Abstract
While hepatitis E is a growing health concern in Europe, epidemiological data on hepatitis E virus (HEV) in Estonia are scarce. Along with imported HEV infections, autochthonous cases are reported from European countries. Both domestic and wild animals can be a source of human cases of this zoonosis. Here, we investigated the presence of anti-HEV antibodies and HEV RNA in domestic pigs and wild boars, as well as in pig farm workers and hunters in Estonia. Anti-HEV antibodies were detected in 234/380 (61.6 %) of sera from domestic pigs and in all investigated herds, and in 81/471 (17.2 %) of meat juice samples from wild boars. HEV RNA was detected by real-time PCR in 103/449 (22.9 %) of fecal samples from younger domestic pigs and 13/81 (16.0 %) of anti-HEV-positive wild boar samples. Analysis of sera from 67 pig farm workers and 144 hunters revealed the presence of HEV-specific IgG in 13.4 and 4.2 % of the samples, respectively. No HEV RNA was detected in the human serum samples. Phylogenetic analyses of HEV sequences from domestic pigs and wild boars, based on a 245 bp fragment from the open reading frame 2 showed that all of them belonged to genotype 3. The present study demonstrates the presence of HEV in Estonian domestic pig and wild boar populations, as well as in humans who have direct regular contact with these animals. Our results suggest that HEV infections are present in Estonia and require attention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatitis E virus (HEV) is a small single-stranded-positive sense RNA virus, which is a causative agent of acute hepatitis in humans. HEV has a genome of approximately 7.2 kb in length, which includes three open reading frames (ORFs), ORF1–ORF3 (Tam et al. 1991). According to the deduced amino acid sequence, ORF1 encodes for the non-structural proteins, ORF2 for the viral capsid protein, and ORF3 for the phosphoprotein (Tam et al. 1991). Based on the genome diversity, four genotypes (1–4) of HEV pathogenic to human are currently distinguished (Wang et al. 1999). Genotypes 1 and 2 are endemic to Africa, Asia, and Mexico and are known to infect only humans, while genotypes 3 and 4 are spread worldwide in humans and animals (Perez-Gracia et al. 2014).
Although HEV is mainly transmitted via the fecal–oral route through contaminated water or food, blood-borne, and vertical transmission also occurs (Gerolami et al. 2008; Khuroo et al. 1995). HEV is an important health concern in developing countries where, due to the poor sanitary conditions, the virus causes large waterborne outbreaks. Until recently, HEV infections in the industrialized parts of the world have been considered as mainly being imported from previously known endemic regions, but sporadic autochthonous cases of unknown origin have also been reported (Balayan 1993). In 1997, HEV was isolated from a domestic pig in the USA, and its homology to the strain obtained from a human patient’s serum in the same country region was demonstrated (Meng et al. 1997). Thus, a domestic source of HEV in developed countries was suspected, and the potential of zoonotic transmission of the virus was suggested. To date, cases of human HEV infections have been reported from a number of European countries, as well as from North America and Japan (Wichmann et al. 2008; Vasickova et al. 2009; Drobeniuc et al. 2013; Yazaki et al. 2003). Numerous reports have suggested a zoonotic nature of HEV in Europe (Wichmann et al. 2008; Schielke et al. 2009; Norder et al. 2009; Thiry et al. 2014; Vasickova et al. 2011). Consumption of undercooked or raw meat of domestic pigs, wild boars, or deer appears to be a major risk factor for local HEV infection in the studied areas (Yazaki et al. 2003; Wichmann et al. 2008; Reuter et al. 2009). Comparison of HEV genome sequences isolated from meat and liver products and those obtained from patients’ sera revealed a high similarity in many sporadic hepatitis E cases (Tei et al. 2003; Colson et al. 2010; Masuda et al. 2005).
Several studies suggest that HEV is widely spread in pigs and wild boars in Europe. For example, HEV-specific antibodies and viral RNA were found both in domestic pigs and wild boars, in France, Germany, and Italy (Caruso et al. 2015; Oliveira-Filho et al. 2014; Adlhoch et al. 2009; Schielke et al. 2009). Moreover, analysis of sera from individuals who were occupationally or recreationally exposed to domestic pigs and wild boars, such as farmers, pig veterinarians, forestry workers and hunters, demonstrated that the seroprevalence to HEV was higher in these risk groups than in individuals without contact to these animals (Chaussade et al. 2013; Dremsek et al. 2013; Drobeniuc et al. 2001). Although there have been few studies that did not detect any significant difference between seroprevalence in humans regularly exposed to domestic pigs or wildlife and those not having any contact with these animals (Olsen et al. 2006; Vulcano et al. 2007).
