Abstract
Application of a drug in the form of eye drops represents easiest, safest and, at the moment, the most common non-invasive method of ocular drug application. Other conventional ophthalmic formulations in the form of aqueous and oily solutions, ointments, suspensions and emulsions were established to increase bioavailability, solubility and pericorneal retention time of a drug, compared to eye drops. But in the last few decades, nanotechnology-based ophthalmic formulations have been intensively analysed in the area of drug delivery to anterior and posterior parts of the eye. Systems based on nanotechnology with adequate nanoparticle size can be formed to achieve lower irritation and inflammation and better bioavailability and interaction of a drug with ocular tissue. The nanocarrier-based approach led to the development of nanoparticles, nanosuspensions, nanoemulsions, liposomes, nanomicelles, niosomes, nanocrystals and dendrimers for ocular drug delivery. These systems have significant advancements compared to conventional systems, particularly if they are observed as systems for drug delivery to the posterior eye part. Besides advantages, the nanoparticle use in these circumstances could be a reason for concern, because of certain toxic effects noticed in some studies. In this article, we aim to provide an overview of the potential of incorporating an active pharmaceutical ingredient into nanoparticles investigated in the therapy of anterior and posterior eye segment conditions. We will discuss the most important improvements that have been accomplished in the development of nanoparticle-based formulations for the treatment of glaucoma, autoimmune uveitis, age-associated macular degeneration and corneal and choroidal neovascularization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
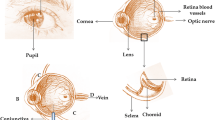
In comparison to the process of drug delivery to the other parts of the body, the delivery of a drug to the eye segments is met with numerous challenges, mostly different eye barriers. Many of these eye barriers are unique and determined by anatomy and physiology of the eye and they are considered to be a significant challenge in the development of ocular drug application technologies [1]. These eye barriers, both anatomical and physiological, exist in the different parts of ocular tissue, especially in the pericorneal space [2].
Conventional ophthalmic formulations usually present poorer bioavailability because of the occurrence of lacrimation, tear dilution and conjunctival absorption [2]. Poorer bioavailability can also be caused because of the transient residence time and the impermeability of corneal epithelium [3]. Eye drops are the basic form of the drug administration to the surface of the eye, due to the good compliance of patients and also economic reasons. Active substances that are dissolved in eye drops are usually absorbed through the cornea and the conjunctiva. Due to the existence of corneal and conjunctival barriers, less than 5% of a drug administered using eye drops reaches the aqueous liquid of the eye, therefore eye drops must be applied more often, for a drug to sustain its therapeutic concentration. Eye drops have shown their efficacy in the treatment of the defects of the cornea, eye irritation and glaucoma. They are less powerful in the treatment of the posterior eye segment conditions, including intraocular melanoma [4]. Blood-retinal barrier restricts the drug transport from the blood to the retina. This eye barrier is made up of narrow bonds of retinal capillary endothelial cells and retinal pigment epithelium (RPE) [5].
In the nanotechnology era, conventional ophthalmic formulations preserve their status, significance and a large share of the market, because they were primarily developed to improve bioavailability, solubility and pericorneal retention time of a drug [6].
Two types of emulsions that are commercially available are water-in-oil type (w/o) and oil-in-water type (o/w). When it comes to the ocular drug delivery, w/o emulsion systems are preferred. Reasons include rare eye irritation and better drug tolerance [7]. Suspensions contain finely divided and dispersed particles of an active pharmaceutical ingredient, that is insoluble in an aqueous solvent. Suspended particles are retained in the pericorneal pocket and therefore pericorneal retention time and duration of drug action are improved [4]. Ointments are also a class of drug delivery systems, developed for the application on the surface of the eye. They are mixtures of solid and semi-solid hydrocarbons (e.g. paraffin) [8].
Although significant efforts are constantly being made to enhance their potency, there is still a demand to conquer the shortcomings linked to conventional ophthalmic formulations. It is known that all of the previously described formulations can cause the occurrence of eye irritation, eye redness and visual impairment. Chronic treatment of a disease may enhance systemic drug concentration, which can lead to serious complications. Dosage forms with preservatives generate the formation of some harmful effects after systemic drug absorption. To surmount the undesirable influence associated with these types of formulations, researchers turned to studying the new strategies for ocular drug delivery, wherein nanotechnology received special attention [8].
In this review, we aim to identify and discuss the different types of nanosystems and nanoparticles used in ophthalmology, their main characteristics, factors affecting their applicability and the strengths and weaknesses of their use. We will first describe the basic aspects of different nanosystems as ocular drug delivery systems, such as nanomicelles, nanoparticles, liposomes, niosomes, dendrimers, nanosuspensions, nanoemulsions, nanocrystals, whilst giving main information about their application as ocular drug delivery systems. In the following section, we will discuss the difference between polymeric and lipid nanoparticles, their subtypes, structure and constituents and methods of the synthesis. In the next section, we will discuss why some drugs are more effective when incorporated into polymeric or lipid nanoparticles, and provide results of recent research on some of the most used drugs in ophthalmology. To continue, we will compare nanoparticles investigated for the treatment of diseases of the anterior and posterior eye segments and their fundamental distinction. Later, we will discuss the application of nanoparticles in glaucoma, corneal diseases, corneal neovascularization, choroidal neovascularization, autoimmune uveitis and age-related macular degeneration, which are the most common eye diseases. Finally, we will make some conclusions and suggest future outlooks about this very current topic.
2 Nanosystems as ocular drug delivery systems
Nanosystems that can be used as ocular drug delivery systems are nanomicelles, nanoparticles, liposomes, niosomes, dendrimers, nanosuspensions, nanoemulsions, nanocrystals, etc. Advantages of nanosystems over conventional ophthalmic formulations include [9]:
Increased permeability and bioavailability of a drug;
Reduced degradation of an unstable drug;
Extended retention of a drug on the surface of the eye;
Improved interaction with the cornea and
Possibility of targeted delivery of a drug.
