lipoprotein lipase (LPL) is one of the important enzymes involved in the catabolism of circulating lipoproteins. It is responsible for the hydrolysis of TAGs packaged in lipoproteins, providing non-esterified fatty acids to peripheral tissues for either energy storage by re-esterification or energy provision via β-oxidation. LPL is anchored to the luminal surface of blood vessels through its interaction with heparan sulfate proteoglycans and/or glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 [5–8]. In addition to its conventional hydrolytic function, LPL is also known to act as a ligand in the uptake of lipoprotein particles via receptor-mediated endocytosis [9, 10]. In their study with LPL knockout mice, Weinstock et al. noted that a null mutation in LPL resulted in hypertriglyceridemia and a low survival rate of newborn mice [11]. These results demonstrated that LPL plays a vital role in the catabolism of circulating TAG-rich lipoproteins.
cDNAs coding for LPL have been cloned and sequenced in a number of vertebrate animals, such as human, rat, mouse, bovine and fish [12–17]. LPL transcripts are widely distributed in mammalian tissues and organs, with relatively high levels in adipose tissues, heart and skeletal muscles, and small amounts in the kidney, lung and small intestine [18, 19]. Interestingly, LPL is expressed in the liver of newborn mammals, but not in adults [20, 21]. In contrast, LPL is expressed in the liver of adult fish, suggesting its functional diversification among vertebrates [16, 22–25]. Moreover, an LPL isoform, called LPL2, has been recently identified in two fish species, red seabream [24] and torafugu [25]. LPL2 transcripts have a widespread distribution in tissues of red seabream as those of LPL1, but the levels of the former are tenfold lower than those of LPL1 in some tissues, including adipose tissue, liver and muscle, suggesting that LPL2 plays a relatively minor role in these tissues.
Medaka Oryzias latipes is a small fish species with a body length of about 3 cm. Due to its short generation time of 2–3 months, short spawning cycle and high transparency of eggs, it has been widely used as a model vertebrate animal in molecular biological studies, especially in those focusing on transgenic research [26–28]. In the study reported here, we cloned the cDNA of LPL from the liver of adult medaka and investigated the expression pattern for further understanding of fish LPL function in circulating lipoprotein metabolism.
Materials and methods
Experimental fish
Medaka specimens (orange–red strain) were obtained from a local fish market (Ichigaya Fish Center, Tokyo, Japan) and reared in indoor tanks at a constant water temperature of 28 ± 0.5 °C. Fish were fed with commercial diets (Kamihata Fish Industry Group; Kyorin Co. Ltd., Himeji, Japan) twice a day. The body weight of fish used for cloning was approximately 200 mg, while specimens of 315–410 mg were used for the quantitative real-time PCR.
RNA isolation
Total RNAs were isolated from approximately 10 mg of liver tissue using an RNeasy Lipid Tissue Mini kit (Qiagen, Hilden, Germany) and stored at −80 °C until further analysis. The quantity and purity of these total RNAs were determined by absorbance measurements at 260 and 280 nm using a nanophotometer (Implen GmbH, Munich, Germany). The integrity of RNA was assessed by 1 % agarose–formaldehyde denaturing gel electrophoresis.
cDNA cloning of medaka LPL
First-strand cDNA was synthesized using 1 μg of extracted total RNA as template and the GeneRacer Oligo (dT)24 primer with SuperScript™ III Reverse Transcriptase (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions.
For amplification of a partial fragment of the LPL gene, including the start codon and 3′ untranslated region (UTR), we first performed alignment searches against medaka expressed sequence tag (EST) databases (http://www.shigen.nig.ac.jp/medaka/top/top.jsp) using the TBLASTN program. Amino acid (AA) sequences of Siniperca chuatsi LPL (GenBank accession no. ACZ55136), Pagrus major LPL1 and LPL2 (BAE95413 and BAB20997, respectively) and Takifugu rubripes LPL1 and LPL2 (AB735414 and AB735415, respectively) were used as reference sequences. A specific primer, LPL-F (Table 1), was designed based on the EST sequences of medaka LPL using Oligo version 7.37 software (MBI, Cascade, CO).
PCR assays were then carried out, each in a 20-μl volume reaction mixture consisting of approximately 80 ng first-strand cDNA, 0.12 μM LPL-F and GeneRacer 3′ primers and 0.4 U of KOD-Plus-Neo DNA polymerase (Toyobo, Osaka, Japan). The cycling parameters for amplification were determined as recommended by the manufacturer. Nested PCR was carried out by the same method except that tenfold-diluted products of the first PCR were used as a template and the GeneRacer 3′ primer was replaced by the GeneRacer 3′ nested primer.