In Estonia, the first acute hepatitis E case was described in 2013, and epidemiological data are still scarce (Prükk et al. 2013). The aim of this study was to assess the presence of HEV infection in Estonian domestic pig and wild boar populations, and in two groups of people that may be exposed to these animals professionally or recreationally, pig farm workers and hunters. In addition, we genotyped the HEV sequences identified in this study.
Materials and Methods
Serum and Fecal Samples Collected from Domestic Pigs
In total, 380 serum samples were collected from adult domestic pigs, Sus scrofa domesticus, originating from 14 Estonian industrial pig farms situated in nine counties, including the largest Estonian island Saaremaa.
A total of 449 pig fecal samples were collected from nine industrial pig farms located in eight Estonian counties, including the island Saaremaa. The fecal samples originated from domestic pigs aged between 1.9 and 4 months. Fresh feces were collected from pen floors of the farm, approximately 50 samples per farm and five samples per pen (Table 1). Both serum and fecal samples were obtained from seven farms (Table 1). In this study, the farms were coded by numbers. All samples were stored at −20 °C until analyzed.
Meat Juice Samples Collected from Hunted Wild Boars
A total of 471 serosanguineous meat juice samples were collected from wild boars, Sus scrofa, hunted in 14 Estonian counties during the hunting season 2013 (Fig. 1). The samples were stored at −20 °C until analysis.
Serum Samples from Pig Farm Workers and Hunters
A total of 211 serum samples were collected in 2013 from two groups of people with exposure to domestic pigs or wild boars. Out of them, 67 samples were from pig farm workers and 144 from hunters. The farm worker sera were obtained from the same nine farms where the pig feces were collected (Table 1). All samples were stored at −20 °C until analyzed.
Serological Assays
Pig serum and wild boar meat juice samples were analyzed for the presence of anti-HEV antibodies with PrioCHECK HEV Ab porcine ELISA kit (Prionics AG, Switzerland), according to the manufacturer’s instructions.
Serum samples obtained from pig farm workers and hunters were analyzed for the presence of immunoglobulin G (IgG) antibodies against HEV (anti-HEV IgG) using the recomWell HEV IgG test (Mikrogen GmbH, Neuried, Germany). Positive sera were consecutively tested for anti-HEV immunoglobulin M (IgM) antibodies using the recomWell HEV IgM (Mikrogen GmbH, Neuried, Germany). For further confirmation of positive sera, recomLine HEV IgG/IgM immunoblot assay for anti-HEV IgG and IgM was applied (Mikrogen GmbH, Germany). The samples were considered IgG positive in case IgG ELISA and IgG immunoblot assay showed positive results and IgM positive if IgM ELISA and IgM immunoblot assay were positive. All procedures, validation, and result interpretation were carried out according to the manufacturer’s instructions.
All serological samples positive for HEV-specific antibodies were analyzed for the presence of HEV RNA by real-time RT-PCR.
Fecal Sample Homogenization
About 1 g of each pig fecal sample was suspended in 10 ml of phosphate buffered saline (PBS), mixed by vortexing, and centrifuged for 30 min at 2000×g. The supernatant was immediately used for RNA isolation.
HEV RNA Extraction
HEV RNA was extracted from 140 µl of pig serum, pig fecal sample supernatant, wild boar meat juice, or human serum with the QIAamp Viral RNA mini kit (Qiagen, Germany), according to the manufacturer’s instructions, and stored at −80 °C.
Real-Time PCR
For detection of HEV RNA, real-time PCR was performed using qScript One-Step Fast qRT-PCR Kit, ROX (Quanta Biosciences, USA) and primers and probe described by Jothinkumar et al. (2006): JHEV-F (5′-GGTGGTTTCTGGGGTGAC-3′), JHEV-R (5′-AGGGGTTGGTTGGATGAA-3′), JHEV-P (5′-GATTCTCAGCCCTTCGC-3′). The TaqMan® probe contained 5′(6)-carboxyfluorescein (FAM) and 3′ tetramethylrhodamine (TAMRA). The reaction was run in 20 µl mix per tube containing 5 µl of RNA, 1 µl of qScript One-Step Fast RT (Quanta BioSciences, Inc., USA), 5 µl of One-Step Fast Master Mix, and the primers and probe at concentrations 900 and 200 nM, respectively. The following cycling conditions were used: 48 °C for 5 min, 95 °C for 30 s followed by two step cycling 45 times at 95 °C for 3 s, and 60 °C for 30 s.
The World Health Organization (WHO) International Standard, code number 6329/10, based on a genotype 3a HEV strain was applied for the evaluation of real-time RT-PCR results (Baylis et al. 2013).
PCR Amplification in ORF2 and Sequencing
Samples positive in real-time PCR were amplified in the ORF2 region (1011 nt) for further sequencing and genotyping. Five microliters of RNA were used for cDNA synthesis in a 20 µl mix that included 200 U of Revert Aid H Minus Reverse Transcriptase (ThermoFisher Scientific, USA) and 100 pmol Random Hexamer Primer (Thermo Fisher Scientific, USA). The synthesis reaction was performed according to the manufacturer’s instructions.
