Abstract
The differences in physicochemical characteristics between infectious and non-infectious viral particles are poorly known. Even for heat, which is known as one of the most efficient treatments to inactivate enteric viruses, the global inactivation mechanisms have not been described yet. Such knowledge would help distinguish between both types of particles and therefore clarify the interpretation of the presence of viral genomes in food after heat treatment. In this study, we examined in particular the differences in electrostatic charge and hydrophobicity between the two particle types. MS2 phage, a common surrogate for enteric viruses, was used as a model virus. The heat-induced inactivation process of the infectious phages caused hydrophobic domains to be transiently exposed and their charge to become less negative. The particles also became progressively permeable to small molecules such as SYPRO Orange dye. The presence of non-infectious phage particles in which the genome was not accessible to RNases has been clearly demonstrated. These observations were done for MS2 phages exposed to a temperature of 60 °C. When exposed to a temperature higher than their critical temperature (72 °C), the particles were disrupted and the genome became available for RNases. At lower temperatures, 60 °C in this study, the transient expression of hydrophobic domains of remaining infectious phages appeared as an interesting parameter for improving their specific detection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last century, environmental virologists were interested in the behaviour and survival of infectious viruses only. The latter are defined as viruses able to complete their entire infectious life cycle inside their host cells. The reference method for their detection is therefore cell culture. The gradual discovery of non-culturable pathogenic viruses (e.g. norovirus, hepatitis E virus) and the use of molecular approaches (e.g. RT-PCR) for the detection of viral genomes in the environment and food commodities have completely changed the frame of reference. Detection of viral genomes includes infectious viruses, and also non-infectious viruses and even naked viral genomes. The latter two were at first neglected because since RNA was considered as not stable; the detected genomes were assumed to be linked to infectious viruses protected by a capsid. Today, such an assumption is no longer acceptable because it has been largely demonstrated that the persistence of the genome is much greater than that of the corresponding infectious virus in most cases (Gassilloud et al. 2003; Ogorzaly et al. 2010; Prevost et al. 2016; Seitz et al. 2011; Simonet and Gantzer 2006). Gaining knowledge about the characteristics of non-infectious viral particles, or more broadly about the molecular mechanisms of viral inactivation, has become a major objective for food and environmental virologists.
Little is known about the mechanisms of inactivation of enteric viruses. Temperature is recognized as one of the main viral inactivation factors (Bertrand et al. 2012). In the case of poliovirus, temperature has been shown to cause structural and conformational changes of its capsid, whose rate depends on the temperature applied. Poliovirus in its native state has a sedimentation coefficient of 160S (Belnap et al. 2000). Two different types of particles, distinguished by their sedimentation coefficients, have been identified after heat treatment: the 135S form followed by the 80S form (Belnap et al. 2000). The 135S particles—also known as “A particles”—have lost ≈73 % of their viral protein 4 (VP4) and externalized the N-terminus of viral protein 1 (VP1), but still contain viral RNA, which is not sensitive to RNase treatment (Curry et al. 1996). Interestingly, VP1 N-terminus externalization is directly linked to an increase in the hydrophobicity of the A particles (Fricks and Hogle 1990). Curry et al. (1996) suggested that despite an increase in hydrophobicity, infectivity is maintained for CHO cells or Murine L cells which are non-permissive to native poliovirus. The 80S particles described as empty capsids from transmission electron microscopy (TEM) experiments are not infectious and have lost mostly their viral RNA and VP4 (Breindl 1971; Levy et al. 2010). The 135S and 80S forms exhibited a 4 % larger size compared to the native poliovirus (Belnap et al. 2000). More recently, Wigginton et al. (2012) showed that temperature has an effect on the capsid because MS2 phage, one of the main surrogates of enteric viruses, lost its ability to recognize its receptor after heat treatment. The 72 °C heat treatment that was applied led to modifications of the capsid. On the other hand, it has been amply demonstrated that genome detection by RT-PCR is not markedly affected by heat (Baert et al. 2008; Wigginton et al. 2012). When considering all the data available, we can hypothesize that heat-inactivated viruses exhibit structural differences in their capsids which may contribute to the loss of infectivity. If so, the physicochemical properties of the viral capsid should be affected.
Thus, the purpose of our work was to evaluate the physicochemical changes that heat treatment induces in MS2 phage. MS2 phage was used as a model because of its similar size (≈22–29 nm), the presence of a positive-sense RNA genome, and an overall structure of the capsid close to enteric pathogenic viruses (Bae and Schwab 2008; Boudaud et al. 2012; Bozkurt et al. 2015; Deboosere et al. 2012; Shirasaki et al. 2009; Sidhu and Toze 2009; Valegård et al. 1990). This phage is non-pathogenic for humans and easy to culture in laboratory. Moreover, a high-phage concentration is easily achieved which was essential for some of the methods used in the present study. The first step was to confirm the presence of non-infectious particles still possessing a genome protected inside the capsid. In a second step, surface property modifications were compared before and after heat treatment by different methods. The selected strategy was to evaluate the changes in charge (electrophoretic mobility and adhesion to charged beads) and hydrophobicity (hydrophobic fluorescent dye labelling and adhesion to hydrophobic beads) that heat induced in infectious phages and in total phage particles (i.e. the whole viral genome detected by RT-PCR corresponding to infectious particles as well as non-infectious ones).
