Abstract
Aqueous Hibiscus sabdariffa extracts possess antimicrobial properties with limited information available on their antiviral effects. Aichi virus (AiV) is an emerging foodborne pathogen that causes gastroenteritis. Vaccines are currently unavailable to prevent their disease transmission. The objective of this study was to determine the antiviral effects of aqueous H. sabdariffa extracts against AiV. AiV at ~5 log PFU/ml was incubated with undiluted (200 mg/ml), 1:1 (100 mg/ml) or 1:5 (40 mg/ml) diluted aqueous hibiscus extract (pH 3.6), phosphate-buffered saline (pH 7.2 as control), or malic acid (pH 3.0, acid control) at 37 °C over 24 h. Treatments were stopped by serially diluting in cell-culture media containing fetal bovine serum and titers were determined using plaque assays on confluent Vero cells. Each treatment was replicated thrice and assayed in duplicate. AiV did not show any significant reduction with 1:1 (100 mg/ml) or 1:5 (40 mg/ml) diluted aqueous hibiscus extracts or malic acid after 0.5, 1, or 2 h at 37 °C. However, AiV titers were reduced to non-detectable levels after 24 h with all the three tested concentrations, while malic acid showed only 0.93 log PFU/ml reduction after 24 h. AiV was reduced by 0.5 and 0.9 log PFU/ml with undiluted extracts (200 mg/ml) after 2 and 6 h, respectively. AiV treated with 1:1 (100 mg/ml) and 1:5 (40 mg/ml) diluted extracts showed a minimal ~0.3 log PFU/ml reduction after 6 h. These extracts show promise to reduce AiV titers mainly through alteration of virus structure, though higher concentrations may have improved effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aichi virus (AiV) is an emerging foodborne enteric virus that was first associated with an outbreak of diarrhea that occurred in March, 1989 in Aichi Prefecture, Japan (Yamashita et al. 1991). It was found in stool specimens of patients who had consumed raw oysters (Yamashita et al. 1991). AiV belongs to the Picornaviridae family under the genus Kobuvirus, with currently three different known species, A, B, and C (Yamashita et al. 1991, 1995, 1998, 2001, 2014). AiV is a single-stranded, positive-sense, non-enveloped RNA virus, small and round in structure with a size of ~30 nM in diameter (Yamashita et al. 1995, 1998, 2001). The length of the RNA excluding the poly (A) tail is 8374 nucleotides, where the nonstructural protein L (leader) is encoded at the N-terminus followed by three structural capsid proteins VPO, VP3, and VP1 and seven nonstructural proteins 2A, 2B, 2C, 3A, 3B, 3C, and 3D (Yamashita et al. 2014).
AiV is primarily transmitted by the fecal-oral route, and contaminated water and shellfish enable the spread of this emerging virus (Le Guyader et al. 2008; Goyer et al. 2008; Yamashita et al. 1991). AiV causes gastroenteritis with an incubation period ranging from 3 to 7 days (Reynolds 2013). The common symptoms of AiV infection include nausea, abdominal pain, fever, vomiting, and diarrhea that can last for 3–10 days (Lodder et al. 2013; Reynolds 2013; Švraka-Latifovic 2011). AiV has been reported to have different circulation and infection rates among different populations, and thus far has been isolated from Japan, Pakistan, Southeast Asia, Brazil, Germany, France, Tunisia, Hungary, China, and Finland indicating its worldwide emergence (Blanton et al. 2006; Hall et al. 2014; Lodder et al. 2013; Reuter et al. 2009; Ribes et al. 2010; Scallan et al. 2011; Yamashita et al. 1991, 1995, 1998, 2001, 2014). AiV is known to have a cytopathic effect on Vero and BSC-1 cells and can be cultivated in the lab (Yamashita et al. 2014). Currently, there are no vaccines available for AiV prophylaxis and the infectious doses for AiV are unknown.