For the first-round, PCR primers 5281_Sense (5′-GGTTGATTCTCAGCCCTTCGC-3′) and 6395_Antisense (5′-GAGAATGCTCAGCAGGATAAGGG-3′) were used, and 5 µl of cDNA were added to the reaction as template. In the second round, the amplification was carried out with primers 5309_Sense (5′-TATATTCATCCAACCAACCCCTT-3′) and 6298_Antisense (5′-AGCCGACGAAATYAATTCTGTC-3′), and 1 µl of the first PCR product was used as template. The four primers were named according to their starting position in the HEV strain Burma genome (GenBank M73218). Both rounds of PCR were performed at the same cycling conditions: 40 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s, and elongation at 72 °C for 45 s. The PCR was carried out in a total volume of 25 µl with 2.5 µl Dream Taq polymerase buffer (Thermo Fisher Scientific, USA), 0.8 mM dNTPs, 2.5 mM MgCl2, 5.6 % DMSO, 0.4 µM of each forward and reverse primer, and Dream Taq polymerase (Thermo Fisher Scientific, USA). Purification and subsequent sequencing of nested PCR products (1011 bp) were done at the core laboratory of Estonian Biocentre (Tartu, Estonia).
Phylogenetic Analysis
Phylogenetic analysis of the obtained sequences was performed using the MEGA6 software (Tamura et al. 2013). Although we managed to sequence a fragment of 1011 nt, we were not able to use it for phylogenetic analysis, since most of the HEV ORF2 sequences deposited in GenBank were around 300 nt. Thus, in order to allow similarity search among a greater number of sequences, a 245 bp fragment of 22 sequences from domestic pigs and 7 from wild boars were aligned with the corresponding region of 21 reference sequences selected from GenBank. Full-size sequences amplified by nested PCR during this study were deposited in GenBank (accession nos. KP871807–KP871835). Tamura–Nei model with Gamma distribution and invariance (TN93 + G + I) was chosen by the likelihood ratio test (LTR) as appropriate for the construction of a maximum-likelihood tree with the given data (Tamura and Nei 1993). Bootstrap testing of phylogeny was performed with 1000 replicates.
Statistical Analysis
Single proportions were calculated with 95 % confidence intervals (CIs). To estimate the significance of the differences between the prevalence estimates, two-tailed Fisher’s exact test was applied, p values <0.05 were considered statistically significant.
Results
HEV Antibodies and HEV RNA in Domestic Pigs and Wild Boars
HEV-specific antibodies were detected in samples from each farm, with farm-level prevalences ranging from 27.3 to 100 % (Table 1). No HEV RNA was detected in the antibody-positive serum samples of domestic pigs.
Of the 449 fecal samples from pigs collected from pen floors, 103 (22.9 %) tested HEV RNA positive. Positive samples were found from each of the nine investigated farms (Table 1). There were 20–100 % of positive pens per farm. The detected quantities varied from 5 to 2,997,964 International Units (IU) per g of fecal samples. Twenty-two positive samples that were successfully amplified by nested PCR were subsequently sequenced in ORF2 region. From each investigated farm at least one sample was sequenced.
Out of the 471 wild boar meat juice samples, 81 (17.2 %) were positive for anti-HEV antibodies (Table 2). HEV-positive animals were detected in each of the 14 investigated counties. No geographical distribution pattern of anti-HEV positivity was observed. The real-time RT-PCR analysis of the seropositive samples showed the presence of HEV RNA in 13 (16.0 %) samples (Table 2). Seven of the HEV RNA-positive samples that were successfully amplified by nested PCR were sequenced in ORF2.
HEV Antibodies in Pig Farm Workers and Hunters
Among the pig farm workers there were 45 females and 22 males, between 21 and 66 years of age, mean 48.5 ± 10.7. Of the 67 pig farm workers, 9 (13.4 %) tested positive for anti-HEV IgG (Table 1), and of those, four were also positive for anti-HEV IgM. All farm workers that were positive for anti-HEV-specific antibodies were over 41 years old.
The 144 hunters were aged 16–66 years, mean 40.6 ± 13.3, and had their hunting areas in all 15 Estonian counties. The majority (113/144; 78.5 %) of them were men. Of the 144 hunters, six (4.2 %) were found seropositive for anti-HEV IgG (Table 2). One of them had also detectable IgM levels. This seroprevalence in hunters was significantly lower than the prevalence observed among pig farm workers (p = 0.02). The positive samples were obtained from hunters between 35 and 48 years of age.
No HEV RNA was detected in the human serum samples.
