Abstract
To enhance our ability to monitor poliovirus circulation and certify eradication, we evaluated the performance of the bag-mediated filtration system (BMFS) against the two-phase separation (TPS) method for concentrating wastewater samples for poliovirus detection. Sequential samples were collected at two sites in Mexico; one L was collected by grab and ~ 5 L were collected and filtered in situ with the BMFS. In the laboratory, 500 mL collected by grab were concentrated using TPS and the sample contained in the filter of the BMFS was eluted without secondary concentration. Concentrates were tested for the presence of poliovirus and non-poliovirus enterovirus (NPEV) using Global Poliovirus Laboratory Network standard procedures. Between February 16, 2016, and April 18, 2017, 125 pairs of samples were obtained. Collectors spent an average (± standard deviation) of 4.3 ± 2.2 min collecting the TPS sample versus 73.5 ± 30.5 min collecting and filtering the BMFS sample. Laboratory processing required an estimated 5 h for concentration by TPS and 3.5 h for elution. Sabin 1 poliovirus was detected in 37 [30%] samples with the TPS versus 24 [19%] samples with the BMFS (McNemar’s mid p value = 0.004). Sabin 3 poliovirus was detected in 59 [47%] versus 49 (39%) samples (p = 0.043), and NPEV was detected in 67 [54%] versus 40 [32%] samples (p < 0.001). The BMFS method without secondary concentration did not perform as well as the TPS method for detecting Sabin poliovirus and NPEV. Further studies are needed to guide the selection of cost-effective environmental surveillance methods for the polio endgame.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental surveillance (ES) allows monitoring poliovirus transmission in populations through the examination of environmental specimens contaminated with human feces (wastewater) (Hovi et al. 2012). The Global Polio Eradication Initiative (GPEI) has used ES to enhance the sensitivity of detection of wild poliovirus (WPV) transmission provided by disease-based acute flaccid paralysis (AFP) surveillance. ES adds sensitivity particularly when there is low-level transmission, there are weaknesses in AFP surveillance performance and when WPV circulates among infected individuals protected from paralysis through vaccination with inactivated polio vaccine (IPV) (Deshpande et al. 2003; Hovi et al. 2005). ES has also provided early indications of poliovirus importations into polio-free areas and of the emergence of vaccine-derived poliovirus (VDPV) circulation (Esteves-Jaramillo et al. 2014; Van Der Avoort et al. 1995; Kopel et al. 2014). As global polio eradication nears, the GPEI is expanding the use of ES to provide evidence for certification of polio-free status in the last endemic countries and for documenting the disappearance of vaccine-related viruses from the environment following cessation of the use of oral polio vaccines (OPV) (Global Polio Eradication Initiative 2013; Cowger et al. 2017; Asghar et al. 2014).
However, ES implementation in low-resource countries has several challenges. The current methodology used for poliovirus ES requires collection of large-volume wastewater samples (i.e. 1 L), which are then transported to one of the laboratories in the Global Polio Laboratory Network (GPLN) furnished with the trained staff, special equipment, and infrastructure to conduct concentration and detection of poliovirus in environmental samples (Hovi et al. 2012; Global Polio Eradication Initiative 2015). The University of Washington (UW) has developed a novel bag-mediated filtration system (BMFS) that allows initial concentration of the sample at the point of collection. Using gravity, about 3–10 L of wastewater pass through a filter which captures virus on a charged membrane (Fagnant et al. 2014). The sample can be eluted from the filter in a local laboratory, and then sent to a reference laboratory for detection of poliovirus through the usual virus isolation and molecular methods (World Health Organization 2004). The initial BMFS has gone through several modifications to facilitate field work and improve poliovirus recovery, including the addition of a secondary concentration step (Fagnant et al. 2017a; Fagnant et al. 2018; Fagnant et al. 2017b; Falman et al. 2019). Some of those modifications were not available when we planned and conducted our study.
We present the results of one of the collaborative studies conducted by the GPEI and UW to evaluate the feasibility and performance of the BMFS for poliovirus ES. The study was conducted in Mexico because of its unique polio vaccination schedule: infants in Mexico receive four doses of IPV through routine immunization services at 2, 4, 6 and 18 months of age, and several doses of OPV up to the age of 5 years through bi-annual campaigns (Esteves-Jaramillo et al. 2014). ES data from Mexico have helped monitor the duration of detection of vaccine poliovirus strains in the environment and the potential for VDPV emergence after OPV campaigns in populations partially vaccinated with IPV (Esteves-Jaramillo et al. 2014).
