Abstract
With development in the area of electronics and artificial intelligence (AI), medical devices (MD) have been sophisticated as well. MD management strategies today are very different than decades ago, so it is reasonable to consider how we can prepare for where we are going in the future. This paper presents the result of application of machine learning (ML) techniques in management of infant incubators in healthcare institutions. A total of 140 samples was used for development of Expert system based on ML classifiers. These samples were collected during 2015–2017 period, as part of yearly inspections of incubators in healthcare institutions by ISO 17020 accredited laboratory. Dataset division 80–20 was used for classifiers development and validation. Performance of the following machine learning algorithms was investigated: Naïve Bayes (NB), Decision Tree (DT), Random Forest (RF), k-Nearest Neighbour (kNN), and Support Vector Machine (SVM). Resulting classifiers were compared by performance and classifier based on Decision Tree algorithm yielded highest accuracy (98.5%) among other tested systems. Obtained results suggest that by introducing ML algorithms in MD management strategies benefit healthcare institution firstly in terms of increase of safety and quality of patient diagnosis and treatments, but also in cost optimization and resource management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
It has been stated multiple times that we live in modern age, where technology has great impact on our lives. We perform a lot of tasks using technology, so we communicate much quicker, plan, organise, pay bills, control appliances at home and all by using one application.
Health has always been, is and will always be the most important thing that for sure should be taken care of. Even with all technological development of modern society, there are still a lot of challenges in maintaining health of population worldwide, such as obesity, cardiovascular diseases and respiratory diseases. On the other hand, major improvements have happened so nowadays major pandemics do not cause such a high mortality rate as they did before.
Development of medical devices (MD) have dramatically changed the way medical care is provided to patients. Even thought, healthcare institutions have roots in ancient time, it was only recently that medical treatments have become more effective so that human life expectancy increased. In comparison, average life expectancy at birth of the global population in 2016 was 72.0 years and in 1841 it was around 55 years. [1, 2] Since 1900 the global average life expectancy has more than doubled, what was caused by development of various MDs. These devices have enabled development of clinical practices because they enabled measurement of parameters that couldn’t be touched or seen manually, such as brain activity, bone structure and fractions, blood infections, and other vital parameters, but also they enabled control of parameters such as respiration, pressure, oxygen level and temperature.
MDs are electronic devices that have a huge impact on the general public. They are used daily in medical practice and medical professionals rely upon their measurements when evaluating the physical state of the patient. Therefore, measurements are crucial for applications in healthcare, and knowing that measurement errors have critical effect on the final outcome it becomes clear that it is not addressed as often as it should.
In time when, healthcare and medical technology is nowadays at one more historical shift and slowly moving toward personalized care, preventive care and home-healthcare medical devices are increasing in the complexity since it is required to support various activities. Also, with increased number of private healthcare institutions, market competitiveness has also become an important issue. The only thing that never changes, though, is the fact that every action in healthcare, every development in MDs, is aimed in ensuring safety and quality of care to patients, whether it is about prevention of diseases or treatment.
Statistics show that annual MD maintenance and management cost in healthcare institution is approximately 1% of the total budget [3]. Healthcare institutions, unfortunately tend to cut these costs so as a consequence usage of MDs results in higher rate of incidents with serious injuries or deaths of patients. Also, they state that numerous optimization models for MD maintenance have been developed, but healthcare institutions still do not benefit from these methods as other industries do. Still, healthcare institutions are burdened by unnecessary and excessive preventive maintenances of questionable quality [4]. In such environment, where healthcare institutions are struggling to keep the balance between cost benefit and safety, it is obvious that traditional approach in healthcare management is becoming inadequate to answer to the rising needs. This problem has been recognized in Bosnia and Herzegovina as well [5], and solved by introducing legal metrology framework based on evidence-based inspections of safety and performance of different groups of medical devices [6, 7].
According to this framework, the MD management is defined through independent safety and performance inspections for 11 different MDs with measuring functions. Inspections are periodical (1 year) and conducted by ISO 17020 accredited inspection laboratory [6,7,8]. All safety and performance measurements and device information, such as serial number, type, manufacturer and location are stored in developed database [9, 10] forming big data structures that can be further analysed extracting useful information about device status and future behaviour, hence turning them from big data to smart data structures.