Amplified DNA fragments were gel purified using a QIAquick Gel Extraction kit (Qiagen) and cloned into the pCR-Blunt II-TOPO vector (Invitrogen) according to the manufacturer’s instructions, followed by the transformation of JM109 competent cells. Purified plasmids were sequenced by an ABI PRISM 3100 genetic analyzer (Applied Biosystems, Foster City, CA).
Rapid amplification of the 5′ end
To obtain the 5′ end, first-strand cDNA was synthesized with the 5′-rapid amplification of cDNA ends (RACE) CDS primer (Table 1) according to the manufacturer’s instructions (SMART™ RACE cDNA Amplification kit; Clontech, BD Biosciences, Palo Alto, CA). The gene-specific primers for amplification of the 5′ end of the cDNA were designed based on the obtained partial sequence of the LPL gene (Table 1). Each PCR assay was carried out a 20-μl volume reaction mixture consisting of approximately 100 ng first-strand cDNA, 0.4 μM LPL-5R primer and nested universal primers (Clontech, BD Biosciences) and 0.5 U Ex Taq DNA polymerase (Takara, Otsu, Japan). After an initial denaturation at 95 °C for 2 min, 35 cycles of PCR were carried out with denaturation at 94 °C for 30 s, annealing at 68 °C for 30 s and extension at 72 °C for 2 min; the final extension step was performed at 72 °C for 7 min. The tenfold-diluted PCR products were used as a template for the second PCR that was carried out under the same conditions except for the primer LPL-5RN. PCR products were cloned into the pCR4-TOPO vector (Invitrogen) and sequenced as described above.
Sequence analyses
The sequence of medaka LPL was obtained through the assembly of the above sequences using SeqMan II software (DNASTAR, Inc., Madison, WI). A multiple alignment of AA sequences was made using Clustal W [29]. The presence of the signal peptide and of potential N-linked glycosylation sites were predicted with SignalP version 3.0 (http://www.cbs.dtu.dk/services/SignalP/) and NetNGlyc version 1.0 (http://www.cbs.dtu.dk/services/NetNGlyc/), respectively. The conserved domains of the LPLs were identified by conserved domain database search (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). The phylogenetic tree based on LPL AA sequences was constructed by the maximum likelihood (ML) method of MEGA software version 5.10 [30] with the JTT + G model, which was determined by the evaluation of best-fit substitution. Bootstrap analysis (10,000 replicates) was used to estimate the branches of the tree.
Real-time PCR assay for LPL transcripts
The expression of LPL at the mRNA level was determined in various tissues of medaka by quantitative real-time PCR. The ribosomal protein L-7 (RPL-7) gene was used as the internal control because of its relatively constant expression in various tissues of medaka [31]. Gene-specific primers and TaqMan probes were designed based on the LPL nucleotide sequence identified in this study, and the sequence of RPL-7 obtained from the GenBank database (DQ118296). Total RNAs were extracted from visceral adipose tissue, brain, gill, heart, intestine, liver and muscle of medaka. First-strand cDNA was synthesized from each tissue sample (n = 10), as described above, except for the GeneRacer Oligo (dT)24 primer substituted by a blend of oligo (dT)18 and random hexamer. The reaction system for real-time PCR was prepared according to the manufacturer’s protocol of the TaqMan Fast Advanced Master Mix kit (Applied Biosystems), followed by running on the ABI 7300 Real Time PCR System (Applied Biosystems). The relative LPL mRNA levels were determined by the comparative C T method.
Statistical analysis
Data were analyzed by one-way analysis of variance, followed by the Tukey–Kramer HSD test using the statistical program JMP version 9 (SAS Institute Inc., Cary, NC).