The primary objective of this study was to assess the performance of the BMFS version that does not include secondary concentration against the current methodology of collection and concentration of wastewater samples for the detection of poliovirus and non-polio enteroviruses (NPEV). Secondary objectives were to assess field implementation logistics for each method, such as cost per sample or staff time involved in collection and laboratory processing, and to monitor the disappearance of type 2 Sabin poliovirus after the last campaign in Mexico that used trivalent OPV before the global switch to bivalent OPV (Hampton et al. 2016).
Materials and Methods
Selection of Collection Sites and Sampling Schedule
We selected two areas, one in Hidalgo State and one in Mexico City, for environmental sampling based upon the following criteria: (1) convergent wastewater system; (2) presence of risk factors for VDPV emergence such as low polio vaccination coverage and high population density; and (3) proximity to a polio laboratory. Coverage by 1 year of age with three doses of IPV in 2013 was 67% for Hidalgo State and 94% for Mexico City. In the absence of water treatment plants in the areas chosen, samples were collected at access points to underground wastewater canals. The catchment population was estimated as 270,000 persons for the Hidalgo site and 4 million for the Mexico City site.
Prospective collection of samples was planned around two OPV campaigns conducted in 2016 and 2017. The 2016 campaign distributing trivalent OPV was implemented between February 16th and March 20th and the 2017 campaign, which distributed bivalent OPV, was conducted between February 25th and March 20th. A campaign planned for October 2016 was canceled because of insufficient bivalent OPV supply. Assuming that Sabin viruses were unlikely to be detected more than 2 months after a campaign (Esteves-Jaramillo et al. 2014; Mas Lago et al. 2003), samples were collected during the week before each campaign, then bi-weekly during the duration of the campaigns and up to 1 month after the first campaign. Samples were collected weekly between April 26 and September 27, 2017; and monthly between September 27 and February 21, 2018.
Sample Collection and Transportation
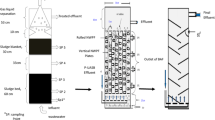
At each study site, trained staff collected two serial samples of wastewater. Using the BMFS, about 5 L of wastewater was collected with a 6 L polyurethane-coated nylon bag. This bag, which was the third-generation BMFS model (Fagnant et al. 2018), had a mesh over the opening to exclude refuse and debris, an open tubing adapter port at the bottom for discarding settled solids into a second vessel, and a 15.5° slope toward the opening that facilitated the bleeding of sediment. After filling with wastewater water, the bag was placed on a tripod and connected to the ViroCap filter (Scientific Methods, Granger, Indiana, USA) to use gravity to force water through the filter. Before starting the filtration, the sample was allowed to sit for 10 min, and the bottom sediment was transferred to a Whirl–Pak bag through the adapter port to avoid solids clogging the filter. The sediment was added back to the bag before finishing the filtration process, if possible. Once all the water in the bag had passed through the filter (or the filter had become obstructed), the filter was disconnected and placed in a cold box with frozen ice packs. ViroCap filter housings were re-used up to ten times for the first 63 samples and were single-use for the remaining samples (Fagnant et al. 2018).
While filtration was taking place with the BMFS, staff collected another sample of wastewater at the same site, using a bucket. The sample was poured into a 1-L container and placed in the same cold box.
The sequential samples were transported directly to the poliovirus laboratory at the Instituto de Diagnóstico y Referencia Epidemiológico (InDRE) in Mexico City. In Hidalgo, the samples were taken to the Laboratorio Estatal de Salud Publica (LESP) in Hidalgo State for administrative processing, and then shipped, within 12 h of collection, to the InDRE in Mexico City.
Sample Concentration
A portion of each 1-L wastewater sample was concentrated in the laboratory using the two-phase separation (TPS) method recommended by the WHO (Global Polio Eradication Initiative 2015). Briefly, the sample was centrifuged for 20 min at 1500×g and 4 °C. Following this, 500 mL of the supernatant were mixed with Polyethylene Glycol 6000 (Sigma-Aldrich, Damstad, Germany) and Dextran T40 (Pharmacocosmos A/S, Holbaek, Denmark). The mixture was shaken vigorously and incubated overnight at 4 °C in a separatory funnel. The lower phase and interphase were collected and treated with chloroform, then centrifuged as above, and the supernatant was supplemented with penicillin and streptomycin before storage at − 20 °C for future testing (Blomqvist et al. 2004; Shulman et al. 2006). Reported recovery efficiency for a sample spiked with 105 CCID50 of type 1 Sabin with this method were between 4 and 39% (CDC, unpublished results). However, wide variations in poliovirus recovery within the same sample have been reported in parallel testing, especially when samples have enterovirus mixtures (Global Polio Eradication Initiative 2015; Hovi et al. 2005).