Very few studies have been reported in literature on methods to control the spread of this virus and its disease that include processing approaches such as heat, high hydrostatic pressure, disinfection by ultraviolet light, chlorine, and various alcohol-based sanitizers (Cromeans et al. 2014; Fino and Kniel 2008; Kingsley et al. 2004). Hence, it is important to study the effects of alternate control measures including natural antimicrobials to control its spread. Hibiscus sabdariffa of the family Malvaceae, commonly referred to as “roselle” is an annual, tropical, or subtropical plant, typically used for ornamental purposes (Morton 1987; Da-Costa et al. 2014). However, their red calyces are also used to prepare beverages, jams, jellies, salad toppings, or coloring, that are rich in bioactives including anthocyanins, phenolic acids, organic acids (malic and tartaric), saponins, and alkaloids (Ali et al. 2005; Joshi et al. 2015; Kakkar and Bais 2014; Morton 1987; Saini et al. 2012; Tsai et al. 2002; Da-Costa et al. 2014). Their aqueous extracts are considered GRAS (generally recognized as safe) as a food additive in beverage flavorings by the U.S. Food and Drug Administration (21 CFR 172.510) (Joshi et al. 2015). H. sabdariffa calyces are known to possess antioxidant, anti-diabetic, anticancer, cardioprotective, and antimicrobial properties (Borges et al. 2013; Cowan 1999; Lin et al. 2005; Liu et al. 2005; McKay et al. 2010; Yang et al. 2013; Joshi et al. 2015). These extracts have been reported to have antimicrobial effects against Staphylococcus aureus (including methicillin-resistant S. aureus (MRSA)), S. epidermidis, Escherichia coli, E. coli O157:H7 (Olaye, 2007; Fullerton et al. 2011); Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii (Liu et al. 2005), Propionibacterium acnes (Chomnawang et al. 2005), Salmonella Typhimurium, Listeria monocytogenes, Campylobacter, and Bacillus cereus (Chao and Yin 2009; Liue et al. 2005; Ravishankar et al. 2012; Yin and Chao 2008). Methanolic extracts of whole plants of dried H. sabdariffa were reported to have antibacterial activity against E. coli, Enterobacter cloacae, E. aerogenes, K. pneumoniae, P. aeruginosa, and P. stuartii with minimum inhibitory concentrations (MIC’s) ranging from 128 to 1024 µg/ml (Djeussi et al. 2013). Water extracts of H. sabdariffa were also shown to inhibit the growth of E. coli, B. subtilis, and S. aureus with higher antimicrobial effects with increasing tested concentrations that ranged from 25 to 200 mg/ml as determined by a paper disk method (Jung et al. 2013).
Literature is currently scarce on the antiviral effects of aqueous H. sabdariffa extracts. Only recently our lab reported that aqueous H. sabdariffa extracts (HE) showed promising results for the reduction of cultivable human norovirus surrogates and hepatitis A virus (Joshi et al. 2015). Feline calicivirus (FCV-F9) titers were shown to be reduced to undetectable levels after 15 min, while murine norovirus (MNV-1) was reported to be reduced by 1.77 ± 0.10 and 1.88 ± 0.12 log PFU/ml after 6 h at 37 °C with 40 and 100 mg/ml HE, respectively (Joshi et al. 2015). MNV-1 was shown to be further reduced to undetectable levels after 24 h at 37 °C by both 40 and 100 mg/ml HE concentrations, and HAV titers were reduced to undetectable levels by both HE concentrations after 24 h at 37 °C (Joshi et al. 2015).
Therefore, the objective of this study was to (1) determine the antiviral effects of aqueous extracts of H. sabdariffa calyces (HE) against the infectious titers of AiV at 37 °C over 24 h using plaque assays; and (2) to gain insights on the mode of action of these extracts on the AiV capsid structure using transmission electron microscopy (TEM) and also using time-of-addition assays to determine their effects on host-cell binding and viral replication.
Materials and Methods
Hibiscus sabdariffa Aqueous Extract Preparation
Dried H. sabdariffa calyces (commercially available, MiCostenita brand) were locally obtained from a grocery store. Twenty-five grams of these dried calyces were added to 125 ml (to make a 200 mg/ml working stock) of deionized distilled water and boiled for 1 min, the supernatant was first filtered, centrifuged at 4000×g for 3 min, and then filter-sterilized through a 0.2-µm filter and stored at 4 °C until further use as reported before (Joshi et al. 2015). The filtered stock was further aseptically diluted in sterile water, stored at refrigeration, or used immediately.
Aichi Virus and Host Cells
Aichi virus (AiV) was obtained as a gift from Dr. David Kingsley (USDA ARS, Delaware). The Vero host cells were propagated in 175 cm2 flasks in Dulbecco’s Modified Eagle’s Medium/Ham’s F-12 (DMEM-F12; HyClone Laboratories, Logan, UT) containing 2 % heat-inactivated fetal bovine serum (FBS, HyClone Laboratories) and 1 × Anti–Anti (Antibiotic–Antimycotic, Invitrogen, Grand Island, NY) at 37 °C under 5 % CO2 in a water-jacketed CO2 incubator. AiV was propagated using previously reported standard procedures (Fino and Kniel 2008). Briefly, AiV stocks were prepared by infecting Vero host cells (when 90 % confluent) in 175 cm2 flasks for 3 days, under 5 % CO2 at 37 °C. The infected cells were then freeze-thawed at least twice, centrifuged at 5000×g for 10 min, and the supernatant was filtered through a 0.2-μm filter, stored at −80 °C until further use, and plaque assayed to determine infectious titers.