Results
Identification of medaka LPL isoforms from ESTs
The medaka EST database search revealed several ESTs that shared high identities with the LPL1 and LPL2 genes from other fish species (Table 2). We used the conserved sequence of olvl25a13 and olli49h22 for primer design because these ESTs were obtained from the adult liver, whereas the other ESTs were from fry, embryo and other tissues. However, our LPL sequence was later revealed to be almost identical (>99 %) with that of other ESTs (MF015DA031e09, MF01FSA036n23, olsp54e15, olbr63h04, olova59e14 and olte29l06), suggesting that the LPL gene cloned in this study is the single and major form expressed during various developmental stages and in various tissues of medaka. Taken together with the results of the alignment and phylogenetic analyses (see below), we denoted this gene medaka LPL1. In comparison, ESTs sharing high identities with LPL2 genes were obtained only from embryonic EST databases (olea56p20 and roleb56p14). It is thus likely that medaka has a second LPL gene (LPL2) which may be mainly expressed in embryonic tissues.
cDNA cloning of medaka LPL1
The PCR assay using the LPL-F primer and GeneRacer 3′ nested primer resulted in specific amplification of a 2,157-bp product containing the whole coding and 3′ UTR regions. 5′ RACE also amplified a single PCR product of 479 bp. These sequences shared a 304-bp overlapping region in which no sequence mismatch was found. 5′ RACE using another gene-specific primer yielded a PCR product with a 1,016-bp overlap identical with the 3′ RACE product (data not shown). Because of the low nucleotide identity between the LPL1 and LPL2 genes and the absence of other LPL isoform genes in the EST- and PCR-amplified fragments, we assembled these sequences to obtain the long medaka LPL1 cDNA sequence that extended from the 5′ UTR to the 3′ UTR. This sequence was 2,259 bp in length [excluding the poly(A) tail] with an open reading frame of 1,551 bp encoding a 516-AA protein, a 145-bp 5′ UTR and a 563-bp 3′ UTR. One AAAATA motif representing the putative polyadenylation signal was found downstream of the 3′ region. This cDNA sequence was deposited in the DDBJ/EMBL/GenBank databases under accession number AB698560.
AA sequence analysis of medaka LPL1
A theoretical molecular mass of 57.98 kDa and isoelectric point (pI) of 7.26 for medaka LPL1 protein were obtained using ExPASy web tools. Based on the conserved domain database search, we determined that medaka LPL1 consists of two structural regions corresponding to LPLs from other species (Fig. 1), i.e., the N-terminal (24–363 residues) and C-terminal domains (364–516 residues). A short polypeptide of 23 AAs, considered to be a putative signal peptide, was identified in front of the N-terminal region. Comparison of AA sequences indicated that the N-terminal region of medaka LPL1 contains several conserved functional sites of vertebrate LPLs that are essential for the structure and functions of LPL [32–36], including one catalytic triad (Ser179, Asp203 and His291), one heparin-binding site (329–332 residues) and a putative polypeptide ‘lid’ (residues 263–289) that covers the active site and contributes to the preference for lipid substrate specificity [33, 37]. We also identified eight conserved cysteine residues in the N-terminal region of medaka LPL1 that play a role in the structural stability of LPL proteins and two conserved motifs in the C-terminal region, i.e., one lipid-binding site (Trp440, Trp443 and Trp444) and one putative N-linked glycosylation site (Asn409). Interestingly, one unpaired cysteine residue (Cys408) was found in the C-terminal domain of medaka LPL1 next to the putative N-linked glycosylation site. The deduced AA sequence of medaka LPL1 showed 78.3–84.7 % identities to LPL1 sequences from mandarin fish, gilthead seabream, European seabass, red seabream and torafugu, while only 45.0 and 46.8 % identities were found with torafugu and red seabream LPL2 sequences, respectively (Table 3).
Multiple alignment of the deduced amino acid sequence of medaka lipoprotein lipase 1 (LPL1) with LPL isoforms (LPL1, LPL2) from other animals. Dots Amino acids identical to those of medaka (Oryzias latipes, top line), dashes alignment gaps, bold underlining the signal peptide, hatch marks conserved cysteine residues, inverted filled triangle active site residues, single underline putative heparin binding domains, bold letters in putative heparin binding domains conserved residues in all species examined, NXT a potential conserved N-linked glycosylation site, box a putative lipid binding site
Phylogenetic analysis
A phylogenetic tree was constructed by the ML method using LPL AA sequences from 16 vertebrate species (Fig. 2). All LPL1 sequences from fish were grouped to one clade, with a separate clade for LPL2 sequences from red seabream and torafugu. Mammalian LPL sequences were in the same clade with the LPL1 sequences from fish.