Particles of a smaller size like nanoparticles are well endured and have mucoadhesive characteristics, responsible for the extended contact time of a drug with ocular tissue and the increase of its bioavailability. Other potential benefits of using nanoparticles in the treatment of eye diseases are [10]:
Possibility of patients’ self-care;
No visual impairment;
Protection against enzyme activity and
Focus on the tissue of interest, which reduces required doses as well as the possibility of the occurrence of adverse effects.
Several researchers have tried to develop drug-filled nanoparticles for its transfer to anterior and posterior eye parts. For this type of delivery, nanoparticles are made of proteins and natural or synthetic polymers (sodium alginate, albumin, polycaprolactone and chitosan) with different types of solid, semi-solid or liquid lipids [11]. Nanoparticles are prospective candidates for these indications, due to their small size (from 10 to 1000 nm) that leads to depleted eye irritability and prolonged drug dispensation, dodging numerous applications per day. Compared to aqueous solutions, nanoparticles can as well be removed quickly from the pericorneal pocket. Therefore, polyethylene glycol (PEG), chitosan and hyaluronic acid were combined in the development of nanoparticles with mucoadhesive properties, for improving pericorneal retention time of a drug [12].
Nanomicelles (see Fig. 1) are mostly used for the formulation of clear aqueous solutions. Generally speaking, they are created using amphiphilic molecules. Research interest is focused on the development of nanomicellar formulations for ocular use. Justification can be assigned to their great drug-entrapment capacity, ease of formation and smaller size. Besides, nanomicellar formulations can improve drug availability in ocular tissues, indicating better therapeutic outcomes [10]. To this date, several studies have been carried out to examine the possibilities of the ocular nanomicelle application. Civiale and associates deployed dexamethasone-filled nanomicelles, using polyhydroxyethylaspartamide (PHEA), for its transfer to the anterior eye segment. Dexamethasone concentration was tested on rabbits as animal models and outcomes demonstrated that these nanomicelles had better bioavailability compared to its suspension. In this regard, it is considered that nanomicellar formulations represent a good option for the surface delivery of small molecules [13]. Nanomicelles were also investigated for drug delivery to the posterior eye part. Voclosporin-filled nanomicelles could deliver this drug to the eye retina in studies conducted on rabbits. These formulations were able to efficiently pass the anterior ocular tissues and deliver this drug to the posterior ocular tissues [10].
Nanosuspensions are proved to be a good approach for the ocular delivery of hydrophilic drugs. They provide various benefits, such as ease of sterilization, rare eye irritation, because of the submicronic size of drug particles, an increase of pericorneal retention time, their stability, because of the use of different surfactants and polymers and improvement of bioavailability of drugs soluble in the lacrimal fluid of the eye [14]. Results of studies conducted so far showed that nanosuspensions can also be an effective ocular delivery system for poorly water-soluble drugs. Glucocorticoids are always selected for the treatment of inflammation in the anterior eye segment. This common treatment calls for frequent dosage regimens and higher doses that promote the production of cataracts, glaucoma and impairment of the optic nerve. The nanosuspension effectiveness in the enhancement of the ocular bioavailability of glucocorticoids has been displayed in many studies. They can be incorporated into hydrogels to achieve prolonged drug release over a specific time. That means that they could improve the ocular bioavailability of glucocorticoids [15].
The size of liposomes (see Fig. 2) is typically from 80 nm to 100 μm and based on the size of phospholipid bilayers, that surround the aqueous base, they can be categorized as small unilamellar vesicles (sizes of 10–100 nm), large unilamellar vesicles (sizes of 100–300 nm) and multilamellar vesicles (containing more than one double layer) [11]. For the ocular drug delivery, liposomes serve as an optimal system, due to the great compatibility, the structure that is similar to the cell membrane structure and the power to entrap hydrophilic and lipophilic drugs. Liposomes have shown acceptable efficacy for the treatment of anterior and posterior eye part diseases in several studies. Development of liposomes is focused on the improvement of the drug half-life, the reduction of its clearance from the vitreous fluids, the protection of labile molecules, such as peptides and oligonucleotides, from their degradation and the provision of extended drug release [4].
Dendrimers (see Fig. 3) are also used as carriers in the ocular drug delivery. These branched and star-shaped polymeric systems have different molecular weights, with terminal amino, hydroxy or carboxyl functional groups. Choices of molecular weight, surface charge and functional groups are crucial for drug delivery. Highly branched structures enable the incorporation of a large number of drugs, both hydrophobic and hydrophilic. In the ocular drug administration, numerous encouraging results for these systems have been shown so far. Polyamidoamine (PAMAM) dendrimers are widely applied in ocular drug delivery. Vandamme and Brobeck pointed out the possibility of using PAMAM dendrimers as vesicles for the ocular delivery of both tropicamide and pilocarpine nitrate [16]. To avoid the formation of scar tissue after the glaucoma filtration surgery, combinations of PAMAM dendrimers with glucosamine-6-sulphate and glucosamine were made, for the expression of immunomodulatory and antiangiogenic activities. Subconjugative administration of these modified conjugates in rabbits showed significant inhibition of proinflammatory and proangiogenic responses and consequently reduced the formation of scar tissue. Results obtained in the study conducted by Yavuz and associates showed that the ocular administration of glucosamine and glucosamine-6-sulphate can be useful and harmless in averting the phenomenon of scar tissue after the glaucoma filtration surgery [17].
Nanocrystals (see Fig. 4) of an active substance belong to a group of therapeutic nanosystems with a crystalline structure [18]. They can be administered exclusively by oral and parenteral routes. Nanocrystals of an active substance are built almost entirely of an active substance, with only a small part being made of excipients (stabilizers or surfactants). In addition to the increase of solubility of an active substance, nanocrystals have a great capacity of adhesion, which may provide longer drug retention in the pericorneal area and consequently its higher bioavailability. Adherence and internalization also depend on surfactants used in the formulation. Most commonly, poloxamers and polysorbates are used [9].