Antiviral Effect of Aqueous H. sabdariffa Extracts
Equal volumes of AiV (~5 log PFU/ml) were mixed with aqueous H. sabdariffa extracts (HE) (40, 100, or 200 mg/ml; pH 3.6), malic acid (10 mM; pH 3.0 as a control), or 10 mM phosphate-buffered saline (PBS, pH 7.2 as another control) and incubated at 37 °C for 0.5, 1, 2, 6, or 24 h as described before for human norovirus surrogates and hepatitis A virus (HAV) (Joshi et al. 2015). The pH of the virus–HE mix was around pH 5.0. Treatments were stopped by diluting in cell-culture media containing 10 % heat-inactivated fetal bovine serum (FBS), followed by serial dilutions in cell-culture media containing 2 % FBS and AiV infectivity was determined using standard plaque assays (Fino and Kniel 2008; Kingsley et al. 2004). Each experiment was replicated thrice and assayed in duplicate.
Time-of-Addition Assays
Time-of-addition assays were conducted in order to determine the mode of action of aqueous HE against infectious AiV using previously described procedures (Joshi et al. 2015; Su and D’Souza, 2013; Su et al. 2010a, 2010b). Aqueous HE (at a 1:40 dilution; 5 mg/ml; at the non-toxic levels to the host cells) was directly added to confluent Vero cells prior to infection in order to determine the effect on blocking of AiV attachment to the Vero host cells.
Cytotoxicity assays using serial dilutions of HE in DMEM-F12 media containing FBS to confluent Vero cells followed by incubation for up to 3 d were carried out and cells were inspected visually and under the light microscope. Cells treated with the various dilutions of the extracts were also overlaid with DMEM-F12 containing 0.75 % agarose, incubated for 3 d, and then stained with neutral red solution. Cytopathic effects were determined by both visual inspection under the optical light microscope and by neutral red staining.
Aqueous HE at 5 mg/ml (at the non-toxic levels to the host cells) was also added after allowing for virus infection of the Vero host cells to determine the effect of HE treatment on viral replication. Standard plaque assay procedures were then followed as described above.
Transmission Electron Microscopy (TEM) Analysis
AiV at ~7 log PFU/ml was mixed with equal volumes of 100 mg/ml HE or sterile distilled water (control) and incubated at 37 °C for 6 and 24 h. The previously described procedures were followed for staining and visualization by TEM. Briefly, fresh glow-discharged, formvar- and carbon-coated copper grids were coated with 10 μl treated and untreated AiV samples, stained with uranyl acetate for 1 min, and allowed to dry (Joshi et al. 2015; Su and D’Souza, 2013; Su et al. 2010a, 2010b). The stained AiV samples were visualized under a Hitachi H800 transmission electron microscope at 75 keV (with the assistance of Dr. Dunlap) at the UT-Knoxville Advanced Microscopy and Imaging Center.
Statistical Analysis
Statistical analysis was carried out using ANOVA with SAS software (version 9.3, SAS Institute, Cary, NC, USA) and Tukey’s test as described earlier on a completely randomized design with data obtained from three replicates of treated AiV and control AiV (Joshi et al. 2015; Su and D’Souza 2013; Su et al. 2010a, 2010b).
Results
Aichi Virus Infectious Titer Reduction by Aqueous H. sabdariffa Extracts
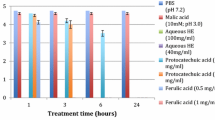
AiV did not show any significant reduction in titers after incubation with 1:1 (100 mg/ml) or 1:5 (40 mg/ml) diluted hibiscus extracts or malic acid after 0.5, 1, or 2 h at 37 °C (p > 0.05). Aqueous HE at 40 mg/ml showed 0.39–0.51 log PFU/ml reduction in AiV titers after 2 and 6 h and reduction to non-detectable levels after 24 h at 37 °C. Similarly, aqueous HE at 100 mg/ml showed minimal reduction in AiV titers from 0.15 to 0.51 log PFU/ml after 2 and 6 h and reduction to non-detectable levels after 24 h at 37 °C. Aqueous HE at 200 mg/ml showed reductions in AiV titers from 0.51 to 0.91 log PFU/ml after 2 and 6 h, and reduction to non-detectable levels after 24 h at 37 °C (Fig. 1). Thus, it appears that higher concentrations of HE are needed to show faster antiviral effects against AiV. Malic acid (10 mM, pH 3.0) resulted in only 0.9 log PFU/ml reduction of AiV titers after 24 h.