Phylogenetic tree based on LPL amino acid sequences, constructed with MEGA v5.10 software using the maximum likelihood method. The topology was tested using bootstrap analyses (10,000 replicates). Numbers at internal branches bootstrap percentages. GenBank accession numbers are as follows: Pagrus major LPL1 (BAE95413), P. major LPL2 (BAB20997), Takifugu rubripes LPL1 (BAM76378), T. rubripes LPL2 (BAM76379), Oncorhynchus mykiss LPL (NP_001118076), Danio rerio LPL (NP_571202), Sparus aurata LPL (AAS75120), Dicentrarchus labrax LPL (CAL69901), Siniperca chuatsi LPL (ACZ55136), Homo sapiens LPL (EAW63764), Pan troglodytes LPL (XP_001149804), Papio anubis LPL (NP_001106082), Tupaia glis LPL (ABQ24000), Mesocricetus auratus LPL (BAD69621), Mus musculus LPL (CAJ18552), Rattus norvegicus LPL (AAH81836), Felis catus LPL (AAB03848)
Expression profile of the medaka LPL1 gene
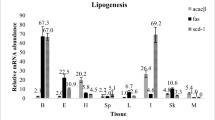
Quantitative real-time PCR was performed to determine the expression levels of LPL1 transcripts in various tissues of medaka. Overall, LPL1 transcripts were ubiquitously expressed in all tissues examined (Fig. 3). The highest expression was found in liver, followed by visceral adipose tissue. The LPL1 transcript levels in these tissues were significantly higher than those in other tissues (P < 0.05). Relatively high mRNA levels of LPL1 were detected in the brain and muscle, and the lowest level of LPL1 transcript expression was found in the intestine.
Discussion
In this study, we cloned the 2,259-bp cDNA encoding medaka LPL isoform 1 from the liver of adult medaka using the RACE approach. The deduced AA sequence of 516 residues was similar to those of LPL1s isolated from other fish species and LPLs from mammals and differed from the AA sequence of LPL2s from red seabream and torafugu. These results, together with those of the phylogenetic analysis, suggest that some conservative structural features are present in fish LPL1s and mammalian LPLs while LPL2 diverged earlier and probably acquired functions different from those of LPL1.
The three-dimensional model of human LPL based on human pancreatic lipase [34] aids our understanding of the functional property of vertebrate LPLs. All LPLs examined to date consist of two domains, i.e., a larger N-terminal domain and a smaller C-terminal domain, separated by Val363 and Phe364 (numbers for medaka LPL1). The N-terminal domain is considered to be a catalytic domain [36, 38], containing a highly conserved Ser-Asp-His catalytic triad on the active site. Two heparin-binding regions (306–309 and 321–327 residues in human LPL) that play a vital role in LPL catalytic function [39, 40] have also been located in the N-terminal domain. These regions are the same as the heparin-binding consensus sequences determined as [B-B-X-B] and [B-X-B-B-X-X-B], where B and X represent basic and hydropathic residues [32, 34], respectively. In all LPLs shown in Fig. 1, three basic residues (Arg306, Lys307 and Arg309, number for human LPL) of the first heparin-binding region are strictly conserved. However, in the second heparin-binding region, only two of the four basic residues (Arg321 and Arg324, number for human LPL) are fully conserved—Lys327 was conserved in fish LPL1s and mammalian LPLs, while Lys323 is substituted by various residues in fish LPLs. Interestingly, we found that the second heparin-binding region in zebrafish LPL exactly matched the consensus sequence (B-X-B-B-X-X-B), with Lys323 (number for human LPL) substituted by Arg342 (number for zebrafish LPL). These findings suggest that the function of the first heparin-binding region is more critical than the second region in vertebrate LPLs.
In contrast to the N-terminal domain, the C-terminal domain has been considered to play a role in substrate binding and specificity [36, 38]. Previous mutagenesis studies for Trp417, Trp420 and Trp421 (numbers for human LPL) by Williams et al. [41] and Krappet al. [42] revealed that these residues play a vital function in the binding of LPL to lipoproteins. In our study, we found that these residues were generally retained in the LPLs examined but that Trp417 was substituted by Val in LPL2s from red seabream and torafugu and Trp420 was substituted by Ile in rainbow trout LPL. These LPL proteins might have different substrate specificity compared to other LPLs.
Disulfide bridges have been shown to play an important role in the stability of LPL structure [16, 43, 44]. Human LPL has been reported to contain five disulfide bridges (Cys54/67, Cys243/266, Cys291/310, Cys302/305 and Cys445/465 in numbering for humans) [45]. The fish LPL1s examined in our study shared eight of the ten cysteine residues, which are all located in the N-terminal domain. Notably, the fish LPL2s retained all cysteine residues, including Cys445 and Cys465 (human numbering), in the C-terminal domain. The C-terminal domain and associated functions of fish LPL2s may thus differ from those of fish LPL1s and mammalian LPLs.