Niosomes (see Fig. 5) are relatively new therapeutic systems that are interesting as carriers in ophthalmology, as they are formed between various types of amphiphilic non-ionic components in an aqueous medium, forming bilayer sacs, sizes of 0,5–10 μm. They are biodegradable, biocompatible, non-immunogenic and non-toxic. They have the capability to incorporate hydrophilic and hydrophobic drugs, but they are characterized by better chemical stability. Niosomes also show similarities with microemulsions, as they contain a surface-active compound, which allows them to increase drug permeability through the cornea. Other advantages are better spreading on the surface of the eye and the increase of viscosity, compared to drug solutions [11, 19].
3 Types of nanoparticles in ocular drug delivery systems
There are two types of nanoparticles: nanospheres and nanocapsules (see Fig. 6). Nanospheres are defines as matrix systems, in which a drug is adsorbed on the surface or evenly dispersed into the matrix [20]. They consist of a solid core with a dense polymer around it and range in size from 100 to 1000 nm [21]. Nanocapsules are defined as vesicular systems, in which the interior of the core can differ from the outer polymeric sheath. In such systems, a drug is usually dissolved in the nucleus of the particle, but it can also be adsorbed on the surface [20]. Nanocapsules consist of an oil-filled cavity surrounded by a firm polymeric envelope and range in size from 10 to 1000 nm [21]. Nanoparticles can be coated with hydrophilic polymers or functional antibodies, which allows drug delivery to the targeted site [20]. It studies carried out so far, it is shown that surface modifications of these nanoparticles can be achieved using targeting molecules, e.g. transferrin and folate, or OX26 monoclonal antibody [21].
In numerous studies, researchers have investigated the possibility of the application of different polymers in the preparation of nanoparticles, such as acrylic polymers (e.g. poly (alkylcyanoacrylate) (PACA)), polyesters (e.g. poly-ɛ-caprolactone), polysaccharides (e.g. hyaluronic acid and chitosan). The first generation of nanoparticles was made of acrylic polymer PACA and the sustained drug release in the pericorneal area was achieved. Besides, prolonged retention on the surface of the eye was achieved, consequently increasing the duration of drug action. After the ocular toxicity of PACA was discovered, polyacrylamide was used (the second generation). Nanospheres made of polyacrylamide with epinephrine prolonged the duration of reduced intraocular pressure [22].
Nanoparticles built of polyesters (poly-ɛ-caprolactone and copolymers of lactic and glycolic acids) were well tolerable in the eye [23]. For example, betaxolol was combined with poly-ɛ-caprolactone to achieve its optimal pharmacological effect, due to the accumulation of hydrophobic nanoparticles and their gradual release. Nanoparticles built of hydrophilic polysaccharides, e.g. hyaluronic acid and chitosan, were very safe for ocular use. Hyaluronic acid is biodegradable and it is the basic component of the vitreous body. Improving the interaction of nanoparticles with the cornea is important for drugs with targeted action site in the inner eye segment and achieving the controlled drug release also provides the possibility of treating diseases of the outer eye segment. This can be achieved by coating nanoparticles with a polymer. The simplest way of making coated nanosystems is by dispersing nanoparticles in an aqueous solution of bioadhesive polymers. The advantages include better permeability of embedded drug (e.g. PACA with cyclosporine-A) and increased stability in the lacrimal fluid (e.g. coating with PEG prevents interactions of nanosystems and proteins (enzymes)) [24].
The third generation includes nanoparticles with modified surface properties. Surface properties change by cladding with specific functional groups, which allows nanosystems to be directed to the targeted site in the eye. An example is lectin, a glycoprotein that recognizes and binds to specific carbohydrate groups located on the surface of the epithelial cells of the cornea [20]. Positively charged nanoparticles penetrate through cell membranes while negatively charged ones do not enter the cell membrane, but prevent it from breaking down [25]. Bioadhesive nanoparticles have been investigated because of their capability to immobilize the particles on the mucosal surface by the process of adhesion. The modification of surface properties is achieved by using different bioadhesive polymers. Between the formulation and the oral mucosa, there is a large specific surface, leading to sustained drug release and its more effective absorption [26].
Nanoparticles, due to their small size, that does not irritate, and achieved sustained release, thus avoiding frequent drug administration, are good systems for ocular drug delivery. Nanoparticles were applied as an alternative method for continual drug delivery to the posterior eye part. For the drug delivery to the posterior eye segment, the disposition of nanoparticles depends on their size and characteristics of the surface. It was observed that, after the periocular application in rats, 20 nm size-nanoparticles were quickly eliminated from the periocular tissue. On the other hand, particles ranging in size from 200 to 2000 nm were kept at the spot for two months. Therefore, we can conclude that, for the extended transscleral drug delivery through the blood and the lymph circulation, nanoparticles were suitable systems [12, 27].
After intravitreal injection, different nanoparticles can drift through the layers of the retina and try to concentrate on the RPE. Zhang and associates explored pharmacokinetics and tolerability of dexamethasone-loaded poly (lactic-co-glycolic acid) (PLGA) nanoparticles (size of 232 ± 5,4 nm, polydispersity index of 0,19 and encapsulation efficacy of 56%) after intravitreal injection in rabbits. They deduced that entrapped dexamethasone exhibits prolonged release for around 50 days. Steady concentration of dexamethasone was kept for around 30 days with a medium concentration of 3,85 mg/L. On the other hand, dexamethasone was only detected in traces on the seventh day after the injection of its solution. There was no fast initial release, 6% of a drug was released the first day, with 97% of a drug released in the next 35 days. Bioavailability of dexamethasone in the vitreous fluid, chorioretina and plasma was higher 4,96, 4,15 and 6,35 times, respectively, compared to the control. This indicates that this type of intravitreal injection can be used to prolong the drug release in the treatment of the posterior eye segment diseases [28].