The ViroCap filters were eluted as described previously (Fagnant et al. 2014, 2018). Sterile 1.5% beef extract (100 mL) with 0.05 M glycine buffer, pH 9.5, was pumped into the filter and allowed to stand for 30 min to allow viruses to detach from the filter. The eluate was collected with a manual bilge pump and pH was adjusted to 7.0–7.5. An aliquot of 12 mL was treated with chloroform and antibiotics as described above, and stored at − 20 °C for future testing. Poliovirus recovery from surface water, secondary effluent and a 50:50 mix using ViroCap filters averaged 44% for type 1, 70% for type 2 and 81% for type 3 (Fagnant et al. 2014).
Poliovirus Isolation and Characterization
The concentrates obtained with both methods were inoculated onto five 25 cm2 flasks (0.5 mL per flask) containing monolayers of L20B cells (mouse fibroblast cells that express the human poliovirus receptor) and onto one 25 cm2 flask containing a monolayer of human rhabdomyosarcoma (RD) cells, following the standard WHO methodology (World Health Organization 2004, 2010). The presence of viral cytopathic effect (CPE) was monitored daily according to the approved virus isolation algorithm. The presence of poliovirus or other enteroviruses and the poliovirus serotype was determined by real-time reverse transcriptase polymerase chain reaction (rRT-PCR) versions 4.0, 4.1 and 5.0 (termed intratypic differentiation; ITD) (Kilpatrick et al. 2009; Gerloff et al. 2018). Sabin isolates were screened by the real-time PCR assay for VDPVs as described previously (Kilpatrick et al. 2009; Gerloff et al. 2018; Kilpatrick et al. 2014), and standard sequencing in the VP1 region was performed at CDC laboratory on a subset of isolates as needed (Burns et al. 2016; Kilpatrick et al. 2014).
Data Collection
Staff involved in the collection and transport of samples recorded information about procedures on standardized forms (one per sample). Relevant data included: (a) dates of sample collection; (b) times when collection started and when sample was placed in the cold box; and (c) volumes collected, and, for BMFS, volumes remaining after removing the sediment and after filtration (using volume markings in the bag). Staff also described problems that arose during sample collection.
Laboratory staff recorded the following information: (a) date, time, and temperature of samples on arrival at the laboratory; (b) volume of sample concentrate obtained with each method; and (c) results per sample of virus isolate, rRT-PCR, and (when performed) sequencing assays.
Statistical Analysis
The primary study outcome was the proportion of samples positive for poliovirus types 1, 2, or 3, or for NPEV, with either the two-phase separation or the BMFS methodology. Secondary outcomes included description of volumes obtained and time invested in sample collection and concentration, and estimates of costs per sample. Proportions are presented with Wilson 95% confidence intervals, and continuous variables are presented as mean ± standard deviation.
We used McNemar’s concordance test to compare the proportion of samples that tested positive for poliovirus or NPEV with each method. Statistical significance was defined as a mid-p value of McNemar’s test below 0.05. Continuous variables were compared using t test.
To determine sample size, we assumed that the difference in percent of positive samples between the two procedures would be 15%, with a discordance between samples of 30%. Under these assumptions, 125 pairs of samples would be required for a power of 85%. Sample size calculations were performed using PASS version 14. Analyses were performed with SAS version 9.3 and R version 3.4.3.
Results
Sample Collection
The 125 pairs of samples were collected between February 16, 2016 and April 18, 2017. Of these, 62 pairs were collected in Mexico City and 63 in Hidalgo. Because of delays in shipment of supplies, five samples that were supposed to have been processed in the field with the BMFS had to be collected (6 L) and transported to the laboratory in cold chain, where they were stored at − 20 °C, and thawed and filtered at a later time. Information on collection and transportation for these five samples was excluded from the analysis for field logistics.
Staff initially collected an average (± standard deviation) of 5.2 ± 0.5 L with the BMFS collection bag and filtered 4.8 ± 0.8 L in situ (Table 1). The volume filtered reached > 4 L for 90% of the samples (111/123), 3–4 L for 9% of the samples (11/123) and < 3 L for one (1%) sample. Staff collected a slightly larger initial sample in the Mexico City site than in the Hidalgo site (5.4 ± 0.7 L in Mexico City vs. 5.1 ± 0.2 L in Hidalgo, p < 0.001), and were able to filter a slightly larger volume (5.0 ± 0.9 vs. 4.7 ± 0.7 L, p = 0.039). Field staff reported that filtration was incomplete because of obstructed filter in six samples and because of leaks in the filter housing in four samples. Reasons were not provided for other occasions when all the volume collected was not filtered. The volume of samples collected by grab was 1 ± 0 L.
Field staff spent 4.3 ± 2.2 min collecting a 1 L sample for TPS, and 73.5 ± 30.5 min collecting and filtering a sample with the BMFS (Table 1). The filtration process took longer in Hidalgo than in Mexico City (87.9 ± 27.6 vs. 58.9 ± 26.1 min, p < 0.001).