The homology analysis of the domains [Electronic Supplementary Material (ESM) Tables 1 and 2] also supported the notion of functional conservation in both domains of LPLs examined. Due to the several highly conserved functional sites, the N-terminal domain of fish LPL1s and mammalian LPLs shared a higher homology (54.0–62.6 %) with fish LPL2s than did the C-terminal domain (28.1–44.8 %). It is possible that these differences in the C-terminal domain indicate that LPL2s interact differently than LPL1s with lipoproteins.
The RPL-7 gene and two other housekeeping genes [encoding elongation factor 1α (EF1α) and β-actin] were used in quantitative real-time PCR assay to increase the accuracy of the analysis (ESM Fig. 1). In general, the gene expression patterns of medaka LPL1 normalized to the three internal control genes were similar to each other. The highest level of LPL1 transcripts were found in the liver followed by visceral adipose tissue, and the lowest levels were found in the heart, gill and intestine. In muscle and brain, there were minor inconsistencies in LPL transcript levels normalized to those of RPL-7, EF1α and β-actin. Our comparison of cycle threshold (C T) values of these housekeeping genes revealed that these inconsistencies were caused by relatively low expressions of the EF1α and RPL-7 transcripts in muscle and brain, respectively (ESM Fig. 2). However, compared with the transcript level of EF1α and β-actin, those of the RPL-7 gene showed less variation among the tissues tested; this result is similar to that reported by Zhang et al. [31]. Thus, among the three housekeeping genes used in our analysis, the RPL-7 gene was the most suitable internal control gene to determine the tissue distribution of LPL transcripts.
Another characteristic of medaka LPL1 was its relatively high expression in the brain. To date, mammalian LPL has been reported to be present in neurons and vascular endothelial cells of various brain regions [46–49]. Although only trace amounts of TAG have been detected in human cerebrospinal fluid due to the blood–brain barrier (BBB) [50], LPL plays a role in the catabolism of TAG-rich lipoproteins in both endothelial cells [51] and neurons of certain brain regions lacking the BBB [52]. Furthermore, the results of several studies suggest that neuronal LPL contributes to the uptake of lipoproteins and vitamin E [53, 54], as well as cell regeneration [55–57], by either conventional or unconventional mechanisms. This has led to the in vivo functions of brain LPL in mammals being the subject of intense investigations. To our knowledge, however, this is the first report to indicate LPL gene expression in the fish brain. Given the unique nature of fish BBB [58], it is therefore interesting to investigate the function of brain LPL in vivo using the transgenic system of medaka.
The results from our EST screening also supported a wide distribution of LPL1 transcripts in medaka tissues. We found that the cloned medaka LPL1 gene shared high homology with ESTs from fry and various tissues of adult medaka other than gill, while the ESTs sharing high homology with LPL2s were found only in embryonic databases. These findings suggest that LPL1 is the major type LPL isoform in adult medaka and that it functions in various developmental stages and tissues, whereas LPL2 may play a role during embryonic developmental stages. It was noted that ESTs cloned from medaka gill shared higher homology with LPL2 than with LPL1 (see Table 2 EST olgi31a18), suggesting that LPL2 is expressed at higher levels than LPL1 in medaka gill. This hypothesis is consistent with the tissue distribution of red seabream LPL isoform genes reported by Oku et al. [24].
In conclusion, we have identified LPL1 as the major LPL isoform in adult medaka. High expressions of LPL1 transcripts were found in the liver and visceral adipose tissue as well as the brain. To obtain insight into the potential functions of LPL1, the transgenic technique will be used in future studies. LPL2 may also be present in medaka and play an important role during embryonic developmental stages. Therefore, the function of this isoform should be investigated as well.