Genotyping of the HEV Sequences
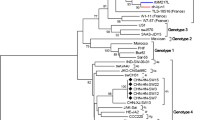
Phylogenetic analysis demonstrated that all HEV sequences detected in Estonian domestic pigs and wild boars belonged to genotype 3 (Fig. 2). The Estonian sequences formed four lineages, which are labeled here with roman numbers I–IV. Six out of seven wild boar sequences belonged to lineage I, together with a domestic pig strain from Kalinigrad Oblast, Russia (HQ380118). Lineage II included sequences from two pig farms that were geographically distant from each other (Fig. 1), farms 2 and 9 located in Northern and South-Eastern Estonia, respectively. These sequences clustered with each other but not with other entries deposited in GenBank.
Genotyping of HEV sequences detected in Estonian domestic pigs and wild boars. The phylogenetic tree was constructed with MEGA6 software using the maximum-likelihood method and bootstrap analysis of 1000 replicates. For the analysis, a 245 bp fragment from ORF2 was chosen. The tree is drawn to scale, with branch lengths measured by the number of substitutions per site. The analysis involved 51 nucleotide sequences. Only bootstrap values ≥66 are shown. All 4 HEV genotypes (1–4) are represented on the tree. Four lineages (I–IV) formed by the Estonian sequences are demonstrated in colored boxes. Sequences isolated from Estonian domestic pigs are designated with farm number and isolate code and the wild boar sequences are designated with Est and the sample code
Lineage III contained samples from a single farm (farm 14) located in the South-Eastern part of the country. The sequences clustered with strains of subtype 3f from Sweden, France, Belgium, Spain, and Japan (Fig. 2). Sequences from six Estonian pig farms and one wild boar formed the lineage IV (Fig. 2). These sequences clustered on a branch with two HEV strains isolated from domestic pigs in Russia, Archangelsk Oblast and Sverdlovsk Oblast (HQ380077, HQ399148; Fig. 2).
The nucleotide sequence similarity ranged between 89.7–96.3, 95.9, 86.9–100, and 84.8–100 % within the lineages I, II, III, and IV, respectively (Table 3). Between the lineages, nucleotide sequence identity ranged from 79.5 to 89.7 % (Table 3).
Discussion
To date, there have been numerous reports suggesting the zoonotic nature of HEV in Europe (Wichmann et al. 2008; Schielke et al. 2009; Norder et al. 2009; Thiry et al. 2014; Vasickova et al. 2011). The present study demonstrates the presence of HEV in the Estonian populations of domestic pigs and wild boars. The overall seroprevalence in domestic pigs was 61.6 %, which is comparable to the situation observed in other European countries, e.g., 42.7 % in Germany and 73 % in Belgium (Dremsek et al. 2013; Thiry et al. 2014). The HEV seroprevalence (17.2 %) among wild boars was significantly lower than that among domestic pigs (p < 0.0001), but in agreement with the seroprevalences of 14 % reported from France and 12 % from The Netherlands (Carpentier et al. 2012; Rutjes et al. 2010). Previously, serum and meat juice of pigs were shown to be equivalent material for serological testing (Wacheck et al. 2012; Casas et al. 2010), and thus a direct comparison of the results obtained from pigs and wild boars in this work was possible. In general, the HEV antibody prevalence in wild boars in Europe has been reported to be between 10.2 and 44.4 %, with the lowest in the Northern Central Italy and the highest in Poland, respectively (Martinelli et al. 2015; Larska et al. 2015). We observed an HEV antibody prevalence variation among different Estonian counties, and geographical variations were also reported from Germany and Italy (Adlhoch et al. 2009; Caruso et al. 2015). No particular pattern of HEV antibody prevalence distribution was identified among counties. The lower seroprevalence in wild boars compared with that in domestic pigs could be attributed to a more intensive interaction between pigs in the farms than between wild boars in their natural habitats.
We detected viral RNA in 22.9 % of pig fecal samples. This prevalence is, however, arbitrary, as the samples were collected from the pen floors and cross-contamination cannot be excluded. A similar detection rate, 23.3 %, was previously reported for domestic pigs in Spain (Fernandez-Barredo et al. 2006), but this comparison should be taken with care, since the fecal samples were collected individually from the rectum of each pig. HEV RNA was also found in 16.0 % of the seropositive wild boar meat juice samples. Although we analyzed only the serologically positive samples, and thus cannot calculate the overall RNA detection rate in Estonian wild boars, the obtained result is in line with a recent report from Germany, where 14.9 % of the samples were found to be HEV RNA positive (Schielke et al. 2009).