Materials and Methods
Production and Purification of Phages
Replication of MS2 bacteriophage (ATCC, 15597-B1) was done according to the procedure ISO 10705-1 (ISO 1995) without the CHCl3 lysis step, using Escherichia coli Hfr K12 (ATCC, 23631) as host cells. The produced phages were twice centrifuged (8000g, 20 min, 4 °C, Beckman Avanti J–E) and the supernatant was filtered using a 0.22-µm filter unit (Stericup-GP, Millipore, SCGPU05RE). The viral suspension was stored at 4 °C before proceeding to the purification steps. MS2 phages were pelleted upon ultracentrifugation of large volumes (≈1 L) of the phage suspension (42,500g, 18 h, 4 °C, Thermo Scientific Sorvall WX Ultra 80, A-621 fixed rotor). The pellets were resuspended in iodixanol solution (20 % final, Axis-Shield, OptiPrep) and laid on a 40 % iodixanol layer in a tube (Cone-Top PA tube 14 × 47 mm, Thermo Fisher Scientific, 75000454). The density gradient was formed upon centrifugation at 160,000g and 15 °C for 7 h (Thermo Scientific Sorvall WX Ultra 80, TH-641 swing rotor). The band corresponding to the position of the phages was then gently removed from the tube with a syringe. This solution was finally dialysed (Float-A-Lyzer G2 dialysis device, 100 kDa, 1 mL, Spectra/Por, G235035) twice (one overnight, the other for 7 h) in phosphate-buffered saline (PBS Fisher BioReagents, BP2944-100) with a concentration of 1 mM (1 mM Na2HPO4 and 14 mM NaCl). The working suspensions of MS2 phages were kept at 4 °C before use.
Infectivity Assay
Infectious MS2 phages were enumerated by plaque assay method using the double-agar-layer technique according to the ISO 10705-1 (ISO 1995). Ten-fold serial dilutions of the MS2 stock solutions were made to the appropriate dilution in PBS (for phage enumeration). Infective MS2 phage concentrations were measured as the number of plaque-forming unit per mL (PFU/mL). The final concentration of purified phages was ≈1014 PFU/mL.
Viral Genome Extraction and Quantification
Viral RNA was extracted from 50 µL samples using the QIAamp viral RNA kit (Qiagen, 52906) and eluted in 100 µL of elution buffer. The extracts were immediately stored at −80 °C before quantification. Reverse transcription was performed according to the manufacturer’s recommendations from 7.5 µL of RNA using 20 pmol of reverse primer (5′-ACCCCGTTAGCGAAGTTGCT), 100 U of SuperScript III (Life Technologies, 18080085) and 20 U of RNaseOUT (Life Technologies, 10777019) in a 20-µL reaction volume. RT step was performed at 50 °C for 60 min and 70 °C for 15 min. Quantitative PCR (Life Technologies, StepOne Plus) was then carried out from 5 µL of cDNA with TaqMan Universal PCR Master Mix (Life Technologies, 4318157) according to the manufacturer’s recommendations in a 25-µL reaction volume, with reverse and forward (5′-TCGATGGTCCATACCTTAGATGC) primers at a concentration of 1 µM and the TaqMan MGB probe (5′-FAM-CTCGTCGACAATGG-MGBNFQ) at a concentration of 0.3 µM. PCR amplification was performed at 50 °C for 2 min and 95 °C for 10 min, followed by 45 cycles of 15 s at 95 °C and 1 min at 60 °C. The primers and probe used in the RT-qPCR (Life Technologies, StepOne Plus) were as described by Ogorzaly and Gantzer (2006). Two negative controls were included in each experiment. One positive control of known quantity of phages was also included to ensure good reproducibility of extractions and RT-qPCR steps. The efficiency of the RT-qPCR stayed between 80 and 95 %.
Determination of MS2 genome copies per mL (gc/mL) used for the standard curve was performed using an MS2 extract quantified by a UV–Vis spectrophotometer (Thermo Scientific, NanoDrop 2000). Integrity of the MS2 RNA was checked by electrophoresis (Agilent, 2100 Bioanalyzer). The calculation for the genome copy per volume was performed with a genome of 3,569 nucleotides. The final concentration of purified phages was around 1015 gc/mL.
Heat Treatment
Purified phages at a concentration of 1014 PFU/mL (250 µL in 1 mM PBS solution with 1 mM Na2HPO4 and 14 mM NaCl) were exposed to different temperatures and time conditions −72 °C for 10 min, 60 °C for 10, 30 or 60 min—in prewarmed Protein LoBind tubes (Eppendorf, 0030108132) in a water bath. Samples were immediately placed on ice after heat treatment to prevent further inactivation.
RNase Assay
Suspensions of MS2 phage, with a final concentration of 107 gc/mL, were treated with RNase A (1 µg/mL final, AppliChem, A3832) at 37 °C for 60 min. Efficiency of the RNase treatment was estimated using RNA extracted from MS2 phage as positive control. MS2 phage suspensions without RNase treatment were used as negative controls for RT-qPCR and plaque assay. Control of the efficiency removal of the RNase was performed by a one ten-fold dilution of samples which gave us between 80 and 120 % of the undiluted RNA concentration from RNase assays.
Transmission Electron Microscopy
For transmission electron microscopy (TEM) experiments, the samples were adsorbed on grids and negatively stained with 2 % of phosphotungstic acid at pH 7.4 for 1 min which had been previously filtered with a 0.22-µm membrane filter. These stained samples were examined using a Philips CM20 electronic microscope operating at 200 kV and with ×50,000 magnification.
Binding of MS2 Phages to E. coli
Binding assays of MS2 phage to its host cell were carried out as described by Wigginton et al. (2012). In short, E. coli Hfr K12 was grown to an optical density of 0.3 according to the procedure ISO 10705-1 (ISO 1995) and then placed on ice. MS2 phages were next added to the bacterial suspension to a final concentration of 106 PFU/mL corresponding to a multiplicity of infection (MOI) of ≈ 0.01 in a 2-mL final volume in each Protein LoBind tube. The samples were incubated at 4 °C for 90 min using Dynal MX1 Mixer (Life Technologies) and then centrifuged at 3000g and 4 °C for 15 min. The pelleted bacteria were washed twice in their culture medium. Viral RNAs were then extracted from the pellet and supernatant (50 µL) using the QIAamp Viral RNA kit, and the RT-qPCR assay was used to quantify the number of phages bound to the bacteria and in the supernatant. The binding capacity of MS2 phage was estimated by \(\log \frac{Q}{{Q_{0} }}\) where Q is the viral RNA found on the bacteria after heat treatment (60 °C for 10, 30 and 60 min) and Q0 before heat treatment.