Reduction of Aichi virus (AiV) titers after treatment with aqueous extracts of hibiscus calyces (40 mg/ml (1:5 dilution), 100 mg/ml (1:1 dilution), and 200 mg/ml (undiluted)) over 24 h at 37 °C. Each experiment was carried out in duplicate and replicated thrice. (Error bars denote standard deviations from three replicate experiments). Reduction to non-detectable levels were obtained after 24 h with undiluted (200 mg/ml) and diluted (40 and 100 mg/ml) HE
Time-of-Addition Assays
Pre- and post-infection assays with the aqueous HE (5 mg/ml) on Vero host cells showed minimal reductions in AiV titers, where mere reductions of only ~0.17 and 0.11 log PFU/ml, respectively were observed from initial titers of 5.8 or 5.6 log PFU/ml (Table 1). Thus, pre-infection treatment results showed that there was minimal to no effect on blocking of binding of virus to the host-cell receptors. Similarly, post-infection treatment assays showed that there was minimal effect on the inhibition of viral replication (note when using the low concentration of 5 mg/ml HE that was not toxic to the host cell).
Transmission Electron Microscopy of Treated and Untreated AiV Samples
Transmission electron microscopy showed coating of the HE-treated AiV particles compared to controls and deformation/reduction in shape/size of the HE-treated AiV particles compared to intact untreated AiV particle images (Fig. 2).
Discussion
Aqueous HE was found to be effective in reducing AiV titers in a concentration and time-dependent manner at 37 °C. All three tested concentrations (40, 100, and 200 mg/ml) of aqueous HE showed reduction of AiV to non-detectable levels after 24 h. Undiluted aqueous extracts (200 mg/ml) showed 0.91 log PFU/ml reduction in AiV titers after 6 h compared to 0.5 log PFU/ml reductions observed after 6 h with 100 mg/ml HE. It is also important to note that our concentrated stock of 200 mg/ml HE was stored at refrigeration temperature (4 °C) and used over a month that showed stability as it resulted in consistent antiviral activity. The working stock was further diluted to freshly prepare lower concentrations from the 200 mg/ml HE just before use. From the results obtained against AiV, higher concentrations and longer treatment times with HE seem to have an improved antiviral effect against AiV titers that may be suitable to decrease or prevent transmission and outbreaks related to AiV. However, these are only preliminary in vitro results and hence, further studies with food systems and simulated gastric conditions as well as feeding studies and clinical trials are needed. Also, appropriate regulatory approvals are needed before any health claims can be made for use.
When comparing the reduction of AiV titers by HE to human norovirus surrogates, murine norovirus (MNV-1) titers were reported to be reduced by 1.19 ± 0.02, 1.30 ± 0.02, and 1.77 ± 0.10 log PFU/ml after treatment with 40 mg/ml HE for 1, 3, and 6 h at 37 °C, respectively and to undetectable levels after 24 h, while reductions of 1.18 ± 0.04, 1.27 ± 0.01, and 1.88 ± 0.12 log PFU/ml were obtained with 100 mg/ml HE after 1, 3, and 6 h, respectively and to undetectable levels after 24 h at 37 °C (Joshi et al. 2015). Aqueous HE was also found to be effective in reducing FCV-F9 titers to undetectable levels (<102 PFU/ml detection limit of the assay) after 15 min at 37 °C with 40 and 100 mg/ml aqueous HE concentrations (Joshi et al. 2015). Thus, it appears that AiV is more resistant than MNV-1 and also FCV-F9 to treatment with aqueous HE at lower concentrations and incubation times at 37 °C.
Similarly, AiV appears to be more resistant to aqueous HE treatment than even the sturdy hepatitis A virus, where HE at 40 mg/ml was shown to reduce HAV by 1.29 ± 0.05 and 1.14 ± 0.01 log PFU/ml after 3 and 6 h and to undetectable levels after 24 h, and HE at 100 mg/ml reduced HAV by 1.37 ± 0.02 and 1.33 ± 0.01 log PFU/ml after 3 and 6 h and reduction to undetectable levels after 24 h (Joshi et al. 2015).
As 10 mM malic acid (pH 3.0) resulted in only 0.9 log PFU/ml reduction after 24 h, the main effects of HE against AiV do not seem to be due to pH alone but are also potentially associated with the bioactivity of the entire aqueous extract together with pH that caused reduction to non-detectable levels after 24 h. The time-of-addition assays did not provide any useful insights on the effects of aqueous HE (at the tested concentrations) on either prevention of AiV attachment to the host cell or on inhibition of AiV replication. However, TEM analysis did reveal coating of the treated AiV particles and deformation in size and shape of the treated AiV particles compared to the observed intact untreated AiV particles. Therefore, it appears that the aqueous HE at higher concentrations can play a role by having an effect on the capsid structure and thus reducing infectivity of AiV particles.