The surface characteristics are the crucial factor that influences nanoparticle disposal from the vitreous fluid into the layers of the retina. Koo and associates examined the parallel between surface properties and nanoparticle disposal in the vitreous fluid and the retina, after intravitreal injection. Polyethyleneimine-glycol chitosan (PEI/GC) (size: 272,1 ± 9,2 nm, zeta potential: 20,7 ± 3,2 mV), human serum albumin-glycol chitosan (HSA/GC) (size: 293,3 ± 11,1 nm, zeta potential: −1,9 ± 4,1 mV) and human serum albumin-hyaluronic acid (HSA/HA) (size: 344,8 ± 10,7 nm, zeta potential: −23,3 ± 4,4 mV) nanoparticles were assembled by mixing polymers. Nanoparticles were instilled into rats’ vitreous cavity and vitreous and retinal distributions were assessed. PEI/GC nanoparticles effortlessly passed through the vitreous barrier and approached the inner limiting membrane. They did not go through the pores of the inner limiting membrane into the layers of the cornea. HSA/GC nanoparticles approached the inner limiting membrane, but could not go through the layers of the retina, which could occur due to the hindrance of interactions between HSA and Müller cells, caused by GC. Negatively charged HSA/HA nanoparticles could go through the full retinal structure and approach the outer layers of the retina, which was assigned to interactions between Müller cells and the anionic surface. Both HA and HSA homogenous nanoparticles, sizes of 200–300 nm, prevail the physical barriers of the inner limiting membrane. Large amounts of HSA nanoparticles reached the inner limiting membrane, with some of them going through the physical barriers of the inner limiting membrane via endocytosis. During the penetration of HSA nanoparticles into the structures of the retina, the Müller cells were observed. The authors concluded that HSA nanoparticles could penetrate through the retina via the specific pathway in the Müller cells, based on endocytosis and exocytosis [29]. In the second study, human serum albumin nanoparticles (HSA-NP) penetrated through the entire retina, following an intravitreal injection into rats’ eyes. Therefore, HSA-NP could be useful carriers of drugs for the therapy of age-associated macular degeneration (AMD), which requires drug distribution in the choroidal region, to inhibit choroidal neovascularization (CNV) [30].
3.1 Polymeric nanoparticles
Biodegradable polymeric nanoparticles of sizes from 10 to 100 nm are the most popular type of nanosystems in ocular therapy. These systems can be composed of several polymers, in which a drug is encapsulated or dissolved. Drugs can be adsorbed on the surface or incorporated into the matrix. Polymeric nanoparticles provide several advantages for ocular use, which primarily relate to their properties: biodegradability, non-toxicity, biocompatibility and mucoadhesiveness. Polymers are suitable materials for the production of a large number of different nanoparticles, which can be made either by the direct process of polymerization of different monomers or from derived polymers. For the production of nanoparticles from derived polymers, methods like classic polymerization or polyreaction, solvent evaporation, salting-out, dialysis and technology of supercritical fluids can be used. Nanoparticles can be directly constructed by the process of polymerization of different monomers using several methods, such as microemulsification, miniemulsification, emulsification without surfactant and interfacial polymerization. The decision on the preparation method is based on a large number of determinants, like the polymer type, the application spot or the required dimensions [31].
Advantages of polymeric nanoparticles are reflected in the way that they [32]:
Increase the stability of easily volatile substances;
Are simply prepared;
Provide a significant improvement over conventional methods in terms of efficiency and effectiveness;
Deliver greater concentration of an active pharmaceutical ingredient to the targeted site and
The choice of polymers and the ability to modify drug release made them ideal candidates for the treatment of cancer.
Drug release mechanisms from polymeric nanoparticles include [32]:
Swelling by hydration, followed by an active substance release by diffusion;
Enzymatic reactions resulting in polymer removal or deterioration at the action spot, thus releasing a drug out of the nanoparticle core and
Detachment of a drug and a polymer and its desorption and liberation from nanoparticles.
Biodegradable nanoparticles are often used due to their higher bioavailability, better encapsulation, controlled release and less toxic properties. Also, polymeric nanoparticles can be used as gene delivery carriers. The development of safe and effective vector carriers for in vivo gene transfer is one of the key challenges of gene therapy [31]. Polymeric nanoparticles increase drug bioavailability by enhancing drug accumulation at the action spot (passive or ligand-mediated mechanisms), thereby reducing its necessary dose and side effects. However, some authors describe the potential drawbacks of these types of nanoparticles, which are related to their toxicity, based on the fact that the polymer deterioration can cause systemic pernicious aftermath and due to the use of organic solvents in the final formulation. Depending on the properties of the used polymer, these types of nanoparticles can be produced using a simple method, such as primary precipitation or ionotropic gelation. Among the most important characteristics required for the use of nanoparticles in ophthalmology is their capability of retaining in ocular tissue, their size and the use of polymers with mucoadhesive properties. These polymers minimize drug clearance from the eye, by interacting with mucin that is present on the surface of the eye. Pericorneal retention time is increased and thus drug bioavailability. Without these properties, nanoparticles are eliminated from the pericorneal area at the same rate as aqueous solutions. Different authors developed nanoparticle-based systems with modified surface characteristics, coated with various mucoadhesive polymers, to improve interaction with mucin in the eye mucosa. Examples of these polymers are PEG, poloxamers, poloxamines, hyaluronic acid, chitosan, polyacrylic acid, sodium alginate, etc. [33].
3.2 Lipid nanoparticles
Lipid nanoparticles were developed out of o/w emulsions, with the replacement of liquid lipids with lipids solid at both room and body temperature. Therefore, the above-mentioned systems consist of an aqueous dispersion of particles, ranging in size from 150 to 300 nm, although particles of larger and smaller dimensions can also be found. When observed with liposomes and polymeric nanoparticles, lipid nanoparticles have been described as superior carriers. Advantages of using lipid nanoparticles are the absence of toxicity, the protection of drug molecules from their degradation, good long-term stability, controlled drug release, improved drug bioavailability, production methods that do not require the use of organic solvents and simplicity of transfer of production to an industrial level. There are two groups of lipid nanoparticles: solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) [34].
SLNs were developed as alternative systems to liposomes, nanoemulsions and polymeric nanoparticles. They are made up of a solid lipid with an incorporated drug. Their standard size is less than 1 μm. Conventional excipients used in SLN formulations are water, co-emulsifiers and emulsifiers. They exhibit benefits like prolonged drug release, higher drug bioavailability, chemical preservation of unstable molecules, good cost-effectiveness ratio, improved drug inclusion and a broad spectrum of applications. Different application routes for SLNs, such as parenteral, oral, rectal, ophthalmic and topical, are possible to achieve [35].