Duration of transportation measured the time between sample placement in the cold box and arrival at the local laboratory (LESP in Hidalgo) and reference laboratory in Mexico City (InDRE). Transportation took less than 2 h for samples collected in Mexico City, which were taken directly to the InDRE. Transportation took less than 4 h for samples collected in Hidalgo, after combining transportations to the LESP, and then to the InDRE (Table 1). Temperature inside the cold boxes on arrival to the InDRE laboratory was 3.8 ± 1.9 °C.
Sample Concentration
Samples collected for TPS were initially stored at − 20 °C, then thawed and concentrated in batches of four using the WHO standard method (Global Polio Eradication Initiative 2015). The average volume of the concentrate obtained from an original sample of 500 mL was 14.4 ± 3.6 mL (concentration factor of 35) for an effective volume of 104 mL. The laboratory staff estimated that the concentration process required about 5 h of hands-on work (2 h for sample processing plus 3 h for reagent preparation and clean-up) and 16 h of overnight incubation.
The ViroCap filters were eluted using the manual bilge pump as described (Fagnant et al. 2014), upon arrival to the laboratory (except for five samples from the Hidalgo site that were collected by grab, frozen, and later filtered at the InDRE laboratory). The average volume of elute obtained was 99.9 ± 5.3 mL, for a concentration factor of 48 from the average 4.8 L of sample filtered and an effective volume assayed of 144 mL. The staff estimated that the process required approximately 3.5 h (1 h for the elution process plus 2.5 h for reagent preparation and clean-up).
Cost Per Sample
Table 2 shows a calculation of the cost of non-reusable supplies and reagents required for collecting and concentrating the sample (up to addition of chloroform and antibiotics) with each method. We excluded supplies required for transportation and storage of samples in cold chain, because they were partially provided by the shipping company. In addition, we separated the cost of supplies for personal protective equipment and for cleaning and disposal of infectious materials, which were similar for the two methods, from the cost of supplies for procedures specific to each method.
The cost of collecting one sample was > 30-fold higher with the BMFS than for the TPS method (~ $101 vs. $3 per sample collected for TPS), whereas the cost of the concentration process was similar for both methodologies ($4 with the BMFS versus $5 with TPS). Adding the two steps, the cost was $105 for each sample collected and processed with the BMFS compared with $8 per sample collected and concentrated with the current TPS method. The total up-front cost of the pump and other reusable materials required for the elution of the sample was about $530. The cost of re-usable materials for the two-phase concentration was $121. We did not include the cost of equipment, because most of it should be available in a virology laboratory (i.e. biosafety cabinet, centrifuges, shakers, etc.). The only specific equipment that most laboratories will not have are high-speed refrigerated centrifuges for large volumes (i.e. 250 mL), which may cost $15,000–20,000 and a separate refrigerator to store the samples during the overnight incubation (estimated cost $6000–8000).
Isolation of Sabin Polioviruses and NPEV
A total of 125 pairs of sequential samples were collected between February 16, 2016 and April 18, 2017; 62 pairs in Mexico City and 63 pairs in Hidalgo (Fig. 1).
Sabin polioviruses and non-polio enteroviruses isolated from environmental samples collected during February 2016 to April 2017 in two sites in Mexico. Poliovirus and non-polio enterovirus could be isolated alone (presented in figure) or in mixtures (only Sabin content shown). Sample collection was conducted before and after two National immunization campaigns providing either tOPV (2016) or bOPV (2017). SL1 Sabin 1, SL2 Sabin 2, SL3 Sabin 3, NPEV non-polio enterovirus
As expected, most of the samples were positive for Sabin poliovirus during the two campaigns and for about 1 month after the campaigns (Fig. 1). Sabin strains could be detected up to 13 weeks after the end of the tOPV campaign (March 20th, 2016), either alone or in mixtures with other Sabin serotypes or NPEV. After that, samples remained negative for polioviruses until the bOPV campaign conducted in March 2017 (1 year after the tOPV campaign).
The comparison of sequentially collected paired samples with McNemar’s test indicated significant discordances between the two concentration methods for detecting Sabin type 1, Sabin type 3 and NPEV, as well as for detecting any poliovirus or NPEV (Table 3). As shown on Table 4, the TPS method identified Sabin 1, Sabin 3 and NPEV in a higher proportion of samples than the BMFS. Sabin 1 poliovirus was detected in 37 samples [30%; 95% confidence interval 22.3–38.1%] with the two-phase method versus 24 samples [19%; 13.3–27.0%] with the BMFS (McNemar’s mid p value = 0.004). Sabin 3 poliovirus was detected in 59 [47%; 38.7–55.9%] versus 49 samples (39%; 31.1–48.0%, p = 0.043), and NPEV was detected in 67 samples [54%; 44.9–62.1%] versus 40 samples [32%; 24.5–40.6%, p < .001). There were no significant differences for detection of type 2 Sabin strains (20%; 13.9–27.9% with TPS vs. 24%; 17.4–32.2% with BMFS, p = 0.18). The difference in detection of NPEV between methods was maintained after exclusion of mixtures of poliovirus and NPEV, with 48/107 [45%] samples positive for NPEV with the two-phase method versus 31/107 [30%] with the BMFS (p = 0.004).