References
Smith LC, Pownall HJ, Gotto AM Jr (1978) The plasma lipoproteins: structure and metabolism. Annu Rev Biochem 47:751–777
Rogie A, Skinner RE (1985) The roles of the intestine and liver in the biosynthesis of plasma lipoproteins in the rainbow trout, Salmo gairdnerii Richardson. Comp Biochem Physiol B 81:285–289
Nilsson-Ehle P, Garfinkel AS, Schotz MC (1980) Lipolytic enzymes and plasma lipoprotein metabolism. Annu Rev Biochem 49:667–693
Mahley RW, Innerarity TL, Rall SC Jr, Weisgraber KH (1984) Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res 25:1277–1294
Goldberg IJ (1996) Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res 37:693–707
Wang H, Eckel RH (2009) Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab 297:E271–E288
Beigneux AP, Davies BS, Gin P, Weinstein MM, Farber E, Qiao X, Peale F, Bunting S, Walzem RL, Wong JS, Blaner WS, Ding ZM, Melford K, Wongsiriroj N, Shu X, de Sauvage F, Ryan RO, Fong LG, Bensadoun A, Young SG (2007) Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab 5:279–291
Berryman DE, Bensadoun A (1995) Heparan sulfate proteoglycans are primarily responsible for the maintenance of enzyme activity, binding, and degradation of lipoprotein lipase in Chinese hamster ovary cells. J Biol Chem 270:24525–24531
Rumsey SC, Obunike JC, Arad Y, Deckelbaum RJ, Goldberg IJ (1992) Lipoprotein lipase-mediated uptake and degradation of low density lipoproteins by fibroblasts and macrophages. J Clin Invest 90:1504–1512
Fernández-Borja M, Bellido D, Vilella E, Olivecrona G, Vilaró S (1996) Lipoprotein lipase-mediated uptake of lipoprotein in human fibroblasts: evidence for an LDL receptor-independent internalization pathway. J Lipid Res 37:464–481
Weinstock PH, Bisgaier CL, Aalto-Setala K, Radner H, Ramakrishnan R, Levak-Frank S, Essenburg AD, Zechner R, Breslow JL (1995) Severe hypertriglyceridemia, reduced high density lipoprotein, and neonatal death in lipoprotein lipase knockout mice—mild hypertriglyceridemia with impaired very low density lipoprotein clearance in heterozygotes. J Clin Invest 96:2555–2568
Gotoda T, Senda M, Gamou T, Furuichi Y, Oka K (1989) Nucleotide sequence of human cDNA coding for a lipoprotein lipase (LPL) cloned from placental cDNA library. Nucleic Acids Res 17:2351
Semenkovich CF, Chen SH, Wims M, Luo CC, Li WH, Chan L (1989) Lipoprotein lipase and hepatic lipase mRNA tissue specific expression, developmental regulation, and evolution. J Lipid Res 30:423–431
Kirchgessner TG, Svenson KL, Lusis AJ, Schotz MC (1987) The sequence of cDNA encoding lipoprotein lipase. A member of a lipase gene family. J Biol Chem 262:8463–8466
Senda M, Oka K, Brown WV, Qasba PK, Furuichi Y (1987) Molecular cloning and sequence of a cDNA coding for bovine lipoprotein lipase. Proc Natl Acad Sci USA 84:4369–4373
Lindberg A, Olivecrona G (2002) Lipoprotein lipase from rainbow trout differs in several respects from the enzyme in mammals. Gene 292:213–223
Saera-Vila A, Calduch-Giner JA, Gomez-Requeni P, Medale F, Kaushik S, Perez-Sanchez J (2005) Molecular characterization of gilthead sea bream (Sparus aurata) lipoprotein lipase. Transcriptional regulation by season and nutritional condition in skeletal muscle and fat storage tissues. Comp Biochem Physiol B 142:224–232
Merkel M, Eckel RH, Goldberg IJ (2002) Lipoprotein lipase: genetics, lipid uptake, and regulation. J Lipid Res 43:1997–2006
Zechner R (1997) The tissue-specific expression of lipoprotein lipase: implications for energy and lipoprotein metabolism. Curr Opin Lipidol 8:77–88
Vilaró S, Llobera M, Bengtsson-Olivecrona G, Olivecrona T (1988) Synthesis of lipoprotein lipase in the liver of newborn rats and localization of the enzyme by immunofluorescence. Biochem J 249:549–556
Gimenez-Llort L, Vilanova J, Skottova N, Bengtsson-Olivecrona G, Llobera M, Robert MQ (1991) Lipoprotein lipase enables triacylglycerol hydrolysis by perfused newborn rat liver. Am J Physiol 261:G641–G647