Size and Electrophoretic Mobility Measurements
Size and electrophoretic mobility measurements were performed in disposable capillary cells (Malvern Instruments, DTS1061) at 23 ± 0.1 °C using a Zetasizer Nano ZS instrument (Malvern Instruments, He–Ne red laser, wavelength 633 nm).
Experiments were driven by Dispersion Technology Software (Malvern Instruments, DTS) as described by Dika et al. (2011). Briefly, electrophoretic mobility measurements were obtained using laser Doppler electrophoresis also known as phase analysis light scattering. The rate of change of the phase shift between the scattered light and a reference beam is correlated with the particle velocity and thus allows for measurement of the electrophoretic mobility. The size distribution of the viral suspensions under investigation was determined using Dynamic Light Scattering (DLS). As a first approximation, the diffusion coefficient was converted into particle size using Stokes–Einstein equation.
The viral concentration used in these experiments ranged from 1011 to 1012 PFU/mL to get enough signal from the Zetasizer instrument. Size and electrophoretic mobility were measured either as a function of pH (from 2 to 7.4 in 1 mM PBS solution with 1 mM Na2HPO4 and 14 mM NaCl) or as a function of ionic strength 1.95, 19.5 and 195 mM (in 0.1, 1 and 10 mM PBS solutions, which contained 0.1 mM Na2HPO4 and 1.4 mM NaCl, 1 mM Na2HPO4 and 14 mM NaCl, and 10 mM Na2HPO4 and 140 mM NaCl, respectively). All solutions were filtered through a 0.22-µm membrane filter prior to use. For one pH or ionic strength, size and electrophoretic mobility measurements were obtained by means of three independent experiments with triplicate measures in each to ensure repeatability of the measurements.
Binding of MS2 Phages to Hydrophobic or Ion Exchange Beads
Two types of beads were used to evaluate binding of the viral particles. Hydrophobic magnetic beads are coated with polystyrene (PolySciences, 19133). Ion exchange beads are either BcMag PSA (Bioclone, FM-105) for anion exchange or BcMag SCX (Bioclone, FV-101) for cation exchange.
Prior to the experiments, the beads were washed twice in the buffer to be used in the assay, which was either 10 mM PBS (10 mM Na2HPO4 and 150 mM NaCl) at pH 7 for hydrophobic beads or 50 mM PBS (50 mM Na2HPO4 and 20 mM NaCl) at different pH values (2.5, 3.0, 4.0, 7.0) for ion exchange beads, as indicated by the manufacturers. The binding assays were performed with a viral concentration of 106 PFU/mL and 108 beads/mL, in a 2-mL final volume in each Protein LoBind tube. The binding assay using hydrophobic beads was carried out twice for 2 h at room temperature using Dynal MX1 Mixer (Life Technologies). Newly washed beads were used for the second run. With ion exchange beads, only one run was done for 10 min at room temperature using Dynal MX1 Mixer too. Then, the beads were separated from the supernatant with a magnet for 2–3 min. Supernatants were analysed by both plaque assay (infectious phages) and RT-qPCR (total phages) methods, and the beads were analysed only by RT-qPCR. The binding capacity of MS2 phage on beads was estimated by \(\log \frac{{C_{x} }}{{C_{0} }}\) where Cx is the concentration of either infectious phages or viral RNA after binding assay in suspension and C0 the initial concentration of either infectious phages or viral RNA in suspension.
Differential Scanning Fluorimetry
Differential scanning fluorimetry (DSF) was performed as previously described with some modifications (Subba-Reddy et al. 2012; Walter et al. 2012). Briefly, thermal melting curves of heated and unheated MS2 phages were obtained in 96-well plates using StepOne Plus qPCR System (Life Technologies) and the fluorescent dye SYPRO Orange, 5000 × concentrate, supplied in DMSO (Life Technologies, S-6650). The final reaction volume was 25 µL with 5 µL of buffer (150 mM Tris–HCl, pH 8), 2 µL of viral suspension from stock or after heat treatment (5.1014–1015 gc/mL), 16 µL of ultrapure water (>18 MΩ/cm) and 2 µL of SYPRO 62.5 × (final concentration = 5×). The experiments were ramped from 20 to 95 °C on a 1 % stepwise gradient corresponding to ≈1 °C/min with fluorescence measurements. Fluorescence intensity was determined by the sum of the readings in all channels of our qPCR system.
Statistical Analysis
Data from the RNase treatment were entered into Microsoft Excel 2010 (Microsoft Corporation) and analysed in StatEL 2.7 (Ad Science). Significant differences between groups were analysed by the Kruskal–Wallis test. A p value of <0.05 was considered as significant.
Results
Overall Effects of Heat on MS2 Phage
The overall effects of heat on the structure and infectivity of MS2 phage were estimated for 60 °C (10, 30 and 60 min) and 72 °C (10 min). The effect on the structure was evaluated by monitoring the capsid integrity, the size, as well as the persistence of the genome and its resistance to RNase treatment (Table 1). First, as shown by TEM, capsid integrity was not affected by any of the 60 °C treatments. In contrast, a 72 °C heat treatment for 10 min was found to disrupt the phage capsid as only a very small number of phage particles could be observed. Secondly, the mean size of MS2 phages did not change at 60 °C compared to the native phages (22 ± 1 nm) but increased at 72 °C to 37 ± 4 nm. The size distribution was also much larger for 72 °C with the presence of particles up to 100 nm in diameter. Thirdly, the persistence of the genome, estimated by RT-PCR, did not exhibit any significant changes for any of the conditions. Finally, RNase treatment did not alter the genome of native phages (−0.1 log10 degradation, Table 1), thus meaning that the difference between infectious and total RNA of 1/5–1/10 observed in stock suspensions was mainly due to the presence of the genome protected inside a capsid and not to the presence of naked RNA. The efficiency of the RNase treatment was checked on extracted MS2 RNA and a decrease of −2.9 ± 0.4 log10 was obtained. After 10 min heating at 60 °C, a transient −0.8 log10 increase in RNase sensitivity (p < 0.05, Kruskal–Wallis test) may be observed. This was not confirmed for either 30 or 60 min heat treatment (−0.2 log10). After 10 min heating at 72 °C, high degradation (−2.1 log10) was observed confirming capsid disruption with such a treatment. An incomplete digestion of the MS2 RNA by the enzyme considering the small size of the RT-qPCR product (169 bp on 3,569 bp) may explain the RNase inability to completely eliminate RNA amplification as previously proposed (Yang and Griffiths 2014).