Discussion
This project showed that Sabin type 2 disappeared from wastewater samples about 3 months after the last tOPV campaign, as observed in a prior study (Esteves-Jaramillo et al. 2014); and one further year of surveillance did not detect VDPV type 2 in wastewater in Hidalgo or Mexico City. For poliovirus surveillance in low-resource settings, the BMFS without secondary concentration tested in this project had some logistical advantages over the WHO-recommended methodology for collection and concentration of environmental samples (two-phase separation or TPS). However, the BMFS did not perform as well as the TPS for the detection of Sabin polioviruses and NPEV. We present the pros and cons of each method and suggest potential changes to improve the BMFS performance as a surveillance tool for the polio endgame and the post-eradication era.
The study was part of a series of projects implemented by GPEI designed to identify feasible and cost-effective methods to improve ES for poliovirus. As global polio eradication nears, the GPEI is expanding the use of ES to provide evidence for certification of polio-free status in the last countries with WPV circulation; for documenting the disappearance of vaccine and vaccine-related viruses from the environment following cessation of the use of oral polio vaccines (OPV) (Global Polio Eradication Initiative 2013), and to detect poliovirus accidental releases or emergences during the post-cessation era. However, many countries at risk of outbreaks following WPV importations or VDPV emergences have insufficient resources, infrastructure, and human capacity to implement laboratory tests required to detect polioviruses in clinical or environmental samples, such as cell culture or real-time PCR. Even if established polio laboratories outside the country could process ES specimens, the complex logistics and high costs involved in the shipment of large volumes of potentially infectious materials from collection sites to outside reference laboratories are also important challenges for ES implementation in some high-risk countries.
The BMFS offered several advantages over the current method used by the GPLN for poliovirus surveillance. First, it allowed collection of larger sample volumes than the current method (5 L instead of 1 L), which theoretically improve the probability of detection. Although the high turbidity of samples slowed the filtration process or prevented its completion in some cases, collectors filtered a sample > 4 L in > 90% of occasions. A second benefit was that the BMFS concentrated the samples by about 50-fold through a combination of in-field and laboratory procedures that did not require complex electronic equipment, continuous power supply, or highly qualified staff, and could, therefore, presumably be performed in many laboratories in low-resource countries. Finally, the 100 mL of eluted sample concentrate obtained after the concentration process (filtration plus elution) with the BMFS would be easier and cheaper to store and transport to reference polio laboratories than the 1000 mL raw wastewater samples required with the current methodology. The filter could also be transported directly to a reference polio laboratory in cold chain if logistics allow for the sample to be processed within 24 h of sample collection. If filter transportation requires more time, preservative agents may be added to the filter after sample collection to avoid bacterial and fungal overgrowth.
The BMFS required a considerable amount of time for in situ sample collection and filtration; besides, collection time and amount of sample collected may be very variable. In our study, 60 to 90 min were required for filtering 4–5 L compared with less than 5 min for sample collection for TPS. The extra field time was partially compensated by the shorter time required to finish the concentration process through elution of the filter in the laboratory. A prolonged collection time can be an important challenge in sites located in security-compromised areas, potentially endangering collection staff. Alternative options, such as carrying the sample immediately after collection to an area close to the laboratory where filtration can proceed safely, were developed for these situations (Zhou et al. 2018). Procedures to minimize heat exposure and risk of contamination during sample transportation will also need to be developed.
The major drawbacks of the BMFS as tested in this study were its lower performance for the detection of Sabin polioviruses and the extra cost per sample processed. The UW investigators have introduced a laboratory-based secondary concentration to be combined with the primary concentration step the BMFS (Falman et al. 2019). This secondary concentration uses PEG/NaCl precipitation, which includes a medium-speed centrifugation step, instead of the PEG/Dextran extraction used in the two-phase concentration method recommended by the WHO GPLN and tested here. Results from recent studies suggest that, with the secondary concentration step, the BMFS has similar or better capacity to detect poliovirus than the TPS method (Falman et al. 2019; Zhou et al. 2018). However, with the addition of this secondary concentration, the cost per sample with the BMFS would increase further, and laboratories would need to re-assess their capacity and resources to include the equipment, staff, and supplies required to perform the concentration. We did not include the secondary concentration step in this project in Mexico because we wanted to compare the TPS concentration to the BMFS primary concentration only (i.e. filter). We also wanted to assess a simpler and less costly BMFS methodology that would be easier to implement in laboratories, requiring less-skilled laboratory staff, less training, and less equipment.