NLCs incorporate liquid and solid lipids as the basic matrix. Because of the use of physiological and biodegradable lipids, these carrier systems also exhibit excellent tolerance. SLNs were primarily designed, while the design of NLCs was the result of attempts to overcome SLNs’ noted drawbacks, such as insufficient drug-loading capacity and drug ejection while being stored [36]. The first type of NLCs is „imperfect type“, in which liquid and solid lipids are blended into several lipid formations. Particular circumstances of crystallization generate very disorganized matrix structures, that represent the gap between the triglycerides and the fatty acid chains in the crystals and therefore extend drug capability to enter the matrix. The second type is „non-shaped type “(with non-crystalline matrix). These NLCs do not have a crystalline structure and therefore prevent drug ejection. Here, crystals are formed during the process of cooling and to prevent it, lipid mixtures should be used. The third class is „multiple type“, in which drug solubility in a liquid lipid is higher compared to the one in a solid lipid, therefore it is used for protection against decomposition caused by that solid lipid. Above-mentioned structure of NLC is comparable to w/o emulsions [37].
SLNs and NLCs provide several advantages, such as scavenging of lipophilic drugs, thereby increasing their retention time, drug protection from enzymatic degradation in the eye, adhesion to the surface of the eye and interaction with the epithelium, all depending on their size, shape and surface charge. Lipid components can reach the tear film’s lipid layer, improving drug delivery, with increased drug bioavailability. The surface composition of colloidal systems may affect their affinity for the eye mucosa. It was observed that a positive surface charge has beneficial effects in prolonging the retention time of emulsion drops on the epithelial layer of the cornea. The preparation of lipid nanoparticles with cationic lipids and surfactants promotes further electrostatic interactions with the anionic layers of ocular tissue [38]. Studies of cytotoxicity of SLNs and NLCs carried out on several cell lines proved that they are well endured and do not irritate ocular tissue. The key point of cytotoxicity research of NLCs is the used surfactant and its characteristics. Hence, non-ionic surfactants need to be chosen, since they provoke less toxic effects and irritation to ocular tissue. On the other hand, nanoparticles have the ability to provide and maintain the non-ionized drug form, when it is in front of the pericorneal film, facilitating the transport through the various eye barriers [37].
Tabular overview of different nanoparticle types with incorporated substances and related sources is presented in Table 1.
4 Drugs incorporated into nanoparticles in ocular drug delivery systems
Different ophthalmic drugs can be incorporated into polymeric or lipid nanoparticles and which type of nanoparticles will be chosen depends on their distinct characteristics.
4.1 Drugs incorporated into polymeric nanoparticles
Giannavola and associates prepared acyclovir-filled poly (d,l-lactic acid) (PLA) nanospheres to study the influence of surfactant concentrations and polymer molecular weights on their size and polydispersity index and to examine the differences in bioavailability and therapeutic efficacy among nanoparticles which were uncoated and coated with PEG and compared with aqueous solutions (maximum concentration: 0,2 ± 0,1 μg/mL; area under the curve concentration – time (AUC): 33,7 ± 10,3 μgh/mL). This study was carried out using rabbit models. Acyclovir levels were observed for six hours. With higher molecular weight of the used polymer, nanosphere size and polydispersity index decreased (for 16.000 Da: 131,5 ± 0,3 nm and 0,112; for 109.000 Da: 92,5 ± 1,7 nm and 0,177; for 209.000 Da: 51,2 ± 0,1 nm and 0,291, respectively). Zeta potential charge ranged from −31,1 mV to −14,7 mV. Both formulations showed prolonged release of acyclovir and were well tolerated, but coated nanoparticles showed greater efficacy (maximum concentration: 1,5 ± 0,1 μg/mL; AUC: 424,1 ± 24,1 μgh/mL) compared to uncoated nanoparticles (maximum concentration: 0,9 ± 0,1 μg/mL; AUC: 235,6 ± 19,2 μgh/mL) [23].
Kao and associates developed pilocarpine-loaded chitosan/carbopol nanoparticles (size of 294 nm). Properties and prolonged-release effects of these nanoparticles were compared to different ophthalmic formulations of pilocarpine, primarily solutions and gels, using in vitro (dissolution test) and in vivo tests (in rabbit models). The main reason for the chitosan/carbopol combination is achieving the nanoparticle stabilization by electrostatic interactions. Chitosan/carbopol nanoparticles had the advantage of their biocompatibility, biodegradability and mucoadhesiveness and showed prolonged release of pilocarpine when compared to other formulations, as a function of time in simulated tear fluid at a pH of 7,4 and at the temperature of 37 °C for 24 h (in vitro dissolution test) and as a function of time at the temperature of 25 °C for 24 h (in vivo rabbits’ pupil decrease), with following results: nanoparticles – AUC: 751,6 μgh/mL, gels – AUC: 324,7 μgh/mL, solutions – AUC: 184,9 μgh/mL. Nanoparticles showed the greatest drug bioavailability and can be considered as a promising ocular drug delivery system [39].
Yuan and associates developed polymeric nanoparticles (size of 300 nm) prepared with chitosan and PLA and loaded with rapamycin. Chitosan modified with cholesterol was used as a stabilizer. Immunosuppressive effects of these nanoparticles were observed. The median survival time of the grafts was 27,2 ± 1,03 days. Half of the grafts survived by the end of this study. This type of nanoparticles gave good retention time and prolonged release of rapamycin using the cornea of rabbits’ eyes as a model, as well as great immunosuppressive effects [40].
Motwani and associates formulated chitosan/sodium alginate nanoparticles loaded with gatifloxacin. Nanoparticle polydispersity index (0,325–0,489), zeta potential charge (17,6–47,8 mV) and size (205–572 nm) were observed. Sodium alginate used in ophthalmic formulations has a number of advantages, primarily biodegradability, biocompatibility and mucoadhesive properties. Results showed that chitosan/sodium alginate nanoparticles had mucoadhesive characteristics. Prolonged release of gatifloxacin over 24 h was achieved (81–85%), which was confirmed by the method of diffusion through the cell membrane, but with rapid drug release in the first hour (10–12%) [41].