Ethanolic extracts of H. sabdariffa leaves (10 and 15 mg/ml) were reported to have antiviral properties against measles virus, as a pre-infection treatment, that caused inhibition of binding to host-cell receptors thus preventing the virus adsorption to host cells (Sunday et al. 2010). However, no significant change in titers was observed in the case of host-cell pre-infection treatment or post-infection treatment with aqueous HE in the current study with AiV. Thus, it could be that the aqueous HE antiviral effect is by direct action on the AiV particles that cause decrease in recovery of infectious titers. Epigallocatechin gallate at 12 mg/ml obtained from green tea extract has also been reported to have significant antiviral activity against the cultivable human norovirus surrogate, FCV-F9 (Oh et al. 2013), which is similar to that obtained after 15 min treatment with 40 mg/ml of aqueous HE that caused FCV-F9 reduction to undetectable levels (Joshi et al. 2015). However, AiV does appear to be more resistant to treatment with aqueous HE than FCV-F9 as it requires 24 h treatment with aqueous HE for reduction to non-detectable levels to occur.
Plant phenolic extracts are also reported to have antiviral effects against foodborne viruses that cause human gastroenteritis. Cranberry proanthocyanidins (C-PAC) at 0.03 % was reported to decrease FCV-F9, MNV-1, as well as bacteriophage MS2 and ϕ-X174 titers by 5.0, 2.8, 0.8, and 3.7 log PFU/ml, respectively from initial viral titers of ~5 log PFU/ml after 1 h at room temperature (Su et al. 2010a, 2010b). Commercial grape seed extract (GSE) at 0.025 % (Gravinol-S) was also reported to reduce FCV-F9, MNV-1, hepatitis A virus, and bacteriophage MS2 at initial ~5 log PFU/ml titer levels by 5.0, 1.5, 1.9, and 1.4 log PFU/ml, respectively after 2 h at 37 °C (Su and D’Souza 2011). Also, pomegranate polyphenols (PP) at 2 mg/ml was reported to decrease FCV-F9 titers from ~5 log PFU/ml to undetectable levels within 30 min at room temperature, and decreased MNV-1 titers by 0.5 and 0.9 log PFU/ml after 10 and 60 min, respectively (Su et al. 2011). For FCV-F9 and MNV-1, the reported activities of the polyphenols based on the tested concentrations, temperatures, and times, appear to follow the order of C-PAC > GSE > PP > HE. Thus, overall AiV appears to be more resistant to the tested concentration of HE at 37 °C than the tested foodborne viruses.
There appears to be a growing consumer demand for hibiscus tea and hibiscus use in the food industry. This study on the antiviral effects of aqueous H. sabdariffa extracts against AiV show promise for their use as additives in food and beverages or for direct consumption to decrease foodborne viral illness incidence or to alleviate human foodborne viral illness symptoms. As reported previously by other researchers and discussed previously (Joshi et al. 2015), hibiscus extracts at 40 mg/ml was also shown to reduce S. aureus to undetectable levels after 168 h in skim and whole milk, while E. coli was shown to be reduced to undetectable levels after 96 h with 60 mg/ml hibiscus extract in skim and whole milk (Higginbotham et al. 2014a). For direct food application as rinses, 1 h treatment of artificially spiked hotdogs with 240 mg/ml hibiscus extracts was shown to reduce L. monocytogenes to 1.5 log CFU/ml and MRSA to undetectable levels from initial levels of 9 log CFU/ml when used as an antimicrobial hot dog rinse (Higginbotham et al. 2014b). Additionally, pectin-based apple, carrot, and hibiscus edible films containing carvacrol and cinnamaldehyde were shown to inactivate L. monocytogenes (Ravishankar et al. 2012). Therefore, as indicated above, further studies need to be carried out with these aqueous Hibiscus sabdariffa extracts in model food systems, and also for their use as washes or edible films, and in simulated gastrointestinal tract conditions to test their effects against AiV titers under in vivo conditions. Higher concentrations of aqueous HE and longer incubation times may be needed for optimal antiviral effects against AiV.
Conclusions
Aqueous Hibiscus sabdariffa calyx extracts show potential to decrease Aichi virus titers after 24 h at 37 °C. However, the antiviral effect is lower than that observed for human norovirus surrogates. The extracts at the tested concentrations did not show inhibition of viral replication or blocking of binding to host-cell receptors, though capsid structure alteration was observed. Thus, the main effect could potentially be due to damage of the viral structure or coating of the viral particles.
References
Ali, B. H., Al Wabel, N., & Blunden, G. (2005). Phytochemical, pharmacological and toxicological aspects of Hibiscus sabdariffa L.: A review. [Review]. Phytotherapy Research, 19(5), 369–375. doi:10.1002/ptr.1628.