We also observed lower performance with the BMFS without secondary concentration for NPEV detection. We assessed NPEV detection because it has been proposed as an important indicator of the adequate performance of the ES system to detect poliovirus after discontinuation of OPV and disappearance of Sabin strains from wastewater worldwide. Although NPEV prevalence varies by country and season (Nakamura et al. 2015; Global Polio Eradication Initiative 2015; Khetsuriani et al. 2006; Dhole et al. 2009; Abedi et al. 2015), NPEV are inactivated by the same environmental factors as poliovirus (Dowdle et al. 2006), and NPEV can be detected by similar assays (World Health Organization 2004, 2010). Other markers of human fecal presence would be less useful for quality control of the performance of ES for poliovirus detection because they may not be affected by high temperatures or high bacteria content in the same way as poliovirus, and they require different assays (Fagnant et al. 2017a). New studies should also assess the effect on NPEV detection of the secondary concentration and other improvements in the BMFS methodology.
This study has several limitations. First, we did not perform a formal head-to-head comparison of the laboratory testing methods in the same sample. Collecting the samples separately was important to replicate field conditions and assess outcomes that are important for future implementation, such as time required to collect a sample or cost per sample. Second, the method for detecting poliovirus in wastewater samples has high intra-assay variability, partly because poliovirus mixtures cause interference among strains for growth in cell cultures. As inoculating several parallel flasks per sample was found to increase poliovirus detection, the WHO standard methodology for cell culture of environmental samples recommends to inoculate five flasks of L20 cells (Global Polio Eradication Initiative 2015; Hovi et al. 2005). Because procedures for cell culture and intratypic differentiation were similar for both methods, we assume that this variability would not be significantly different between methods. Finally, the estimates of cost per sample only include cost of reagents and supplies, which may vary by country. Other expenses such as transportation, cold chain equipment, and human resources that will also impact the final cost of the sample are likely to be similar for both methods, but these will also vary by country.
In summary, we showed that the BMFS without a secondary concentration could allow low-resource local laboratories to perform an initial concentration of large wastewater samples of for poliovirus surveillance, which could facilitate and reduce the cost of shipping samples to reference laboratories. However, BMFS requires a secondary concentration step or other modifications to improve its performance for the detection of poliovirus and NPEV. In our study, BMFS use without secondary concentration incurred > 30 times higher processing costs compared to the current TPS method; the relative final cost per sample processed using the BMFS with the secondary concentration would likely be higher. The results from several ongoing studies in different countries assessing the feasibility and cost of BMFS with different modifications against the TPS method will guide the GPEI in the selection of new methodologies for ES with the sensitivity and quality standards required to certify and maintain a polio-free world.
References
Abedi, G. R., Watson, J. T., Pham, H., Nix, W. A., Oberste, M. S., & Gerber, S. I. (2015). Enterovirus and human parechovirus surveillance—United States, 2009–2013. Morbidity and Mortality Weekly Report,64(34), 940–943. https://doi.org/10.15585/mmwr.mm6434a3.
Asghar, H., Diop, O. M., Weldegebriel, G., Malik, F., Shetty, S., El Bassioni, L., et al. (2014). Environmental surveillance for polioviruses in the Global Polio Eradication Initiative. Journal of Infectious Diseases,210(Suppl 1), S294–S303. https://doi.org/10.1093/infdis/jiu384.
Blomqvist, S., Savolainen, C., Laine, P., Hirttio, P., Lamminsalo, E., Penttila, E., et al. (2004). Characterization of a highly evolved vaccine-derived poliovirus type 3 isolated from sewage in Estonia. Journal of Virology,78(9), 4876–4883. https://doi.org/10.1128/JVI.78.9.4876-4883.2004.
Burns, C. C., Kilpatrick, D. R., Iber, J. C., Chen, Q., & Kew, O. M. (2016). Molecular properties of poliovirus isolates: nucleotide sequence analysis, typing by PCR and real-time RT-PCR. In J. Martin (Ed.), Poliovirus: methods and protocols, methods in molecular biology (Vol. 1387, pp. 177–212). New York: Springer.
Cowger, T. L., Burns, C. C., Sharif, S., Gary, H. E., Jr., Iber, J., Henderson, E., et al. (2017). The role of supplementary environmental surveillance to complement acute flaccid paralysis surveillance for wild poliovirus in Pakistan—2011–2013. PLoS ONE,12(7), e0180608. https://doi.org/10.1371/journal.pone.0180608.