The inactivation rate during heat treatment was evaluated by plaque assay and by the capacity of MS2 phage to bind to its receptor (F-pili of E. coli). The results are given in (Table 1). Infectivity decreased as temperature increased: −1 ± 0.1 log10 at 60 ± 0.2 °C and −8 log10 at 72 ± 0.2 °C. The inactivation rate was also correlated with contact time at 60 °C: −1.0 ± 0.1 log10 for 10 min, −2.0 ± 0.2 log10 for 30 min and −2.7 ± 0.4 log10 for 60 min. The reduction in the binding capacity to the host cells correlated fairly well with that in infectivity, except for 60 min where a slight difference was observed. Since disruption of the MS2 phage capsid was observed for viral particles at 72 °C using TEM assays, the binding assays were not performed at this temperature.
To sum up, the 60 °C heat treatments had no effect on the structure of MS2 phage (capsid aspect, size and genome), except for a suspected transient permeability to RNase after 10 min exposure. Moreover, it can be assumed with confidence that some changes occurred at the capsid level since high inactivation was observed, possibly due to the reduction in the capacity of the phage to bind to its host cell. As for the 72 °C heat treatment applied for 10 min, it disrupted the phage capsid. This led us therefore to use the 60 °C heat treatments only to evaluate the differences in charge and hydrophobicity between native and heat-inactivated phages.
Effect of Heat on the Charge of MS2 Phage Particles
The charge of MS2 phage was evaluated by electrophoretic mobility (µ) and by its adhesion capacity to positively or negatively charged beads.
Electrophoretic mobility experiments were performed on native and heated phage particles. These experiments were carried out as a function of ionic strength or of pH. No difference in behaviour was noted between native and heated particles whatever the conditions (Fig. 1a, b). The very same electrophoretic mobility profile was obtained after heating at 60 °C for 10, 30 and 60 min. The isoelectric points (IEP) for native and heated phage particles were between pH 3.0 and 3.5. These results showed that electrophoretic mobility was not suitable to identify charge differences for the total particles in suspension. It is important to note that total particles include infectious as well as non-infectious particles.
Electrophoretic mobility (µ) of MS2 bacteriophage a as a function of pH in 1 mM PBS or b as a function of the ionic strength (mM) at pH 7.4. The solid lines are only guides to the eye. Three independent experiments were performed, each time with three measurements. Error bars indicate standard deviations
The capacity of the phages to adhere to charged beads was assessed by plaque assay (PFU) and RT-qPCR. The two methods, as opposed to the electrophoretic mobility assay, allowed the charge of the infectious phages (PFU) and that of the total phages (RT-qPCR) to be estimated independently.
Two types of ion exchange magnetic beads were used in our experiments: one was negatively charged with a pKa of ≈2.3 and the other was positively charged with a pKa of ≈10. The experiments were performed in triplicate in 50 mM PBS for different pH values, for the two bead types, as well as for native and heated phages. Only one heating condition was selected: 60 °C for 10 min. Binding profiles of the native and heated phages were obtained on the positively and negatively charged beads (Fig. 2a, b respectively).
Binding assay of MS2 bacteriophage to charged beads in the case of native phages (empty forms) or heated phages (full forms) at 60 °C for 10 min in 50 mM PBS. The results obtained with positively and negatively charged beads are represented in A and B, respectively. The circle forms represent the total phages detected by RT-qPCR and the square forms the infectious phages detected by plaque assay. The dotted lines are only guides to the eye. Three independent experiments were performed. Error bars indicate standard deviations. CX, concentration after binding assay, C0, initial concentration
For both native and heated phages, the lowest adhesion capacity was obtained on negatively charged beads above the IEP (pH 4 and 7) and on positively charged beads below the IEP (3.0 and 2.5). Inversely, the highest adhesion capacity was obtained on negatively charged beads below the IEP and on positively charged beads above the IEP. It clearly confirmed that the IEP was between 3.0 and 4.0 for all types of particles as previously determined. For pH 7, differences in negative charge were observed between infectious (−2.6 log10 ± 0.1) and non-infectious (−1.6 log10 ± 0.1) phages in the native suspension. After heating, the negative charge of the infectious phages decreased, approaching the adhesion capacity of the non-infectious particles. For pH 3.0 and 2.5, results were more difficult to analyse because of (i) the proximity of the IEP of the phage particles, (ii) the possible aggregation of the phages and (iii) the proximity to the pKa of the negatively charged beads.
Overall, both native (infectious and non-infectious) and heated phages (infectious and non-infectious) had a negative charge for pH above 4 but native infectious phages had the highest negative charge.
Effect of Heat on the Hydrophobicity of MS2 Phage Particles
The hydrophobicity of MS2 phage was evaluated by DSF and by its adhesion capacity to hydrophobic beads.