No direct comparison between the RNA detection rate in domestic pig and wild boar samples can, however, be achieved. Firstly, because all provided domestic pig fecal samples were analyzed for the presence of HEV RNA, while among the wild boar samples, only those positive for anti-HEV antibodies were tested. Secondly, the sample material used for RNA isolation was different for domestic pigs and wild boars. As demonstrated by Di Bartolo et al. (2012) for domestic pigs, fecal samples show higher HEV viral load, compared to liver or meat. Thus, the RNA detection rate in our study could be partly influenced by the sample material.
Phylogenetic analysis showed that all the strains detected in animals belonged to genotype 3. This result was expected, as the majority of studies conducted in Europe have detected this genotype (Kamar et al. 2012). Similarly to Oliveira-Filho et al. (2014), we did not find any geographic clustering of the HEV sequences from wild boars.
We observed four lineages (I–IV) among the Estonian HEV sequences. As expected, lineages III and IV consisting mainly from domestic pig sequences had slightly higher sequence identity to each other than to lineage I which comprised sequences from wild boars. Viruses undergo particular mutations depending on the host organism, and thus the HEV strains detected in the same host species are expected to be more similar (Lara et al. 2014). Within lineage III, sequences from Farm 14, located in South-Eastern Estonia, clustered with HEV strains from France, Spain, Belgium, Sweden, and Japan, and all of them were of the HEV 3f subtype. Although the bootstrap value was 66 %, somewhat below the desirable 70 %, we can assume that these strains form their own lineage. Subtype 3f is common in European pigs and has been found in France, Sweden, and Belgium (Colson et al. 2010; Widen et al. 2010; Thiry et al. 2014). The sequence of the Japanese strain, which appeared to be similar to the farm 14 sequences, was also shown by Widen et al. to cluster with Swedish as well as other European sequences (Widen et al. 2010). No recently imported animals from any European farms were reported from farm 14. Nevertheless, pigs are purchased and transported to Estonia from Sweden and other European countries, and we can speculate that there is a trade-related link between Estonian and Swedish HEV strains.
Recently, Japanese scientists have performed molecular tracing of HEV 3e subtype and found that the virus entered Japan by importation of domestic pigs from Europe and then likely spread further to wild boars (Nakano et al. 2013). The clustering of Estonian sequences in lineages I and IV with Russian sequences from domestic pigs has no clear explanation, but we can assume that this could be a consequence of animal trading between the countries, especially considering the fact that until 25 years ago it was a common economic area.
There appeared to be no specific geographic clustering of HEV sequences among the Estonian farms, which can be explained in two ways; either there is an absence of farm-to-farm virus transmission, or the virus is frequently transmitted between farms and due to the presence of an abundance of strains, no clusters can be distinguished.
The Russian HEV strain, with sequences similar to the Estonian wild boar sequences in lineage I, originated from Kalinigrad Oblast, which is located in the Southern Baltic region. Since the wild boar populations in the Baltic region roam freely, it is possible that HEV was transmitted among migrating animals and can thus now be found both in Kalinigrad Oblast and in Estonia.
In lineage IV, there is one wild boar sequence among all the domestic pig sequences. The samples for this study were collected from the farms where pigs were properly isolated from the surrounding environment. However, piglets are often sold to smaller backyard farms where the contact between them and wild animals is possible. Another opportunity for the wild boar to encounter the virus could be contact with the waste from pig farms, or pig feces distributed to the fields as fertilizer.
Human populations in contact with both domestic and wild animals have been shown to have an increased risk of HEV zoonotic infection. Recent studies have reported a higher prevalence of anti-HEV antibodies in forestry and pig farm workers in France and Germany (Krumbholz et al. 2011; Dremsek et al. 2011; Carpentier et al. 2012; Chaussade et al. 2013). We assessed the prevalence of HEV-specific antibodies in Estonian pig farm workers and hunters who have occupational or recreational exposure to domestic pigs and wild boars, respectively. The results show that the HEV seroprevalence in Estonian pig farm workers (13.4 %) belongs among the lowest reported in Europe among people with regular direct contact with pigs. Similar values for individuals professionally exposed to pigs were observed in Sweden and in Spain, 13 and 18.8 %, respectively (Galiana et al. 2008; Olsen et al. 2006). Higher anti-HEV levels, 44 %, were demonstrated for France (Chaussade et al. 2013). However, one should take into account that our study included only sera from persons employed at large farms, and only reflects the situation in industrial pig farming. Among the hunter serum samples, 4.2 % tested positive for HEV antibodies. Mansuy et al. analyzed a group of blood donors from Midi-Pyrénées region in France and reported that out of 25 persons who practiced hunting, 20 (80 %) had anti-HEV IgG antibodies, which was probably due to the traditional consumption of raw game meat in that area (Mansuy et al. 2011). The lack of this custom in Estonia makes thus a comparison of the results difficult.