So, having this database and following the practice of usage variety of machine learning (ML) techniques, for the development of predictive models in biomedicine [11, 12] and for MD management [13, 14] authors of this paper have decided to conduct the research of developing classifiers (Expert systems) based on ML techniques and use them for MD management optimisation in healthcare institutions. The aim of this study is to investigate how measured data from individual MD can be used to predict it’s performance and future failures in order to optimize current management strategies in healthcare institutions. As a case study, real data of infant incubator inspections were used during this research, to compare the results obtained by Spahic et al. [15].
2 Materials and methods
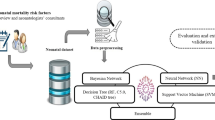
Expert system developed in this research consists of three layers, input layer, classifier and output layer, Fig. 1. Input layer consists of 30 inputs and 1 output parameter. System inputs are device information and measurements of safety-performance parameters. System inputs are defined according to ISO/IEC 60601 international standard for MDs [15] and established rules within inspection framework for MDs [4]. System output is a class with two possible values: (1) device requires maintenance and (2) device performance is safe for usage until the next inspection.
For development of classifier, five different ML algorithms were used: (1) Naïve Bayes (NB), (2) Decision Tree (DT), (3) Random Forest (RF), (4) k-Nearest Neighbour (k-NN), and (5) Support Vector Machine (SVM) [16,17,18,19,20]. The reason for choosing these algorithms is because they are representatives of filter method, stochastic general search method and wrapper method.
The dataset for development of the classifiers consisted of 140 samples. These are real data acquired during 2015–2017 period, as part of yearly inspections of incubators in 7 healthcare institutions (2 clinical centres and 5 hospitals) by ISO 17020 accredited laboratory.
Each sample consists of seven groups of features (overall 30 attributes) that are organized in the following manner: (1) device age, (2) device manufacturer, (3) device type, (4) preventive/corrective maintenance history, (5) safety inspection measurement results (mains voltage, protective earth resistance, insulation resistance, earth leakage current, enclosure leakage current, patient applied parts leakage current), (6) performance inspection measurement results (temperature, relative humidity and sound measurements in 6 measuring points), and (7) inspection decision (Class 1 or 2).
For system development 80% samples were used, and for performance validation 20% samples were used. The distribution into training and validation class was random following previous practice [11,12,13,14] and is represented in Table 1. In total dataset, 101 samples depicted satisfactory MD performance and 39 were depicting MDs with failure that required maintenance.
The safety parameters according to ISO/IEC 60601 [15] were measured with Fluke Biomedical ESA 620 etalon [16], while performance parameters (temperature at given point) was measured using Fluke Biomedical INCU I etalon [17]. Both etalons were calibrated according to the ISO 17025 [18]. Step-by-step procedure for infant incubator inspection according to mentioned framework [6,7,8] is explained in the paper by Gurbeta et al. [3].
Overall, system performance was evaluated by:
Where: TN (true negative), TP (true positive), FN (false negative), FP (false positive) - representing the number of correctly/incorrectly classified instances belonging to output negative/positive group of instances.
3 Results and discussion
The results of development of expert system for infant incubator performance and possible failure prediction is presented in Table 2. As it can be seen from the Table 1, all classifiers, except the one based on k-NN algorithm yielded better training results than validation. However, the validation performance of the developed classifiers was within 1% of training performance.
The results presented in Table 2. suggest that ES based on DT classifier has the highest accuracy of 98.5%. Computational time needed for generation of these classifiers was measured in mili-seconds (ms). Since it is relativelty small dataset involve in the research, there wasn’t significant difference in computational time needed for generation of these classifiers. However, significant acceleration is observed when compared to generation of neural networks and fuzzy classifiers as discussed in work by Spahic [15].
Compared to the results obtained in [19] it can be concluded that performance is acceptable but the classifier needs improvement in terms of balancing training dataset with more samples of failure status. Also, this study differs than the previous study [19] by the input information fed into the system. Previous study included fuzzy analysis of the parameters such as number of additional parts/parts that are most susceptible to damage and utilization coefficient. These values were estimates and are removed from the dataset, therefore this developed expert system is based only on objective and measured data, available at any point from the individual medical device.