In the second study, gatifloxacin/prednisolone-loaded Eudragit RS 100 and RL 100 nanoparticles were prepared. Hyaluronic acid was used as a polymer. The molar ratio of a drug and a polymer and the molar ratio of Eudragit RS 100 and RL 100 were observed. Nanoparticle size ranged from 315,2 to 973,65 nm. Drug-release efficacy decreased when the polymer concentration increased as well as the molar ratio of Eudragit RS 100 and RL 100. Encapsulation efficacy decreased when the molar ratio of a drug and a polymer increased. Results of this study showed sustained drug release for more than six hours (AUC: 54,75 ± 1,78 μgh/mL), while the effects of commercial eye drops lasted about two hours (AUC: 30,88 ± 1,51 μgh/mL). The authors of this study (Ibrahim and associates) concluded that this type of strategy increased pericorneal retention time and improved drug penetration through the cornea [42].
Mahmoud and associates combined advantages of chitosan with advantages of cyclodextrin, polyanionic polymers and solubilizing agents for poorly water-soluble drugs. These authors prepared chitosan/sulphobutyl ether-ß-cyclodextrin nanoparticles filled with econazole nitrate. Nanoparticle polydispersity index (0,16–0,64), zeta potential charge (22–33 mV), size (90–673 nm) and mucoadhesiveness were observed to investigate the influence of the chitosan molecular weight (150 and 360 Da). Lower the molecular weight and viscosity of chitosan were, higher was the ability to form smaller nanoparticles (150 Da – AUC: 170 ± 6 μgh/mL; 360 Da – AUC: 127 ± 5 μgh/mL). Studies performed on rabbits showed that these nanoparticles gave a prolonged release of econazole nitrate and hence antifungal effects over a longer period compared to the administration of the same drug dissolved in the solution, therefore its bioavailability when incorporated into polymeric nanoparticles would be exponentially increased [43].
Nagarwal and associates developed chitosan-coated sodium alginate/chitosan nanoparticles filled with 5-fluorouracil, sizes from 329 to 505 nm. Firstly low zeta potential charge (7,5–13,8 mV) increased because of the nanoparticle coating with chitosan (18,5–28,9 mV). The influence of the molar ratio of sodium alginate and chitosan on nanoparticle size and encapsulation efficacy was investigated. AUC values were 27,62 μgh/mL, 134,23 μgh/mL and 198,42 μgh/mL for mere 5-fluorouracil, sodium alginate/chitosan nanoparticles and chitosan-coated sodium alginate/chitosan nanoparticles, respectively. In this study, nanoparticle coating with chitosan caused an increase in their size, viscosity and mucoadhesiveness, compared to uncoated nanoparticles. A study using rabbits’ eyes as a model showed an increase of bioavailability of 5-fluorouracil in the aqueous liquid of the eye and therefore an increase of its therapeutic efficacy. The prolonged release was also achieved, compared to 5-fluorouracil solution [44].
Bhatta and associates prepared lecithin/chitosan mucoadhesive nanoparticles with natamycin. Nanoparticle size was 213 nm with zeta potential charge of 43 mV and encapsulation efficacy of 73,57%. Studies proved that drug release had biphasic characteristics, firstly fast release which was followed by a very slow, prolonged release. On the other hand, studies performed on rabbits showed excellent mucoadhesive characteristics that affect retention time. The retention time increased 1,47 times and clearance reduced 7,4 times compared to commercially available suspensions. AUC values were 13.348,90 ± 1293,48 μgh/mL and 9047,35 ± 132,15 μgh/mL for natamycin suspension and natamycin-loaded nanoparticles, respectively. The authors concluded that the antifungal activity of lecithin/chitosan mucoadhesive nanoparticles with natamycin was comparable to natamycin suspension (the zone of inhibition and MIC90 were similar or higher), but the main advancement was the less frequent dosing need [45].
Gupta and associates presented two studies related to PLGA nanoparticles filled with levofloxacin and sparfloxacin. In both studies with rabbit models, due to the small size (180–190 nm), zeta potential charge (−22 mV), encapsulation efficiency (86,6%) and polydispersity index (0,238), there was a long retention period on the surface of the eye and reduced drug elimination compared to traditional formulations available on the market. These formulations allowed prolonged drug release and exhibited good ocular tolerance. In two hours, 37,8% of sparfloxacin was released from nanoparticles compared to commercially available formulations (75,33%), and in six hours, 57,13% (nanoparticles) compared to 96,7% (commercially available formulations) [46]. Gupta and associates developed PLGA nanoparticles with sparfloxacin, which was dispersed into chitosan gel. These researchers called the combination of nanoparticles and in situ gelation systems „nanoparticle-filled in situ gels“. Gelation took place at a pH of 7,2. This study proved the influence of the small size of PLGA nanoparticles (180 nm) on mucoadhesiveness, improvement of penetration and pH response, due to the characteristics of chitosan gel (it behaves as a penetration stimulator). Results showed that nanoparticle-loaded in situ gel was entrapped in the eye longer, promoting sustained drug release and prolonged retention time, when compared to nanosuspensions and in situ gels without nanoparticles [47]. In the second study, Gupta and associates prepared PLGA nanoparticles with levofloxacin scattered into chitosan gel (size: 199,3 ± 1,86 nm; polydispersity index: 0,344 ± 0,037). Results were identical to those obtained in the previous study, which showed that the combination of these two strategies increased the bioavailability of these drugs [48].
4.2 Drugs incorporated into lipid nanoparticles
Drugs incorporated into lipid nanoparticles are ordinarily used on the surface of the eye. Cavalli and associates made the SLN nanosystem with tobramycin (size: less than 100 nm, polydispersity index: less than 0,2) for the ocular drug administration. Rabbits were used as animal models. Characteristics of tobramycin-loaded SLNs (size: 80 nm, polydispersity index: 0,12) were compared to the ones of fluorescent SLNs without tobramycin (size: 70 nm, polydispersity index: 0,15). Tests also showed prolonged drug release and prolonged retention time over six hours (AUC: 155,08 ± 4,31 μgh/mL), compared to shorter drug release of the same dose when using eye drops (AUC: 37,19 ± 1,14 μgh/mL). Consequently, the bioavailability of tobramycin was higher in the SLN system [49].