The present study revealed that domestic pigs and wild boars represent two possible sources for human HEV infection in Estonia. The local circulation of the virus was confirmed by the presence of both specific antibodies and HEV RNA. We also showed that antibodies against HEV are present in the human populations with direct contact to these animals. It is evident from our study that HEV is present and should be a concern to public health in Estonia.
References
Adlhoch, C., Wolf, A., Meisel, H., Kaiser, M., Ellerbrok, H., & Pauli, G. (2009). High HEV presence in four different wild boar populations in East and West Germany. Veterinary Microbiology, 139(3–4), 270–278.
Balayan, M. S. (1993). Hepatitis E virus infection in Europe: Regional situation regarding laboratory diagnosis and epidemiology. Clinical and Diagnostic Virology, 1(1), 1–9.
Baylis, S. A., Blumel, J., Mizusawa, S., Matsubayashi, K., Sakata, H., Okada, Y., et al. (2013). World Health Organization International Standard to harmonize assays for detection of hepatitis E virus RNA. Emerging Infectious Diseases, 19(5), 729–735.
Carpentier, A., Chaussade, H., Rigaud, E., Rodriguez, J., Berthault, C., Boue, F., et al. (2012). High hepatitis E virus seroprevalence in forestry workers and in wild boars in France. Journal of Clinical Microbiology, 50(9), 2888–2893.
Caruso, C., Modesto, P., Bertolini, S., Peletto, S., Acutis, P. L., Dondo, A., et al. (2015). Serological and virological survey of hepatitis E virus in wild boar populations in northwestern Italy: Detection of HEV subtypes 3e and 3f. Archives of Virology, 160(1), 153–160.
Casas, M., Pina, S., Peralta, B., Mateu, E., Casal, J., & Martin, M. (2010). Comparison of muscle fluid and serum for detection of antibodies against hepatitis E virus in slaughter pigs. The Veterinary Journal, 190(1), 179–180.
Chaussade, H., Rigaud, E., Allix, A., Carpentier, A., Touze, A., Delzescaux, D., et al. (2013). Hepatitis E virus seroprevalence and risk factors for individuals in working contact with animals. Journal of Clinical Virology, 58(3), 504–508.
Colson, P., Borentain, P., Queyriaux, B., Kaba, M., Moal, V., Gallian, P., et al. (2010). Pig liver sausage as a source of hepatitis E virus transmission to humans. Journal of Infectious Diseases, 202(6), 825–834.
Di Bartolo, I., Diez-Valcarce, M., Vasickova, P., Kralik, P., Hernandez, M., Angeloni, G., et al. (2012). Hepatitis E virus in pork production chain in Czech Republic, Italy, and Spain, 2010. Emerging Infectious Diseases, 18(8), 1282–1289.
Dremsek, P., Joel, S., Baechlein, C., Pavio, N., Schielke, A., Ziller, M., et al. (2013). Hepatitis E virus seroprevalence of domestic pigs in Germany determined by a novel in-house and two reference ELISAs. Journal of Virological Methods, 190(1–2), 11–16.
Dremsek, P., Wenzel, J. J., Johne, R., Ziller, M., Hofmann, J., Groschup, M. H., et al. (2011). Seroprevalence study in forestry workers from eastern Germany using novel genotype 3- and rat hepatitis E virus-specific immunoglobulin G ELISAs. Medical Microbiology and Immunology, 201(2), 189–200.
Drobeniuc, J., Favorov, M. O., Shapiro, C. N., Bell, B. P., Mast, E. E., Dadu, A., et al. (2001). Hepatitis E virus antibody prevalence among persons who work with swine. Journal of Infectious Diseases, 184(12), 1594–1597.
Drobeniuc, J., Greene-Montfort, T., Le, N. T., Mixson-Hayden, T. R., Ganova-Raeva, L., Dong, C., et al. (2013). Laboratory-based surveillance for hepatitis E virus infection, United States, 2005-2012. Emerging Infectious Diseases, 19(2), 218–222.
Fernandez-Barredo, S., Galiana, C., Garcia, A., Vega, S., Gomez, M. T., & Perez-Gracia, M. T. (2006). Detection of hepatitis E virus shedding in feces of pigs at different stages of production using reverse transcription-polymerase chain reaction. Journal of Veterinary Diagnostic Investigation, 18(5), 462–465.
Galiana, C., Fernandez-Barredo, S., Garcia, A., Gomez, M. T., & Perez-Gracia, M. T. (2008). Occupational exposure to hepatitis E virus (HEV) in swine workers. American Journal of Tropical Medicine and Hygeine, 78(6), 1012–1015.
Gerolami, R., Moal, V., & Colson, P. (2008). Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. New England Journal of Medicine, 358(8), 859–860.
Jothikumar, N., Cromeans, T. L., Robertson, B. H., Meng, X. J., & Hill, V. R. (2006). A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. Journal of Virological Methods, 131(1), 65–71.