Deshpande, J., Shetty, S., & Siddiqui, Z. (2003). Environmental surveillance system to track wild poliovirus transmission. Applied and Environmental Microbiology,69(5), 2919–2927. https://doi.org/10.1128/AEM.69.5.2919-2927.2003.
Dhole, T. N., Ayyagari, A., Chowdhary, R., Shakya, A. K., Shrivastav, N., Datta, T., et al. (2009). Non-polio enteroviruses in acute flaccid paralysis children of India: vital assessment before polio eradication. Journal of Paediatrics and Child Health,45(7–8), 409–413. https://doi.org/10.1111/j.1440-1754.2009.01529.
Dowdle, W., van der Avoort, H., de Gourville, E., Delpeyroux, F., Desphande, J., Hovi, T., et al. (2006). Containment of polioviruses after eradication and OPV cessation: characterizing risks to improve management. Risk analysis,26(6), 1449–1469. https://doi.org/10.1111/j.1539-6924.2006.00844.x.
Esteves-Jaramillo, A., Estivariz, C. F., Peñaranda, S., Richardson, V. L., Reyna, J., Coronel, D. L., et al. (2014). Detection of vaccine-derived polioviruses in Mexico using environmental surveillance. Journal of Infectious Diseases,210(Suppl 1), S315–S323. https://doi.org/10.1093/infdis/jiu183.
Fagnant, C. S., Beck, N. K., Yang, M. F., Barnes, K. S., Boyle, D. S., & Meschke, J. S. (2014). Development of a novel bag-mediated filtration system for environmental recovery of poliovirus. Journal of Water and Health,12(4), 747–754. https://doi.org/10.2166/wh.2014.032.
Fagnant, C. S., Kossik, A. L., Zhou, N. A., Sanchez-Gonzalez, L., Falman, J. C., Keim, E. K., et al. (2017a). Use of preservative agents and antibiotics for increased poliovirus survival on positively charged filters. Food and Environmental Virology,9(4), 383–394. https://doi.org/10.1007/s12560-017-9306-4.
Fagnant, C. S., Sanchez-Gonzalez, L. M., Zhou, N. A., Falman, J. C., Eisenstein, M., Guelig, D., et al. (2018). Improvement of the bag-mediated filtration system for sampling wastewater and wastewater-impacted waters. Food and Environmental Virology,10(1), 72–82. https://doi.org/10.1007/s12560-017-9311-7.
Fagnant, C. S., Toles, M., Zhou, N. A., Powell, J., Adolphsen, J., Guan, Y., et al. (2017b). Development of an elution device for ViroCap virus filters. Environmental Monitoring and Assessment,189(11), 574. https://doi.org/10.1007/s10661-017-6258-y.
Falman, J. C., Fagnant-Sperati, C. S., Kossik, A. L., Boyle, D. S., & Meschke, J. S. (2019). Evaluation of secondary concentration methods for poliovirus detection in wastewater. Food and Environmental Virology,11(1), 20–31. https://doi.org/10.1007/s12560-018-09364-y.
Gerloff, N., Sun, H., Mandelbaum, M., Maher, C., Nix, W. A., Zaidi, S., et al. (2018). Diagnostic assay development for poliovirus eradication. Journal of Clinical Microbiology,56(2), e01624. https://doi.org/10.1128/jcm.01624-17.
Global Polio Eradication Initiative (2013). Polio Eradication & Endgame Strategic Plan 2013–2018. WHO/POLIO/13.02. Retrieved from http://www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/PEESP_EN_US.pdf.
Global Polio Eradication Initiative (2015). Guidelines on environmental surveillance for detection of polioviruses. March, 2015. Retrieved from http://polioeradication.org/wp-content/uploads/2016/07/GPLN_GuidelinesES_April2015.pdf.
Hampton, L. M., Farrell, M., Ramirez-Gonzalez, A., Menning, L., Shendale, S., Lewis, I., et al. (2016). Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine—Worldwide, 2016. Morbidity & Mortality Weekly Report,65(35), 934–938. https://www.cdc.gov/mmwr/volumes/65/wr/mm6535a3.htm?s_cid=mm6535a3_w.
Hovi, T., Blomqvist, S., Nasr, E., Burns, C. C., Sarjakoski, T., Ahmed, N., et al. (2005). Environmental surveillance of wild poliovirus circulation in Egypt—Balancing between detection sensitivity and workload. Journal of Virological Methods,126(1–2), 127–134. https://doi.org/10.1016/j.jviromet.2005.02.002.
Hovi, T., Shulman, L. M., van der Avoort, H., Deshpande, J., Roivainen, M., & De Gourville, E. M. (2012). Role of environmental poliovirus surveillance in global polio eradication and beyond. Epidemiology and Infection,140(1), 1–13. https://doi.org/10.1017/S095026881000316X.