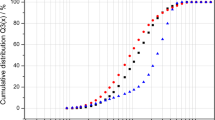
DSF experiments were performed with SYPRO Orange, a small dye with affinity for the hydrophobic regions of the proteins that become exposed upon heating. This method was able to measure the temperature at which proteins unfold (Niesen et al. 2007; Subba-Reddy et al. 2012; Walter et al. 2012). The DSF profile (fluorescence as a function of temperature) permitted the determination of the temperature at which the viral hydrophobic domains were accessible and therefore the melting temperature (Tm) of the phage particles. For native MS2 phages, Tm was estimated to be 66–67 °C meaning that significant conformational changes occurred around such temperatures (Fig. 3). After exposure to 60 °C (i.e. from 10 to 60 min), the initial fluorescence of the DSF profile progressively increased with heating time (Fig. 3). It meant that the dye was in part initially fixed on hydrophobic domains which could be located either on the capsid surface or/and inside the viral particle thus implying possible dye penetration. On the DSF profile, the decrease in fluorescence between 20 and 60 °C for heated phages could be due to probe quenching by water (Cimmperman and Matulis 2011). The Tm value of 66–67 °C was still observed on the DSF profile after a 60 °C treatment. On the other hand, no Tm could be determined after a 72 °C treatment which was certainly due to the low concentration of remaining intact phage particles. Such an observation confirmed the results obtained with TEM and RNase treatment regarding the disruption of the viral particles after exposure to 72 °C.
The binding capacity to hydrophobic beads was compared between native and heated phages (Fig. 4). To minimize the influence of charge, hydrophobicity was studied at pH 7 and high ionic strength (10 mM PBS with 10 mM Na2HPO4 and 150 mM NaCl). As described above on global charge, binding assays allowed the determination of hydrophobicity of the infectious phages (PFU) and that of the total phages (RT-qPCR) to be estimated independently. Due to the ratio of infectious phages on total phages of 1/5–1/10, total phages represented before and after heat treatment mainly non-infectious phages. For native phages, no adhesion was observed on these beads for both total and infectious phages. Therefore, few hydrophobic domains were expressed on the surface of MS2 particles as previously described (Dika et al. 2013). Subjecting phages to 60 °C for 10 min (Fig. 4) highly increased the binding of the remaining infectious phages (initially in suspension, 105 PFU/mL). The adhesion level reached 0.9 ± 0.4 log10 in the first run and 1.5 ± 0.2 log10 in the second run. For total phages, detected by RT-qPCR, no adhesion was observed during the two runs. Thus, the hydrophobicity of heat-inactivated phages was the same as that of native phages. It meant that heat treatment transiently increased the hydrophobicity of infectious MS2 phages before inactivating them.
Binding of native (empty forms) or heated (full forms) MS2 phages to hydrophobic magnetic beads at pH 7 in 10 mM PBS (10 mM Na2HPO4 and 150 mM NaCl) after 10 min heating at 60 °C. The circle forms represent the total phages detected by RT-qPCR and the square forms the infectious phages detected by plaque assay. Three independent experiments were performed. Error bars indicate standard deviations. CX, concentration after binding assay, C0, initial concentration
To conclude, these approaches showed a transitory increase in hydrophobicity at the surface of MS2 particles induced by heating at 60 °C for infectious particles only. An increase in permeability may have also contributed to the increase in hydrophobicity that was shown by the DSF profile. Heat treatment may, therefore, induce the expression of hydrophobic domains at the surface of infectious MS2 phages and increase permeability of the particles due to modifications of the phage capsid.
Discussion
The purpose of this study was to identify possible differences in surface properties between native and heated MS2 phages. To do so, overall charge and hydrophobicity were evaluated before and after heat treatment at 60 and 72 °C. We also studied the structure of the phage particles (physical aspect, size, genome degradation and resistance to RNase treatment) and their inactivation (loss of infectivity and host cell binding capacity).
For such a purpose, it was essential to carefully characterize the native MS2 phages prior to any treatment. In our study, infectious phages accounted for only 10–20 % of the total phages (i.e. genomes of infectious and non-infectious phages estimated by RT-qPCR using standard curve). The difference was not due to free viral RNA since our RNase treatment had no influence on native MS2 phages. Such a ratio could therefore be explained by the presence of incomplete and non-infectious phages that may not have been removed by the density gradient purification protocol. However, TEM observation results showed homogenous particles with a diameter of 22 nm. Previous studies had already identified such different particles (Krahn and Paranchych 1971; Paranchych et al. 1970). As observed after CsCl density gradient purification, ≈10–20 % of phages R17 were infectious for E. coli (Paranchych et al. 1970). A 10 % fraction of these non-infectious phages failed to bind to the F-pilus of E. coli due to a lack of the maturation protein (Krahn and Paranchych 1971). On the other hand, we could not exclude overestimation of the number of genome copies in the MS2 extract used for the standard curve and quantified by UV–Vis spectrophotometry, which measures all RNA including fragmented RNA. However, our initial phage suspension contained homogenous particles without free RNA but some of them were non-infectious for undefined reasons.
The first issue discussed here is the destruction of the phage particles by heat treatment. Heat is known to inactivate viruses but the conditions needed to disrupt the capsid still need to be specified. In this study, disruption is defined as a modification of the capsid that makes its observation by TEM impossible or the genome accessible to large molecules like RNase. Three major conclusions from our work allow identifying heat treatment conditions that disrupt the capsid: (i) few phage particles were observed by TEM after heating at 72 °C for 10 min, (ii) the sensitivity of the genome to RNase after heating at 72 °C was similar to that of the genome chemically extracted by nucleic acid extraction procedure and (iii) the DSF profile (≈1 °C/min) showed only one major conformational change for a Tm of ≈ 66–67 °C for native MS2 phages. Taking into account that heating at 60 °C did not disrupt the phage capsid whatever the exposure time (10–60 min), the critical temperature at which MS2 phage particles were disrupted may have been near 66–67 °C but surely strictly above 60 °C. Some literature data confirm such a conclusion but only for MS2 VLPs (Virus-Like Particles). Using differential scanning calorimetry (DSC), performed with a temperature increase of 5 °C/min, Bundy et al. (2008) and Ashcroft et al. (2005) obtained Tm values of 72.3 and 68.8 °C, respectively. In the case of Norwalk VLPs, similar temperature disruption was reported by TEM at 65 °C with an increase in temperature of 15 °C/h (Ausar et al. 2006). For bacteriophage λ, the release of its DNA was observed at ≈68 °C and seemed to follow the loss of the phage tail (Qiu 2012). Exposure to 72 °C led to genome accessibility to RNases for viruses such as hepatitis A virus or poliovirus (Nuanualsuwan and Cliver 2002; Pecson et al. 2009). For other viruses, the critical temperature seemed lower. Such was the case for echovirus 1, which was disrupted at 60 °C for 10 min (Ruokola et al. 2014), as well as for Feline Calicivirus, whose genome became accessible to RNases after a treatment at 60 °C for 2 min (Topping et al. 2009).