Kamar, N., Bendall, R., Legrand-Abravanel, F., Xia, N. S., Ijaz, S., Izopet, J., et al. (2012). Hepatitis E. Lancet, 379(9835), 2477–2488.
Khuroo, M. S., Kamili, S., & Jameel, S. (1995). Vertical transmission of hepatitis E virus. Lancet, 345(8956), 1025–1026.
Krumbholz, A., Mohn, U., Lange, J., Motz, M., Wenzel, J. J., Jilg, W., et al. (2011). Prevalence of hepatitis E virus-specific antibodies in humans with occupational exposure to pigs. Medical Microbiology and Immunology, 201(2), 239–244.
Lara, J., Purdy, M. A., & Khudyakov, Y. E. (2014). Genetic host specificity of hepatitis E virus. Infection, Genetics and Evolution, 24, 127–139.
Larska, M., Krzysiak, M. K., Jablonski, A., Kesik, J., Bednarski, M., & Rola, J. (2015). Hepatitis E virus antibody prevalence in wildlife in Poland. Zoonoses and Public Health, 62(2), 105–110.
Mansuy, J. M., Bendall, R., Legrand-Abravanel, F., Saune, K., Miedouge, M., Ellis, V., et al. (2011). Hepatitis E virus antibodies in blood donors, France. Emerging Infectious Diseases, 17(12), 2309–2312.
Martinelli, N., Pavoni, E., Filogari, D., Ferrari, N., Chiari, M., Canelli, E., et al. (2015). Hepatitis E Virus in Wild Boar in the Central Northern Part of Italy. Transboundary and Emerging Diseases, 62(2), 217–222.
Masuda, J., Yano, K., Tamada, Y., Takii, Y., Ito, M., Omagari, K., et al. (2005). Acute hepatitis E of a man who consumed wild boar meat prior to the onset of illness in Nagasaki, Japan. Hepatology Research, 31(3), 178–183.
Meng, X. J., Purcell, R. H., Halbur, P. G., Lehman, J. R., Webb, D. M., Tsareva, T. S., et al. (1997). A novel virus in swine is closely related to the human hepatitis E virus. Proceedings of the National Academy of Sciences of the United States of America, 94(18), 9860–9865.
Nakano, T., Takahashi, K., Arai, M., Okano, H., Kato, H., Ayada, M., et al. (2013). Identification of European-type hepatitis E virus subtype 3e isolates in Japanese wild boars: Molecular tracing of HEV from swine to wild boars. Infection, Genetics and Evolution, 18, 287–298.
Norder, H., Sundqvist, L., Magnusson, L., Ostergaard Breum, S., Lofdahl, M., Larsen, L. E., et al. (2009). Endemic hepatitis E in two Nordic countries. Eurosurveillance Weekly, 14(19), 20–28.
Oliveira-Filho, E. F., Bank-Wolf, B. R., Thiel, H. J., & Konig, M. (2014). Phylogenetic analysis of hepatitis E virus in domestic swine and wild boar in Germany. Veterinary Microbiology, 174(1–2), 233–238.
Olsen, B., Axelsson-Olsson, D., Thelin, A., & Weiland, O. (2006). Unexpected high prevalence of IgG-antibodies to hepatitis E virus in Swedish pig farmers and controls. Scandinavian Journal of Infectious Diseases, 38(1), 55–58.
Perez-Gracia, M. T., Suay, B., & Mateos-Lindemann, M. L. (2014). Hepatitis E: An emerging disease. Infection, Genetics and Evolution, 22, 40–59.
Prükk, T., Proshkina, S., & Pihlak, R. (2013). Äge E-viirushepatiit. Haigusjuhu kirjeldus. Eesti Arstist, 92(10), 587–589.
Reuter, G., Fodor, D., Forgach, P., Katai, A., & Szucs, G. (2009). Characterization and zoonotic potential of endemic hepatitis E virus (HEV) strains in humans and animals in Hungary. Journal of Clinical Virology, 44(4), 277–281.
Rutjes, S. A., Lodder-Verschoor, F., Lodder, W. J., van der Giessen, J., Reesink, H., Bouwknegt, M., et al. (2010). Seroprevalence and molecular detection of hepatitis E virus in wild boar and red deer in The Netherlands. Journal of Virological Methods, 168(1–2), 197–206.
Schielke, A., Sachs, K., Lierz, M., Appel, B., Jansen, A., & Johne, R. (2009). Detection of hepatitis E virus in wild boars of rural and urban regions in Germany and whole genome characterization of an endemic strain. Virology Journal, 6, 58.
Tam, A. W., Smith, M. M., Guerra, M. E., Huang, C. C., Bradley, D. W., Fry, K. E., et al. (1991). Hepatitis E virus (HEV): Molecular cloning and sequencing of the full-length viral genome. Virology, 185(1), 120–131.