Cheng HL, Sun SP, Peng YX, Shi XY, Shen X, Meng XP, Dong ZG (2010) cDNA sequence and tissues expression analysis of lipoprotein lipase from common carp (Cyprinus carpio Var. Jian). Mol Biol Rep 37:2665–2673
Cheng HL, Wang X, Peng YX, Meng XP, Sun SP, Shi XY (2009) Molecular cloning and tissue distribution of lipoprotein lipase full-length cDNA from Pengze crucian carp (Carassius auratusvar. Pengze). Comp Biochem Physiol B 153:109–115
Oku H, Koizumi N, Okumura T, Kobayashi T, Umino T (2006) Molecular characterization of lipoprotein lipase, hepatic lipase and pancreatic lipase genes: effects of fasting and refeeding on their gene expression in red sea bream Pagrus major. Comp Biochem Physiol B 145:168–178
Kaneko G, Yamada T, Han Y, Hirano Y, Khieokhajonkhet A, Shirakami H, Nagasaka R, Kondo H, Hirono I, Ushio H, Watabe S (2013) Differences in lipid distribution and expression of peroxisome proliferator-activated receptor gamma and lipoprotein lipase genes in torafugu and red seabream. Gen Comp Endocrinol 184:51–60
Wittbrodt J, Shima A, Schartl M (2002) Medaka—a model organism from the far East. Nat Rev Genet 3:53–64
Kinoshita M, Toyohara H, Sakaguchi M, Inoue K, Yamashita S, Satake M, Wakamatsu Y, Ozato K (1996) A stable line of transgenic medaka (Oryzias latipes) carrying the CAT gene. Aquaculture 143:267–276
Ono Y, Kinoshita S, Ikeda D, Watabe S (2010) Early development of medaka Oryzias latipes muscles as revealed by transgenic approaches using embryonic and larval types of myosin heavy chain genes. Dev Dyn 239:1807–1817
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Zhang ZB, Hu JY (2007) Development and validation of endogenous reference genes for expression profiling of medaka (Oryzias latipes) exposed to endocrine disrupting chemicals by quantitative real-time RT-PCR. Toxicol Sci 95:356–368
Cardin AD, Weintraub HJ (1989) Molecular modeling of protein–glycosaminoglycan interactions. Arteriosclerosis 9:21–32
Dugi KA, Dichek HL, Santamarina-Fojo S (1995) Human hepatic and lipoprotein lipase: the loop covering the catalytic site mediates lipase substrate specificity. J Biol Chem 270:25396–25401
van Tilbeurgh H, Roussel A, Lalouel JM, Cambillau C (1994) Lipoprotein lipase. Molecular model based on the pancreatic lipase x-ray structure: consequences for heparin binding and catalysis. J Biol Chem 269:4626–4633
Wang CS, Hartsuck J, McConathy WJ (1992) Structure and functional properties of lipoprotein lipase. Biochim Biophys Acta 1123:1–17
Wong H, Davis RC, Thuren T, Goers JW, Nikazy J, Waite M, Schotz MC (1994) Lipoprotein lipase domain function. J Biol Chem 269:10319–10323
Henderson HE, Ma Y, Liu MS, Clark-Lewis I, Maeder DL, Kastelein JJ, Brunzell JD, Hayden MR (1993) Structure-function relationships of lipoprotein lipase: mutation analysis and mutagenesis of the loop region. J Lipid Res 34:1593–1602
Wong H, Davis RC, Nikazy J, Seebart KE, Schotz MC (1991) Domain exchange: characterization of a chimeric lipase of hepatic lipase and lipoprotein lipase. Proc Natl Acad Sci USA 88:11290–11294
Hata A, Ridinger DN, Sutherland S, Emi M, Shuhua Z, Myers RL, Ren K, Cheng T, Inoue I, Wilson DE et al (1993) Binding of lipoprotein lipase to heparin. Identification of five critical residues in two distinct segments of the amino-terminal domain. J Biol Chem 268:8447–8457
Ma Y, Henderson HE, Liu MS, Zhang H, Forsythe IJ, Clarke-Lewis I, Hayden MR, Brunzell JD (1994) Mutagenesis in four candidate heparin binding regions (residues 279–282, 291–304, 390–393, and 439–448) and identification of residues affecting heparin binding of human lipoprotein lipase. J Lipid Res 35:2049–2059
Williams SE, Inoue I, Tran H, Fry GL, Pladet MW, Iverius PH, Lalouel JM, Chappell DA, Strickland DK (1994) The carboxyl-terminal domain of lipoprotein lipase binds to the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor (LRP) and mediates binding of normal very low density lipoproteins to LRP. J Biol Chem 269:8653–8658