Attama and associates developed lipid nanoparticles containing diclofenac sodium in combination with phospholipids, with high encapsulation efficacy (61,7 ± 2,6%). Nanoparticle size was around 100 nm and zeta potential charge around −29,2 ± 0,5 mV. Penetration through the cornea of diclofenac sodium and prolonged drug release were enhanced by the nanoparticle surface-coating with phospholipids, which influenced the efficacy of these formulations for ocular drug administration [50].
Gökçe and associates made cyclosporine-A-filled SLNs with Tween 80, Compritol 888 ATO and Poloxamer 188 as surfactants. Nanoparticle size was 225,9 ± 5,5 nm with polydispersity index of 0,253 ± 0,05, zeta potential charge of −16,9 ± 0,7 mV and encapsulation efficacy of 95,6%. Both in vitro and in vivo tests were carried out. Results showed that the release of cyclosporine-A was lipase-dependent. Different results can be shown at different sterilization temperatures. The temperature of 110 °C was more adequate for maintaining the optimal nanoparticle size and polydispersity index, compared to 121 °C. Tween 80 can cause cell deterioration by leaking at the sterilization temperature of 121 °C. Cell retention tests and cytotoxicity tests carried out on the rabbits’ cornea showed that cyclosporine-A-filled SLNs were not harmful and that permeation characteristics through the cornea were improved (15–24%) [51].
Araujo and associates prepared triamcinolone-filled NLCs (size of 100–300 nm, polydispersity index of 0,1, zeta potential charge of 45 mV and encapsulation efficacy of 95%), using Lutrol F 68 as a surfactant, squalene as a liquid lipid and Precirol ATO 5 as a solid lipid, for the ocular drug application. Tests did not show signs of ocular toxicity (mean total score after the Draize test was zero) [52].
Baicalin-filled SLNs were prepared by Liu and associates. Rabbits were used as animal models. Nanoparticle size was 91,42 ± 1,02 nm with zeta potential charge of −33,5 ± 1,28 mV and encapsulation efficacy of 62,45 ± 1,67%. Studies showed that SLNs retained the drug better in ocular tissue than baicalin solution, while achieving prolonged drug release and higher permeation through the cornea. The ocular irritation test was also performed, showing the average result of zero and proving that these formulations are not irritating. In pharmacokinetic studies, AUC of baicalin-filled SLNs (0,1229 ± 0,0312 μgh/mL) was 4 times higher compared to baicalin solution (0,0314 ± 0,0136 μgh/mL) [53].
Gonzales-Mira and associates made flurbiprofen-filled NLCs, containing Miglyol 812 and castor oil as liquid lipids and stearic acid as a solid lipid, to advance drug bioavailability. Best NLC formulation with the molar ratio of stearic acid to oils of 70:30 had a standard size of 228,3 nm, polydispersity index of 0,156, zeta potential charge of −33,3 mV and encapsulation efficacy of 90%. They noted that these NLCs exhibited prolonged drug release and that an ideal NLC never shows toxicity to ocular tissue (mean total scores were 5 and 0,1 for NLCs and eye drops, respectively, with both formulations classified as minimally irritant) [54].
Hao and associates developed chloramphenicol-filled SLNs using Poloxamer 188 as a surfactant and glycerol monostearate as a solid lipid. Nanoparticle size was around 248 nm with polydispersity index of 0,277 ± 0,058, zeta potential charge of −8,74 mV and encapsulation efficacy of 83,29% ± 1,23%. Studies demonstrated fast release in the first stage, which was followed by a sustained release for 48 h. The authors found that chloramphenicol-filled SNLs should be further explored as the ocular drug delivery systems with improved incorporation efficacy and controlled drug release [55].
Liu and associates prepared mangiferin-filled NLC formulations for the potential cataract treatment using rabbits as animal models. Nanoparticle size was 51,39 ± 3,53 nm with polydispersity index of 0,25 ± 0,02, zeta potential charge of −36,5 ± 1,5 mV and encapsulation efficacy of 88,10 ± 1,58%. NLC formulations showed prolonged drug release (mangiferin release of 96,52% from solution versus mangiferin release of 25,92% in the first hour and 86,98% after 12 h from NLCs) and 2,61–4,31 times the increase of penetration through the cornea. Studies demonstrated great ocular tolerance (irritation indicators were all zero, consequently these NLCs were classified as non-irritant) and extended drug retention on the surface of the eye (retention time: 0,20 ± 0,31 h for solution and 3,50 ± 0,33 h for NLCs). A pharmacokinetic study showed 5,69 times the enhancement in bioavailability in the eye in comparison to mangiferin solution (AUC: 0,11 ± 0,03 μgh/mL for solution and 0,62 ± 0,09 μgh/mL for NLCs) [56].
Hippalgaonkar and associates prepared indomethacin-filled SLNs for its delivery to ocular tissue, using Compritol 888 as a lipid phase. Nanoparticle size was around 140 nm with polydispersity index of 0,16, zeta potential charge of −21 mV and encapsulation efficacy of 72% before sterilization at 110 °C and 149 nm, 0,17, −22 mV and 71% after sterilization at 110 °C. They showed a significant rise in drug stability and drug permeability through the cornea compared to indomethacin solution (12,2 – 1,85*10−6 cm/s for SLNs and 2,74 – 0,49*10−6 cm/s for solution) [57].
Zhang and colleagues prepared genistein-filled Eudragit RS 100-modified NLCs. Nanoparticle size was 148,4 ± 1,38 nm with polydispersity index of 0,258 ± 0,02, zeta potential charge of 5,12 ± 0,53 mV and encapsulation efficacy of 91,6 ± 0,8%. They demonstrated 1,22 times greater raise of AUC in comparison to regular NLCs (8696 ± 881,23 μgh/mL and 7155,11 ± 820,11 μgh/mL for modified NLCs and regular NLCs, respectively). Eudragit RS 100 modifications increased zeta potential charge (−7,46 mV to 13,60 mV) and also enhanced corneal penetration, resulting in 1,6–3,3 times the increase of permeability and also stability. The cytotoxicity test and the ocular irritation test confirmed that this NLC type was neither irritating nor toxic for the corneal cells (classified as non-irritant and non-toxic) [58].