Tamura, K., & Nei, M. (1993). Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution, 10(3), 512–526.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30(12), 2725–2729.
Tei, S., Kitajima, N., Takahashi, K., & Mishiro, S. (2003). Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet, 362(9381), 371–373.
Thiry, D., Mauroy, A., Saegerman, C., Thomas, I., Wautier, M., Miry, C., et al. (2014). Estimation of hepatitis E virus (HEV) pig seroprevalence using ELISA and Western blot and comparison between human and pig HEV sequences in Belgium. Veterinary Microbiology, 172(3–4), 407–414.
Vasickova, P., Psikal, I., Widen, F., Smitalova, R., Bendova, J., Pavlik, I., et al. (2009). Detection and genetic characterisation of Hepatitis E virus in Czech pig production herds. Research in Veterinary Science, 87(1), 143–148.
Vasickova, P., Slany, M., Chalupa, P., Holub, M., Svoboda, R., & Pavlik, I. (2011). Detection and phylogenetic characterization of human hepatitis E virus strains, Czech Republic. Emerging Infectious Diseases, 17(5), 917–919.
Velström, K., Lassen, B., & Jokelainen, P. (2013). Toxoplasma gondii antibody prevalence in Estonian wild boars. In Tropical medicine and international health: 8th European Congress on tropical medicine and international health & 5th conference of the Scandinavian-Baltic Society for parasitology, Copenhagen, Denmark (pp. 226–226), 10–13 September 2013, Wiley-Blackwell.
Vulcano, A., Angelucci, M., Candelori, E., Martini, V., Patti, A. M., Mancini, C., et al. (2007). HEV prevalence in the general population and among workers at zoonotic risk in Latium Region. Annali di Igiene, Medicina preventiva e di comunità, 19(3), 181–186.
Wacheck, S., Werres, C., Mohn, U., Dorn, S., Soutschek, E., Fredriksson-Ahomaa, M., et al. (2012). Detection of IgM and IgG against hepatitis E virus in serum and meat juice samples from pigs at slaughter in Bavaria, Germany. Foodborne Pathogens and Disease, 9(7), 655–660.
Wang, Y., Ling, R., Erker, J. C., Zhang, H., Li, H., Desai, S., et al. (1999). A divergent genotype of hepatitis E virus in Chinese patients with acute hepatitis. Journal of General Virology, 80(Pt 1), 169–177.
Wichmann, O., Schimanski, S., Koch, J., Kohler, M., Rothe, C., Plentz, A., et al. (2008). Phylogenetic and case-control study on hepatitis E virus infection in Germany. Journal of Infectious Diseases, 198(12), 1732–1741.
Widen, F., Sundqvist, L., Matyi-Toth, A., Metreveli, G., Belak, S., Hallgren, G., et al. (2010). Molecular epidemiology of hepatitis E virus in humans, pigs and wild boars in Sweden. Epidemiology and Infection, 139(3), 361–371.
Yazaki, Y., Mizuo, H., Takahashi, M., Nishizawa, T., Sasaki, N., Gotanda, Y., et al. (2003). Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. Journal of General Virology, 84(Pt 9), 2351–2357.
Acknowledgments
The authors would like to thank Sofia Sidorova, senior laboratory specialist, for technical assistance as well as Elina Shatova and Jana Muravjova for analyzing pig sera and wild boar meat juice as a part of their Bachelor theses, Nurses Anu Kuusmann, Marge Reiss for taking blood samples. The farm workers and hunters are warmly thanked for their contributions and interest in this study. The study was financially supported by the European Regional Development Fund (Estonian Research Council (http://www.etag.ee/), programme TerVe, Projects ZoonRisk 3.2.1002.11-0002, 3.2.1002.11-0002 EKZE_SS and by Ministry of Education and Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Human rights and animals and informed consent
All procedures of this work were approved by Tallinn Medical Research Committee, No. 2921. The pig sera used in this study were collected for a regular survey on porcine pathogens performed at the Estonian University of Life Sciences. The fecal samples from pigs were collected from the pen floors with no physical contact with the animals. Serosanguineous meat juice samples were primarily collected for a study of Toxoplasma gondii in Estonian wild boars (Velström et al. 2013). Approval from the Research Ethics Committee of the University of Tartu (No. 216/T-15) as well as written informed consents was obtained from volunteer pig farm workers and hunters for the use of serum samples.
Rights and permissions
About this article
Cite this article
Ivanova, A., Tefanova, V., Reshetnjak, I. et al. Hepatitis E Virus in Domestic Pigs, Wild Boars, Pig Farm Workers, and Hunters in Estonia. Food Environ Virol 7, 403–412 (2015). https://doi.org/10.1007/s12560-015-9210-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-015-9210-8