Individual validation performance of each classifier is presented by the confusion matrix in Table 3.
Overlooking the successful application of machine learning techniques in other fields it is logic to raise the question and research the possibilities of implementing them in medical device management. Machine learning techniques are not strange in healthcare, since they have been successfully used for prediction of various diseases and conditions [21,22,23].
So this study confirmed that expert systems based on machine learning algorithms yielded high prediction accuracy rates as in other fields where used. This approach is novel and currently investigated under post-market surveillance and medical device inspection activities in healthcare institutions in Bosnia and Herzegovina. It is in line with statements that predictive analysis is one of the three main areas in which healthcare will benefit from artificial intelligence. [24, 25, 26, 27] This is a step up from the conventional medical device management tools based on software solutions.
4 Conclusion
This paper presents expert system based on machine learning algorithms for performance prediction of infant incubators. For system development 5 different machine learning algorithms were used. Out of all tested expert system, the one with Decision tree algorithm yielded highest accuracy of 99.2%. This is similar to results obtained in research of Spahic et al. [15], but using less computational resources.
This study proves that such expert systems combined with real-time updated database of medical devices safety and performance parameters, can be powerful tool of post-market surveillance by National Notified Bodies as instructed by new EU Medical Device Regulation. Such systems are easily scalable to other types of medical devices and can optimize the costs of medical device maintenance in healthcare institutions. Also, since safety parameters are similar for variety of medical devices next research aim of the authors is to build integrated expert system that will be able to perform in real-time clinical settings during periodical inspections of medical devices.
Expert system is already being developed to the level of high fidelity minimal viable product (MVP) based on existing dynamic database which contains and is continuously filled with newly measured MD output parameters. Method for collecting data from MD brings new operational capability to traditional approach, and offers critical information for maintaining MD functionality. The proposed approach can be used to addres the post-market surveillance issues defined by new MD Regulations that were published in April 2017 and will become obligatory for all EU member states until May 2020. For this purpose, developed system can be transferred to the hardware components such as FPGA or linked with other IoT devices that would make it accessible on sight during periodical inspections on sight. Implementing an expert system in hardware enables better feasibility of the system and its application in real-time. Such approach would make system portable for easier on-sight performance predictions on a single device located in healthcare institution.
References
CIA – The World Factbook Life Expectancy At Birth. Available at: https://www.cia.gov/library/publications/the-world-factbook/rankorder/2102rank.html
United Nations Department of Economic and Social Affairs (29 July 2015). "United Nations World Population Prospects: 2015 revision”.
Sharareh Taghipour, Dragan Banjevic and Andrew K. S. Jardine, reliability analysis of maintenance data for complex medical devices.
Badnjević A, Cifrek M, Magjarević R, Džemić Z, (2018), Inspection of medical devices for regulatory purposes, series in biomedical engineering ISBN 978-981-10-6649-8.
Gurbeta L, Izetbegović S, Badnjević-Čengić A. Inspection and testing of infant incubators. In: Badnjević A, Cifrek M, Magjarević R, Džemić Z, editors. Inspection of medical devices. Singapore: Series in Biomedical Engineering. Springer; 2018.
Badnjevic A, Gurbeta L, Jimenez ER, Iadanza E. Testing of mechanical ventilators and infant incubators in healthcare institutions. Technol Health Care. 2017;25(2):237–50.
Gurbeta L, Dzemic Z, Bego T, Sejdic E, Badnjevic A. Testing of anesthesia machines and defibrillators in healthcare institutions. J Med Syst. 2017:41, 133. https://doi.org/10.1007/s10916-017-0783-7.
Gurbeta L., Dzemic, Z., Badnjevic A., Establishing traceability chain of infusion and perfusor pumps using legal metrology procedures in Bosnia and Herzegovina, IUPESM – The World Congress on Medical Physics & Biomedical Engineering in Prague, June 3—8, 2018.
Gurbeta L, Badnjević A. Inspection process of medical devices in healthcare institutions: software solution. Health Technol. 2017;7(1):109–17. https://doi.org/10.1007/s12553-016-0154-2.