Khetsuriani, N., Lamonte-Fowlkes, A., Oberste, S., & Pallansch, M. A. (2006). Enterovirus surveillance—United States, 1970–2005. MMWR: Surveillance Summaries,55(8), 1–20.
Kilpatrick, D. R., Ching, K., Iber, J., Chen, Q., Yang, S. J., De, L., et al. (2014). Identification of vaccine-derived polioviruses using dual-stage real-time RT-PCR. Journal of Virological Methods,197, 25–28. https://doi.org/10.1016/j.jviromet.2013.11.017.
Kilpatrick, D. R., Yang, C. F., Ching, K., Vincent, A., Iber, J., Campagnoli, R., et al. (2009). Rapid group-, serotype-, and vaccine strain-specific identification of poliovirus isolates by real-time reverse transcription-PCR using degenerate primers and probes containing deoxyinosine residues. Journal of Clinical Microbiology,47(6), 1939–1941. https://doi.org/10.1128/jcm.00702-09.
Kopel, E., Kaliner, E., & Grotto, I. (2014). Lessons from a public health emergency–importation of wild poliovirus to Israel. New England Journal of Medicine,371(11), 981–983. https://doi.org/10.1056/NEJMp1406250.
Mas Lago, P., Gary, H. E., Jr., Perez, L. S., Caceres, V., Olivera, J. B., Puentes, R. P., et al. (2003). Poliovirus detection in wastewater and stools following an immunization campaign in Havana, Cuba. International Journal of Epidemiology,32(5), 772–777.
Nakamura, T., Hamasaki, M., Yoshitomi, H., Ishibashi, T., Yoshiyama, C., Maeda, E., et al. (2015). Environmental surveillance of poliovirus in sewage water around the introduction period for inactivated polio vaccine in Japan. Applied and Environmental Microbiology,81(5), 1859–1864. https://doi.org/10.1128/aem.03575-14.
Shulman, L. M., Manor, Y., Sofer, D., Handsher, R., Swartz, T., Delpeyroux, F., et al. (2006). Neurovirulent vaccine-derived polioviruses in sewage from highly immune populations. PLoS ONE,1, e69. https://doi.org/10.1371/journal.pone.0000069.
Van Der Avoort, H. G., Reimerink, J. H., Ras, A. R., Mulders, M. N., & Van Loon, A. M. (1995). Isolation of epidemic poliovirus from sewage during the 1992-3 type 3 outbreak in The Netherlands. Epidemiology and Infection,114, 481–491.
World Health Organization (2004). Department of Immunization, Vaccines and Biologicals. Polio laboratory manual, 4th Edition 2004. Retrieved from http://whqlibdoc.who.int/hq/2004/WHO_IVB_04.10.pdf.
World Health Organization (2010). Department of Immunization, Vaccines and Biologicals. Supplement 1to the WHO Polio Laboratory Manual. An alternative test algorithm for poliovirus isolation and characterization. Retrieved from http://www.who.int/immunization_monitoring/Supplement_polio_lab_manual.pdf.
Zhou, N. A., Fagnant-Sperati, C. S., Shirai, J. H., Sharif, S., Zaidi, S. Z., Rehman, L., et al. (2018). Evaluation of the bag-mediated filtration system as a novel tool for poliovirus environmental surveillance: Results from a comparative field study in Pakistan. PLoS ONE,13(7), e0200551. https://doi.org/10.1371/journal.pone.0200551.
Acknowledgements
We are grateful to the local field staff at Hidalgo (Víctor Manuel Bustos Zamora, Gabriela Muños Villegas, Carlos Alberto de la Guardia Ángeles) and Mexico City (José Antonio Herrera Cobos, Jorge Morales López) for their contribution to the collection and initial processing of the environmental samples. We would also like to acknowledge Alexandra L Kossik for supporting the training of field and laboratory staff in the use of the bag-mediated filtration system; Dr. Steven G. F. Wassilak, Dr. Mark A. Pallansch and Dra. Tamara Mancero Buchelli, for their contributions to the study design and planning; Hongmei Liu and Jane Iber for sequencing; and Dr. Angela Coulliette-Salmond, and Jeff Shirai for their support to the coordination of logistics for study implementation.
Disclaimer
The findings and conclusions in this Article are those of the authors and do not necessarily represent the views of the Centers for Disease control and Prevention.
Funding
This study was supported by funding from the Centers for Disease Control and Prevention.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Estívariz, C.F., Pérez-Sánchez, E.E., Bahena, A. et al. Field Performance of Two Methods for Detection of Poliovirus in Wastewater Samples, Mexico 2016–2017. Food Environ Virol 11, 364–373 (2019). https://doi.org/10.1007/s12560-019-09399-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-019-09399-9