Krapp A, Zhang HF, Ginzinger D, Liu MS, Lindberg A, Olivecrona G, Hayden MR, Beisiegel U (1995) Structural features in lipoprotein lipase necessary for the mediation of lipoprotein uptake into cells. J Lipid Res 36:2362–2373
Lo JY, Smith LC, Chan L (1995) Lipoprotein lipase: role of intramolecular disulfide bonds in enzyme catalysis. Biochem Biophys Res Commun 206:266–271
Keiper T, Schneider JG, Dugi KA (2001) Novel site in lipoprotein lipase (LPL415-438) essential for substrate interaction and dimer stability. J Lipid Res 42:1180–1186
Raisonnier A, Etienne J, Arnault F, Brault D, Noe L, Chuat JC, Galibert F (1995) Comparison of the cDNA and amino acid sequences of lipoprotein lipase in eight species. Comp Biochem Physiol B 111:385–398
Bessesen DH, Richards CL, Etienne J, Goers JW, Eckel RH (1993) Spinal cord of the rat contains more lipoprotein lipase than other brain regions. J Lipid Res 34:229–238
Ben-Zeev O, Doolittle MH, Singh N, Chang CH, Schotz MC (1990) Synthesis and regulation of lipoprotein lipase in the hippocampus. J Lipid Res 31:1307–1313
Vilaro S, Camps L, Reina M, Perez-Clausell J, Llobera M, Olivecrona T (1990) Localization of lipoprotein lipase to discrete areas of the guinea pig brain. Brain Res 506:249–253
Gong H, Dong W, Rostad SW, Marcovina SM, Albers JJ, Brunzell JD, Vuletic S (2013) Lipoprotein lipase (LPL) is associated with neurite pathology and its levels are markedly reduced in the dentate gyrus of Alzheimer’s disease brains. J Histochem Cytochem 61:857–868
Koch S, Donarski N, Goetze K, Kreckel M, Stuerenburg HJ, Buhmann C, Beisiegel U (2001) Characterization of four lipoprotein classes in human cerebrospinal fluid. J Lipid Res 42:1143–1151
Brecher P, Kuan HT (1979) Lipoprotein lipase and acid lipase activity in rabbit brain microvessels. J Lipid Res 20:464–471
Wang H, Astarita G, Taussig MD, Bharadwaj KG, DiPatrizio NV, Nave KA, Piomelli D, Goldberg IJ, Eckel RH (2011) Deficiency of lipoprotein lipase in neurons modifies the regulation of energy balance and leads to obesity. Cell Metab 13:105–113
Goti D, Balazs Z, Panzenboeck U, Hrzenjak A, Reicher H, Wagner E, Zechner R, Malle E, Sattler W (2002) Effects of lipoprotein lipase on uptake and transcytosis of low density lipoprotein (LDL) and LDL-associated α-tocopherol in a porcine in vitro blood-brain barrier model. J Biol Chem 277:28537–28544
Xian X, Liu T, Yu J, Wang Y, Miao Y, Zhang J, Yu Y, Ross C, Karasinska JM, Hayden MR, Liu G, Chui D (2009) Presynaptic defects underlying impaired learning and memory function in lipoprotein lipase-deficient mice. J Neurosci 29:4681–4685
Nuñez M, Peinado-Onsurbe J, Vilaró S, Llobera M (1995) Lipoprotein lipase activity in developing rat brain areas. Biol Neonate 68:119–127
Blain JF, Paradis E, Gaudreault SB, Champagne D, Richard D, Poirier J (2004) A role for lipoprotein lipase during synaptic remodeling in the adult mouse brain. Neurobiol Dis 15:510–519
Paradis E, Clement S, Julien P, Ven Murthy MR (2003) Lipoprotein lipase affects the survival and differentiation of neural cells exposed to very low density lipoprotein. J Biol Chem 278:9698–9705
Bundgaard M, Abbott NJ (2008) All vertebrates started out with a glial blood–brain barrier 4–500 million years ago. Glia 56:699–708
Acknowledgments
We acknowledge the National Institute for Basic Biology for the medaka EST data. This work was partly supported by a grant from the Scientific Technique Research Promotion Program for Agriculture, Forestry, Fisheries and Food industry and by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, L., Kaneko, G., Takahashi, SI. et al. Identification and gene expression profile analysis of a major type of lipoprotein lipase in adult medaka Oryzias latipes . Fish Sci 81, 163–173 (2015). https://doi.org/10.1007/s12562-014-0826-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-014-0826-7