Blanton, L. H., Adams, S. M., Beard, R. S., Wei, G., Bulens, S. N., Widdowson, M. A., et al. (2006). Molecular and epidemiologic trends of caliciviruses associated with outbreaks of acute gastroenteritis in the United States, 2000–2004. Journal of Infectious Diseases, 193(3), 413–421. doi:10.1086/499315.
Borges, A., Ferreira, C., Saavedra, M. J., & Simoes, M. (2013). Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. [Research Support, Non-U.S. Gov’t]. Microbial Drug Resistance, 19(4), 256–265. doi:10.1089/mdr.2012.0244.
Chao, C. Y., & Yin, M. C. (2009). Antibacterial effects of roselle calyx extracts and protocatechuic acid in ground beef and apple juice. Foodborne Pathogens and Disease, 6(2), 201–206. doi:10.1089/fpd.2008.0187.
Chomnawang, M. T., Surassmo, S., Nukoolkarn, V. S., & Gritsanapan, W. (2005). Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. [Research Support, Non-U.S. Gov’t]. Journal of Ethnopharmacology, 101(1–3), 330–333. doi:10.1016/j.jep.2005.04.038.
Cowan, M. M. (1999). Plant products as antimicrobial agents. [Review]. Clinical Microbiology Reviews, 12(4), 564–582.
Cromeans, T., Park, G. W., Costantini, V., Lee, D., Wang, Q., Farkas, T., et al. (2014). Comprehensive comparison of cultivable norovirus surrogates in response to different inactivation and disinfection treatments. Applied and Environment Microbiology, 80(18), 5743–5751. doi:10.1128/AEM.01532-14.
Da-Costa-Rocha, I., Bonnlaender, B., Sievers, H., Pischel, I., & Heinrich, M. (2014). Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chemistry, 165, 424–443. doi:10.1016/j.foodchem.2014.05.002. Epub 2014 May 27.
Djeussi, D. E., Noumedem, J. A., Seukep, J. A., Fankam, A. G., Voukeng, I. K., Tankeo, S. B., et al. (2013). Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complementary and Alternative Medicine, 1013, 164. doi:10.1186/1472-6882-13-164.
Fino, V. R., & Kniel, K. E. (2008). UV light inactivation of hepatitis A virus, Aichi virus, and feline calicivirus on strawberries, green onions, and lettuce. Journal of Food Protection, 71, 908–913.
Fullerton, M., Khatiwada, J., Johnson, J. U., Davis, S., & Williams, L. L. (2011). Determination of antimicrobial activity of sorrel (Hibiscus sabdariffa) on Escherichia coli O157:H7 isolated from food, veterinary, and clinical samples. Journal of Medicinal Food, 14(9), 950–956. doi:10.1089/jmf.2010.0200. Epub 2011 May 6.
Goyer, M., Aho, L. S., Bour, J. B., Ambert-Balay, K., & Pothier, P. (2008). Seroprevalence distribution of Aichi virus among a French population in 2006–2007. Archives of Virology, 153, 1171–1174.
Hall, A. J., Wikswo, M. E., Pringle, K., Gould, L. H., Parashar, U. D., Division of Viral Diseases, N. C. f. I., et al. (2014). Vital signs: Foodborne norovirus outbreaks—United States, 2009–2012. MMWR. Morbidity and Mortality Weekly Report, 63(22), 491–495.
Higginbotham, K. L., Burris, K. P., Zivanovic, S., Davidson, P. M., & Stewart, C. N, Jr. (2014a). Antimicrobial activity of Hibiscus sabdariffa aqueous extracts against Escherichia coli O157:H7 and Staphylococcus aureus in a microbiological medium and milk of various fat concentrations. [Research Support, Non-U.S. Gov’t]. Journal of Food Protection, 77(2), 262–268. doi:10.4315/0362-028X.JFP-13-313.
Higginbotham, K. L., Burris, K. P., Zivanovic, S., Davidson, P. M., & Stewart, C. N, Jr. (2014b). Aqueous extracts of Hibiscus sabdariffa calyces as an antimicrobial rinse on hot dogs against Listeria monocytogenes and methicillin-resistant Staphylococcus aureus. Food Control, 40, 274–277. doi:10.1016/j.foodcont.2013.12.011.
Joshi, S. S., Dice, L., & H D’Souza, D. (2015). Aqueous extracts of Hibiscus sabdariffa decrease hepatitis A virus and human norovirus titers. Food and Environmental Virology. E-pub ahead of print.
Jung, E., Kim, Y., & Joo, N. (2013). Physicochemical properties and antimicrobial activity of Roselle (Hibiscus sabdariffa L.). Journal of the Science of Food and Agriculture, 293(15), 3769–3776. doi:10.1002/jsfa.6256.