Gurbeta L., Badnjević A., Kurta E. (2020) eVerlab: Software Tool for Medical Device Safety and Performance Inspection Management. In: Badnjevic A., Škrbić R., Gurbeta Pokvić L. (eds) CMBEBIH 2019. CMBEBIH 2019. IFMBE proceedings, vol 73. Springer, Cham.
Das S, Dey A, Pal A, Roy N. Applications of artificial intelligence in machine learning: review and Prospect. International Journal of Computer Applications. 2015;115(9):31–41.
Horvitz, E. (2006) Machine learning, reasoning, and intelligence in daily life: directions and challenges. USA.
Beam AL, Kohane IS. Big data and machine learning in health care. Jama. 2018;319(13):1317–8.
Badnjevića A, Pokvić LG, Hasičić M, Bandić L, Mašetić Z, Kovačević Ž, et al. Evidence-based clinical engineering: machine learning algorithms for prediction of defibrillator performance. Biomedical Signal Processing and Control Volume. 2019;54:101629.
L Spahić, E Kurta, S Ćordić, M Bećirović, L Gurbeta, Z Kovacevic, S Izetbegovic, A Badnjevic, Machine learning techniques for performance prediction of medical devices: infant incubators. In: Badnjevic A., Škrbić R., Gurbeta Pokvić L. (eds) CMBEBIH 2019. CMBEBIH 2019. IFMBE proceedings, vol 73. Springer, Cham.
ESA620 Electrical Safety Analyzer by Fluke Biomedical. Available at: https://www.flukebiomedical.com/products/biomedical-test-equipment/electrical-safety-analyzers/esa620-electrical-safety-analyzer
INCU incubator analyser by Fluke Biomedical. Available at: https://www.flukebiomedical.com/products/biomedical-test-equipment/incubator-radiant-warmer-analyzers/incu-incubator-analyzer
Traceability pyramid. Available at: https://www.isobudgets.com/measurement-traceability-complying-iso-17025-requirements/
International Electrotechnical Commission, IEC 62353, available at: https://webstore.iec.ch/publication/6913
Novakovic J, Strbac P, Bulatovic D. Toward optimal feature selection using ranking methods and classification algorithms. Yugoslav journal of operations research. An International Journal Dealing with Theoretical and Computational Aspects of Operations Research, Systems Science, and Management Science. 2011;21(1):119–35.
Guidi, G., Pettenati, M. C., Miniati, R., & Iadanza, E. (2013). Random Forest for automatic assessment of heart failure severity in a telemonitoring scenario. Conference Proceedings: ... Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference , 2013, 3230–3233.
LD Mustafić, L Gurbeta, A Badnjevic-Cengic, A Badnjević, BB Hukeljić, Diagnosis of Severe Aortic Stenosis Using Implemented Expert System, International Conference on Medical and Biological Engineering, 149–153.
Alić B, Gurbeta L, Osmanovic A, Badnjević A. "machine learning techniques for classification of diabetes and cardiovascular diseases," 2017 6th Mediterranean conference on embedded computing (MECO). Bar: Montenegro; 2017, pp. 1-4. https://doi.org/10.1109/MECO.2017.7977152.
Machine Learning Healthcare Applications – 2018 and Beyond, available at: https://emerj.com/ai-sector-overviews/machine-learning-healthcare-applications/
Shaikhina T, Lowe D, Daga S, Briggs D, Higgins R, Khovanova N. Decision tree and random forest models for outcome prediction in antibody incompatible kidney transplantation. Biomedical Signal Processing and Control. 2017. https://doi.org/10.1016/j.bspc.2017.01.012.
Zhang, Z. (2016). Introduction to machine learning: k-nearest neighbors. Annals of Translational Medicine, 4(11), 218–218.
Calix, R., & Sankaran, R. (2013). Feature ranking and support vector machines classification analysis of the NSL-KDD intrusion detection corpus. TwentySixth International Florida Artificial Intelligence Research Society Conference.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not relevant for this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kovačević, Ž., Gurbeta Pokvić, L., Spahić, L. et al. Prediction of medical device performance using machine learning techniques: infant incubator case study. Health Technol. 10, 151–155 (2020). https://doi.org/10.1007/s12553-019-00386-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12553-019-00386-5