To sum up, above the critical temperature, non-infectious particles were mainly represented by free genomes. As a consequence, discrimination between the genome of infectious and non-infectious viruses can easily be obtained by an RNase treatment. Such treatment reduces false positive up to 3 log10 of genome. The critical temperature seemed to be strictly over 60 °C for the previous cited viruses, including for MS2 phage.
For temperatures lower than the critical limit (60 °C in our experiments), the presence of non-infectious structured particles in which the genome was still protected inside the capsid was clearly shown. Even after 60 min heating at 60 °C, no effect was identified on the overall particle structure (TEM), size (DLS) or genome (RT-PCR) or on the accessibility of the genome to RNase. On the other hand, inactivation of MS2 phage was significant and the capsid lost its capacity to bind to the host cell. A correlation between loss of infectivity and loss of host cell binding was observed as previously noted (Wigginton et al. 2012). This shows that modifications of the capsid were the cause of heat inactivation. Conformational changes at the capsid level could modify the charge or hydrophobicity of the particle.
The overall charge of the viruses could be determined by measuring their electrophoretic mobility and their adhesion to positively or negatively charged beads. Such an approach allowed monitoring, by a plaque assay, infectious phages among the total particles whose genomes were quantified by RT-qPCR. The electrophoretic mobility results concerning the IEP and overall charge of MS2 phages were in full agreement with literature data in the field (Dika et al. 2011; Langlet et al. 2008). No differences were observed using this method whatever the used conditions (i.e. as a function of ionic strength or of pH) which concerned mainly non-infectious phages. Nevertheless, other studies reported drastic changes. For example, heat treatment caused the IEP of native poliovirus to decrease from 7.5 to 4.5 (O’Brien and Newman 1979). This IEP decrease could have been related to a loss of VP4 and viral genomes and might have corresponded to the conversion from the 160S to the 80S form (Breindl 1971; O’Brien and Newman 1979). The capsid rearrangement after the loss of VP4 and genomes might have led to this lower IEP. In our study, the adhesion profiles of the total particles (mainly non-infectious) also confirmed the IEP of MS2 phage. They also showed that the charge did not change at pH 7 after heating at 60 °C for 10 min. They confirmed that the IEP of MS2 phage was between 3.0 and 4.0 as well. More interestingly, the results clearly showed that before heating, infectious phages exhibited higher adhesion capacity to positively charged beads than that of total phages which were mainly non-infectious phages. This modification of the charge density for infectious viruses was never described until yet.
With respect to the determination of the particle hydrophobicity, different approaches were used: interaction with a fluorescent probe and adhesion to hydrophobic support (Dika et al. 2013). Our results confirm data found in the literature saying that MS2 phage expresses relatively low hydrophobicity (Dika et al. 2013), but they also provide new knowledge on MS2 hydrophobicity through comparison between native and heated phages. The adhesion experiments showed that a 60 °C heat treatment leads to an increase in the hydrophobicity of the remaining infectious MS2 phages compared to the native infectious phages. Interestingly, non-infectious phages had low hydrophobicity like native phages. Therefore, a transient higher hydrophobic but still infectious state could be hypothesized. We propose a transient state between low hydrophobic infectious particles and low hydrophobic non-infectious particles composed of particles still infectious but with higher hydrophobicity than the other two particle types. This transitory state may potentially explain the facilitated accessibility of RNase to viral genome for the latter particles after 10 min heating at 60 °C. The DSF profile gave additional information as the initial fluorescence was found to correlate with the heat exposure time at 60 °C. The more the heat exposure time was increased, the more the hydrophobic domains were accessible to the probe. Nevertheless, this relationship was not gradual for adhesion capacity to hydrophobic beads so it may have been due to the diffusion of the SYPRO probe inside the capsid (SYPRO probe is smaller than RNase). Disruption of the capsid was still observed on the DSF profile at 66–67 °C whatever the heating time.
In the light of our results, this study demonstrated that heating at 60 °C slightly decreases the negative charge of the remaining infectious phages and transiently increases their hydrophobicity. The permeability to small molecules such as SYPRO probe, but not RNase, may be increased by heat exposure, thus establishing the existence of non-infectious particles in which the genome is still protected from RNases. At 72 °C, the particles are disrupted and the genome becomes accessible to RNase. Critical temperatures, permeability and expression of hydrophobic domains should now be explored for other enteric viruses to determine the application range of pretreatments able to discriminate infectious from non-infectious viruses prior to their detection by molecular tools. Nonetheless, some of the method used in this study will not be applicable to the low concentration of enteric viruses found in food samples.
References
Ashcroft, A. E., Lago, H., Macedo, J. M. B., Horn, W. T., Stonehouse, N. J., & Stockley, P. G. (2005). Engineering thermal stability in RNA phage capsids via disulphide bonds. Journal of Nanoscience and Nanotechnology, 5(12), 2034–2041. doi:10.1166/jnn.2005.507.
Ausar, S. F., Foubert, T. R., Hudson, M. H., Vedvick, T. S., & Middaugh, C. R. (2006). Conformational stability and disassembly of Norwalk virus-like particles. effect of pH and temperature. The Journal of biological chemistry, 281(28), 19478–19488. doi:10.1074/jbc.M603313200.