Kakkar, S., & Bais, S. (2014). A review on protocatechuic Acid and its pharmacological potential. [Review]. ISRN Pharmacology, 2014, 952943, doi:10.1155/2014/952943.
Kingsley, D. H., Chen, H., & Hoover, D. G. (2004). Inactivation of selected picornaviruses by high hydrostatic pressure. Virus Research, 102, 221–224.
Le Guyader, F. S., Le Saux, J. C., Ambert-Balay, K., Krol, J., Serais, O., Parnaudeau, S., et al. (2008). Aichi virus, norovirus, astrovirus, enterovirus, and rotavirus involved in clinical cases from a French oyster-related gastroenteritis outbreak. Journal of Clinical Microbiology, 46(12), 4011–4017. doi:10.1128/JCM.01044-08.
Lin, H. H., Huang, H. P., Huang, C. C., Chen, J. H., & Wang, C. J. (2005). Hibiscus polyphenol-rich extract induces apoptosis in human gastric carcinoma cells via p53 phosphorylation and p38 MAPK/FasL cascade pathway. Molecular Carcinogenesis, 43(2), 86–99. doi:10.1002/mc.20103.
Liu, K. S., Tsao, S. M., & Yin, M. C. (2005). In vitro antibacterial activity of roselle calyx and protocatechuic acid. Phytotherapy Research, 19(11), 942–945. doi:10.1002/ptr.1760.
Lodder, W. J., Rutjes, S. A., Takumi, K., & de Roda Husman, A. M. (2013). Aichi virus in sewage and surface water, the Netherlands. Emerging Infectious Diseases, 19, 1222–1230.
McKay, D. L., Chen, C. Y., Saltzman, E., & Blumberg, J. B. (2010). Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. [Randomized Controlled TrialResearch Support, Non-U.S. Gov’tResearch Support, U.S. Gov’t, Non-P.H.S.]. Journal of Nutrition, 140(2), 298–303. doi:10.3945/jn.109.115097.
Morton, J. (1987). Roselle. In Fruits of warm climates (pp. 281–286). Miami, FL.
Oh, E.-G., Kim, K.-L., Shin, S., Son, K.-T., Lee, H.-J., Kim, T., et al. (2013). Antiviral activity of green tea catechins against feline calicivirus as a surrogate for norovirus. Food Science and Biotechnology, 22(2), 593–598. doi:10.1007/s10068-013-0119-4.
Olaleye, M. (2007). Cytotoxicity and antibacterial activity of methanolic extract of Hibiscus sabdariffa. Journal of Medicinal Plants Research, 1, 9–13.
Ravishankar, S., Jaroni, D., Zhu, L., Olsen, C., McHugh, T., & Friedman, M. (2012). Inactivation of Listeria monocytogenes on ham and bologna using pectin-based apple, carrot, and hibiscus edible films containing carvacrol and cinnamaldehyde. [Research Support, Non-U.S. Gov’tResearch Support, U.S. Gov’t, Non-P.H.S.]. Journal of Food Science, 77(7), M377–M382. doi:10.1111/j.1750-3841.2012.02751.x.
Reuter, G., Boldizsar, A., Papp, G., & Pankovics, P. (2009). Detection of Aichi virus shedding in a child with enteric and extraintestinal symptoms in Hungary. Archives of Virology, 154, 1529–1532.
Reynolds, K. (2013). On tap: Aichi virus: Possible agent of unexplained cases of waterborne diarrhea. On Tap 55, 1, January 2013.
Ribes, J. M., Montava, R., Tellez-Castillo, C. J., Fernandez-Jimenez, M., & Buesa, J. (2010). Seroprevalence of Aichi virus in a Spanish population from 2007 to 2008. Clinical and Vaccine Immunology, 17, 545–549.
Saini, P., Gayen, P., Nayak, A., Kumar, D., Mukherjee, N., Pal, B. C., et al. (2012). Effect of ferulic acid from Hibiscus mutabilis on filarial parasite Setaria cervi: Molecular and biochemical approaches. [Research Support, Non-U.S. Gov’t]. Parasitology International, 61(4), 520–531. doi:10.1016/j.parint.2012.04.002.
Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V., Widdowson, M. A., Roy, S. L., et al. (2011). Foodborne illness acquired in the United States—Major pathogens. Emerging Infectious Diseases, 17(1), 7–15. doi:10.3201/eid1701.091101p1.
Su, X., & D’Souza, D. H. (2011). Grape seed extract for control of human enteric viruses. Applied and Environment Microbiology, 77(12), 3982–3987. doi:10.1128/AEM.00193-11.
Su, X., & D’Souza, D. H. (2013). Grape seed extract for foodborne virus reduction on produce. Food Microbiology, 34(1), 1–6. doi:10.1016/j.fm.2012.10.006.