Bae, J., & Schwab, K. J. (2008). Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model of viral persistence in surface water and groundwater. Applied and Environmental Microbiology, 74(2), 477–484. doi:10.1128/AEM.02095-06.
Baert, L., Wobus, C. E., Van Coillie, E., Thackray, L. B., Debevere, J., & Uyttendaele, M. (2008). Detection of murine norovirus 1 by using plaque assay, transfection assay, and real-time reverse transcription-PCR before and after heat exposure. Applied and Environmental Microbiology, 74(2), 543–546. doi:10.1128/AEM.01039-07.
Belnap, D. M., Filman, D. J., Trus, B. L., Cheng, N., Booy, F. P., Conway, J. F., et al. (2000). Molecular tectonic model of virus structural transitions: the putative cell entry states of poliovirus. Journal of virology, 74(3), 1342–54. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=111469&tool=pmcentrez&rendertype=abstract
Bertrand, I., Schijven, J. F., Sánchez, G., Wyn-Jones, P., Ottoson, J., Morin, T., et al. (2012). The impact of temperature on the inactivation of enteric viruses in food and water: a review. Journal of Applied Microbiology, 112(6), 1059–1074. doi:10.1111/j.1365-2672.2012.05267.x.
Boudaud, N., Machinal, C., David, F., Fréval-Le Bourdonnec, A., Jossent, J., Bakanga, F., et al. (2012). Removal of MS2, Qβ and GA bacteriophages during drinking water treatment at pilot scale. Water Research, 46(8), 2651–2664. doi:10.1016/j.watres.2012.02.020.
Bozkurt, H., D’Souza, D. H., & Davidson, P. M. (2015). Thermal inactivation of foodborne enteric viruses and their viral surrogates in foods. Journal of Food Protection, 78(8), 1597–1617. doi:10.4315/0362-028X.JFP-14-487.
Breindl, M. (1971). The structure of heated poliovirus particles. The Journal of general virology, 11(3), 147–156. doi:10.1099/0022-1317-11-3-147.
Bundy, B. C., Franciszkowicz, M. J., & Swartz, J. R. (2008). Escherichia coli-based cell-free synthesis of virus-like particles. Biotechnology and Bioengineering, 100(1), 28–37. doi:10.1002/bit.21716.
Cimmperman, P., & Matulis, D. (2011). Protein thermal denaturation measurements via a fluorescent dye. Biophysical Approaches Determining Ligand Binding to Biomolecular Targets: Detection, Measurement and Modelling, (22), 247–273. http://pubs.rsc.org/en/content/chapter/bk9781849730099-00247/978-1-84973-009-9
Curry, S., Chow, M., & Hogle, J. M. (1996). The poliovirus 135S particle is infectious. Journal of virology, 70(10), 7125–31. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=190765&tool=pmcentrez&rendertype=abstract. Accessed 5 December 2014
Deboosere, N., Pinon, A., Caudrelier, Y., Delobel, A., Merle, G., Perelle, S., et al. (2012). Adhesion of human pathogenic enteric viruses and surrogate viruses to inert and vegetal food surfaces. Food Microbiology, 32(1), 48–56. doi:10.1016/j.fm.2012.04.007.
Dika, C., Duval, J. F. L., Ly-Chatain, H. M., Merlin, C., & Gantzer, C. (2011). Impact of internal RNA on aggregation and electrokinetics of viruses: comparison between MS2 phage and corresponding virus-like particles. Applied and Environmental Microbiology, 77(14), 4939–4948. doi:10.1128/AEM.00407-11.
Dika, C., Ly-Chatain, M. H., Francius, G., Duval, J. F. L., & Gantzer, C. (2013). Non-DLVO adhesion of F-specific RNA bacteriophages to abiotic surfaces: importance of surface roughness, hydrophobic and electrostatic interactions. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 435, 178–187. doi:10.1016/j.colsurfa.2013.02.045.
Fricks, C. E., & Hogle, J. M. (1990). Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. Journal of virology, 64(5), 1934–45. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=249347&tool=pmcentrez&rendertype=abstract. Accessed 5 December 2014
Gassilloud, B., Schwartzbrod, L., & Gantzer, C. (2003). Presence of viral genomes in mineral water: a sufficient condition to assume infectious risk? Applied and Environmental Microbiology, 69(7), 3965–3969. doi:10.1128/AEM.69.7.3965.
ISO. (1995). ISO 10705-1. Water quality: detection and enumeration of bacteriophages. Part 1: Enumeration of F-specific RNA bacteriophages.
Krahn, P. M., & Paranchych, W. (1971). Heterogeneous distribution of A protein in R17 phage preparations. Virology, 43(2), 533–5. http://www.ncbi.nlm.nih.gov/pubmed/5543844
Langlet, J., Gaboriaud, F., Duval, J. F. L., & Gantzer, C. (2008). Aggregation and surface properties of F-specific RNA phages: implication for membrane filtration processes. Water Research, 42(10–11), 2769–2777. doi:10.1016/j.watres.2008.02.007.
Levy, H. C., Bostina, M., Filman, D. J., & Hogle, J. M. (2010). Catching a virus in the act of RNA release: a novel poliovirus uncoating intermediate characterized by cryo-electron microscopy. Journal of Virology, 84(9), 4426–4441. doi:10.1128/JVI.02393-09.
Niesen, F. H., Berglund, H., & Vedadi, M. (2007). The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nature Protocols, 2(9), 2212–2221. doi:10.1038/nprot.2007.321.
Nuanualsuwan, S., & Cliver, D. O. (2002). Pretreatment to avoid positive RT-PCR results with inactivated viruses. Journal of virological methods, 104(2), 217–25. http://www.ncbi.nlm.nih.gov/pubmed/12088831
O’Brien, R. T., & Newman, J. (1979). Structural and compositional changes associated with chlorine inactivation of polioviruses. Applied and Environmental Microbiology, 38(6), 1034–1039. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=291240&tool=pmcentrez&rendertype=abstract
Ogorzaly, L., Bertrand, I., Paris, M., Maul, A., & Gantzer, C. (2010). Occurrence, survival, and persistence of human adenoviruses and F-specific RNA phages in raw groundwater. Applied and Environmental Microbiology, 76(24), 8019–8025. doi:10.1128/AEM.00917-10.
Ogorzaly, L., & Gantzer, C. (2006). Development of real-time RT-PCR methods for specific detection of F-specific RNA bacteriophage genogroups: application to urban raw wastewater. Journal of Virological Methods, 138(1–2), 131–139. doi:10.1016/j.jviromet.2006.08.004.
Paranchych, W., Krahn, P. M., & Bradley, R. D. (1970). Stages in phage R17 infection. Virology, 41(3), 465–473. doi:10.1016/0042-6822(72)90552-1.
Pecson, B. M., Martin, L. V., & Kohn, T. (2009). Quantitative PCR for determining the infectivity of bacteriophage MS2 upon inactivation by heat, UV-B radiation, and singlet oxygen: advantages and limitations of an enzymatic treatment to reduce false-positive results. Applied and Environmental Microbiology, 75(17), 5544–5554. doi:10.1128/AEM.00425-09.
Prevost, B., Goulet, M., Lucas, F. S., Joyeux, M., Moulin, L., & Wurtzer, S. (2016). Viral persistence in surface and drinking water: suitability of PCR pre-treatment with intercalating dyes. Water Research, 91, 68–76. doi:10.1016/j.watres.2015.12.049.
Qiu, X. (2012). Heat induced capsid disassembly and DNA release of bacteriophage λ. PLoS ONE, 7(7), e39793. doi:10.1371/journal.pone.0039793.
Ruokola, P., Dadu, E., Kazmertsuk, A., Häkkänen, H., Marjomäki, V., & Ihalainen, J. A. (2014). Raman spectroscopic signatures of echovirus 1 uncoating. Journal of Virology, 88(15), 8504–8513. doi:10.1128/JVI.03398-13.
Seitz, S. R., Leon, J. S., Schwab, K. J., Lyon, G. M., Dowd, M., McDaniels, M., et al. (2011). Norovirus infectivity in humans and persistence in water. Applied and Environmental Microbiology, 77(19), 6884–6888. doi:10.1128/AEM.05806-11.
Shirasaki, N., Matsushita, T., Matsui, Y., Urasaki, T., & Ohno, K. (2009). Comparison of behaviors of two surrogates for pathogenic waterborne viruses, bacteriophages Qbeta and MS2, during the aluminum coagulation process. Water Research, 43(3), 605–612. doi:10.1016/j.watres.2008.11.002.
Sidhu, J. P. S., & Toze, S. G. (2009). Human pathogens and their indicators in biosolids: a literature review. Environment International, 35(1), 187–201. doi:10.1016/j.envint.2008.07.006.
Simonet, J., & Gantzer, C. (2006). Degradation of the Poliovirus 1 genome by chlorine dioxide. Journal of Applied Microbiology, 100(4), 862–870. doi:10.1111/j.1365-2672.2005.02850.x.
Subba-Reddy, C. V., Yunus, M. A., Goodfellow, I. G., & Kao, C. C. (2012). Norovirus RNA Synthesis Is Modulated by an Interaction between the Viral RNA-Dependent RNA Polymerase and the Major Capsid Protein, VP1. Journal of Virology, 86(18), 10138–10149. doi:10.1128/JVI.01208-12.
Topping, J. R., Schnerr, H., Haines, J., Scott, M., Carter, M. J., Willcocks, M. M., et al. (2009). Temperature inactivation of Feline calicivirus vaccine strain FCV F-9 in comparison with human noroviruses using an RNA exposure assay and reverse transcribed quantitative real-time polymerase chain reaction-A novel method for predicting virus infectivity. Journal of Virological Methods, 156(1–2), 89–95. doi:10.1016/j.jviromet.2008.10.024.
Valegård, K., Liljas, L., Fridborg, K., & Unge, T. (1990). The three-dimensional structure of the bacterial virus MS2. Nature, 345(6270), 36–41. doi:10.1038/345036a0.
Walter, T. S., Ren, J., Tuthill, T. J., Rowlands, D. J., Stuart, D. I., & Fry, E. E. (2012). A plate-based high-throughput assay for virus stability and vaccine formulation. Journal of Virological Methods, 185(1), 166–170. doi:10.1016/j.jviromet.2012.06.014.
Wigginton, K. R., Pecson, B. M., Bosshard, F., Kohn, T., & Sigstam, T. (2012). Virus inactivation mechanisms: impact of disinfectants on virus function and structural integrity. Environmental Science and Technology, 46(21), 12069–12078. doi:10.1021/es3029473.
Yang, Y., & Griffiths, M. W. (2014). Enzyme Treatment Reverse Transcription-PCR To Differentiate Infectious and Inactivated F-Specific RNA Phages. Applied and Environmental Microbiology, 80(11), 3334–3340. doi:10.1128/AEM.03964-13.
Acknowledgments
The results of this study were obtained within the scope of CapsiVir, a project coordinated by ACTALIA and funded by the “Conseil Régional de Basse Normandie”. This study, labelled by the competitiveness cluster VALORIAL, was also supported by the Joint Technical Unit ACTIA VIROcontrol and the “Syndicat des Fabricants des Produits Frais Prêts à l’Emploi” (cluster of ready-to-eat food industries: Florette, Bonduelle, Crudettes and Rosée des Champs). We thank Sylvie Migot (IJL, UMR 7198, Nancy, France) and Dr. Céline Caillet (LIEC, UMR 7360, Vandoeuvre-les-Nancy, France) for their help and support with TEM and DLS/electrophoretic mobility experiments, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brié, A., Bertrand, I., Meo, M. et al. The Effect of Heat on the Physicochemical Properties of Bacteriophage MS2. Food Environ Virol 8, 251–261 (2016). https://doi.org/10.1007/s12560-016-9248-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-016-9248-2