Su, X., Howell, A. B., & D’Souza, D. H. (2010a). Antiviral effects of cranberry juice and cranberry proanthocyanidins on foodborne viral surrogates—A time dependence study in vitro. Food Microbiology, 27(8), 985–991. doi:10.1016/j.fm.2010.05.027.
Su, X., Howell, A. B., & D’Souza, D. H. (2010b). The effect of cranberry juice and cranberry proanthocyanidins on the infectivity of human enteric viral surrogates. Food Microbiology, 27(4), 535–540. doi:10.1016/j.fm.2010.01.001.
Su, X., Sangster, M. Y., & D’Souza, D. H. (2011). Time-dependent effects of pomegranate juice and pomegranate polyphenols on foodborne viral reduction. Foodborne Pathogens and Disease, 8(11), 1177–1183. doi:10.1089/fpd.2011.0873. Epub 2011 Jul 21.
Sunday, O. A., Munir, A. B., Akeeb, O., Bolanle, A., & Badaru, S. O. (2010). Antiviral effect of Hibiscus sabdariffa and Celosia argen tea on measles virus. African Journal of Microbiology Research, 4(4), 293–296.
Švraka-Latifovic, Sanela. (2011). Thesis on: A systematic approach to elucidate causes of gastroenteritis outbreaks of suspected viral etiology. Tergooiziekenhuizen: RIVM, Erasmus University Rotterdam.
Tsai, P.-J., McIntosh, J., Pearce, P., Camden, B., & Jordan, B. R. (2002). Anthocyanin and antioxidant capacity in Roselle (Hibiscus sabdariffa L.) extract. Food Research International, 35(4), 351–356. doi:10.1016/S0963-9969(01)00129-6.
Yamashita, T., Adachi, H., Hirose, E., Nakamura, N., Ito, M., Yasui, Y., et al. (2014). Molecular detection and nucleotide sequence analysis of a new Aichi virus closely related to canine kobuvirus in sewage samples. Journal of Medical Microbiology, 63(5), 715–720. doi:10.1099/jmm.0.070987-0.
Yamashita, T., Ito, M., Tsuzuki, H., & Sakae, K. (2001). Identification of Aichi virus infection by measurement of immunoglobulin responses in an enzyme-linked immunosorbent assay. Journal of Clinical Microbiology, 39, 4178–4180.
Yamashita, T., Kobayashi, S., Sakae, K., Nakata, S., Chiba, S., Ishihara, Y., & Isomura, S. (1991). Isolation of cytopathic small round viruses with BS-C-1 cells from patients with gastroenteritis. Journal of Infectious Diseases, 164, 954–957.
Yamashita, T., Sakae, K., Kobayashi, S., Ishihara, Y., Miyake, T., Mubina, A., & Isomura, S. (1995). Isolation of cytopathic small round virus (Aichi virus) from Pakistani children and Japanese travelers from Southeast Asia. Microbiology and Immunology, 9(6), 433–435.
Yamashita, T., Sakae, K., Tsuzuki, H., Suzuki, Y., Ishikawa, N., Takeda, N., et al. (1998). Complete nucleotide sequence and genetic organization of Aichi virus, a distinct member of the Picornaviridae associated with acute gastroenteritis in humans. Journal of Virology, 72, 8408–8412.
Yang, Y. S., Wang, C. J., Huang, C. N., Chen, M. L., Chen, M. J., & Peng, C. H. (2013). Polyphenols of Hibiscus sabdariffa improved diabetic nephropathy via attenuating renal epithelial mesenchymal transition. [Research Support, Non-U.S. Gov’t]. Journal of Agriculture and Food Chemistry, 61(31), 7545–7551. doi:10.1021/jf4020735.
Yin, M. C., & Chao, C. Y. (2008). Anti-Campylobacter, anti-aerobic, and anti-oxidative effects of roselle calyx extract and protocatechuic acid in ground beef. International Journal of Food Microbiology, 127(1–2), 73–77. doi:10.1016/j.ijfoodmicro.2008.06.002.
Acknowledgments
The authors gratefully acknowledge the funding provided partly by the University of Tennessee-Institute of Agriculture (Multistate Project S1056). The authors also acknowledge Dr. Dunlap at UT-Advanced Microscopy Center for his assistance with the Transmission electron microscopy sample preparations and observations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals that were performed by any of the authors.
Informed Consent
As this article does not contain any studies with human participants or animals performed by any of the authors, informed consent was not required.
Rights and permissions
About this article
Cite this article
D’Souza, D.H., Dice, L. & Davidson, P.M. Aqueous Extracts of Hibiscus sabdariffa Calyces to Control Aichi Virus. Food Environ Virol 8, 112–119 (2016). https://doi.org/10.1007/s12560-016-9229-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-016-9229-5