To further enhance pericorneal retention time, the surface of lipid nanoparticles filled with ofloxacin was coated with chitosan. Ethanol was used as a cosurfactant with 0,75% chitosan, Tween 80 as a surfactant, oleic acid as a liquid lipid and Compritol HD5 ATO as a solid lipid. Rabbits’ cornea was used as an animal model. Nanoparticle size was 153,5 ± 2,38 nm with polydispersity index of 0,188 ± 0,01, zeta potential charge of −4,63 ± 0,259 mV and encapsulation efficacy of 98,441 ± 0,023%. Üstundag-Okur and associates noticed that these NLCs stayed on the surface of the eye for 20–40 min. The maximum pericorneal retention time was 40–60 min. Penetration rate of 23,872 ± 2349% and permeation rate of 2928 ± 0,092% were also achieved [59]. In the second study, the authors prepared NLC-based implants using 0,75% chitosan. They were added to the ofloxacin system for the treatment of bacterial keratitis. Implants made with glycerin as a plasticizer were optimal. Nanoparticle size was 153,5 ± 2,3 nm with polydispersity index of 0,188 ± 0,01 and zeta potential charge of −4,63 ± 0,259 mV. The periocular retention time was improved up to 24 h and the AUC value was 1,84 ± 0,1 μgh/mL. The maximum drug concentration was nearly six times higher compared to commercial formulations in conducted studies [60].
5 Comparison between nanoparticles investigated for the treatment of diseases of different parts of the eye
Usually, eye diseases can be classified into two categories: diseases of the anterior segment and diseases of the posterior segment, therefore different drugs can be incorporated into nanoparticles in order to improve the treatment of both types of diseases.
5.1 Nanoparticles investigated for the treatment of diseases of the anterior eye segment
The size of flurbiprofen-loaded PLGA nanoparticles created using Poloxamer 188 as a stabilizer was 232,8–277,6 nm with zeta potential charge of −24,48 - –27,45 mV and encapsulation efficacy of 93,5–94,6%. It showed fast initial drug release while providing gradual drug release after. This approach showed an improvement of anti-inflammatory effects after the addition of sodium arachidonate for the inflammation induction, compared to commercial flurbiprofen eye drops, in studies conducted on eye inflammation models in rabbits. These formulations were shown to be non-toxic and non-irritant (mean total score was zero) and stable for six months [61].
Additionally, charged nanoparticles of flurbiprofen with a size of 100 nm, polydispersity index of 0,26 and zeta potential charge of 40–60 mV, created using Eudragit RS 100 and RL 100, were investigated for their anti-inflammatory effects and exhibited similar inhibitory impact on the miotic feedback in the surgical trauma model (after paracentesis in rabbits) with lower drug concentration in the conjunctiva and higher one in the aqueous liquid, compared to eye drops. This action is credited to an enhanced drug release and consequent passage in the aqueous liquid of the eye. Such advances indicate a great influence of colloidal nanoparticles on improving the bioavailability of drugs like flurbiprofen. Different tests were performed, such as stability tests (resulting in an optimized formulation), in vitro dissolution tests (resulting in controlled drug release) and toxicity tests (showing that these formulations were non-toxic) [22].
In situ gel systems became a significant subject of research, especially micellar supramolecular hydrogels, created with PEG and cyclodextrin, that react to stimuli like pH, as well as temperature- and ion-sensitive hydrogels. When a hydrogel was spread on the surface of the eye, nanoparticles could come out after blinking and then release drugs in an extended manner, meaning these systems extend drug retention time on the surface of the eye and achieve greater drug bioavailability. These types of gels have thixotropic characteristics, are non-irritant nor cytotoxic. As expected, a range of drugs that contain nanobuffer could withstand blinking of the eye and stay on the surface of the cornea for a couple of hours. This system not only provided prolonged drug release but also improved effectiveness in the treatment of corneal neovascularization in rabbit animal models [62].
The corneal epithelium is proved to be a significant permeation and penetration eye barrier, which can limit the passage of particles tinier than 21 ± 11 nm into the ocular area. In the early studies on the bovine eye with its epithelium removed, chemically-dependent permeation characteristics were tested on two groups of nanoparticles, which had following characteristics: size of 21–45 nm (thiolated), 27–54 nm (PEGylated 750 Da) and 43–64 nm (PEGylated 5000 Da) and polydispersity index of 0,263–0,332 (thiolated), 0,194–0,254 (PEGylated 750 Da) and 0,145–0,231 (PEGylated 5000 Da), but a different number of thiol groups. Correlation between the stroma of the cornea coated with collagen and these functional groups concerning the nanoparticle size was a significant resistance factor during the process of drug permeation and penetration. Excelling absorption in the stroma of the cornea was observed by PEGylating with PEG of higher molecular weight (5000 Da) compared to the one of molecular weight (750 Da), but thiolated and PEGylated nanoparticles with PEG of 750 Da were more adhesive so longer retention on the surface of the eye was achieved [63].
5.2 Nanoparticles investigated for the treatment of diseases of the posterior eye segment
Unlike diseases of the anterior part of the eye, diseases of the posterior part of the eye most commonly occur in the retina. Those include AMD, CNV, glaucoma, retinoblastoma and posterior uveitis [64].
Numerous efforts were attempted to upgrade the drug delivery process for the therapy of diseases of the posterior part of the eye. Nanoimplants and hydrogels are a good way to reduce the frequency of application. Light-activated solutions prepared using polycaprolactone dimethacrylate and hydroxymethylmethacrylate were successfully produced and injected into rabbits’ eyes for the therapy of CNV. After fast cross-linking activated by light, solution could produce an in situ hydrogel for prolonged bevacizumab release for 60 days. However, this system is limited due to its toxicity. Natarajan and associates developed latanoprost-filled unilamellar nanovesicles (size of 54–100 nm), which could reduce intraocular pressure up to 20% and achieve prolonged drug release for 120 days, via a single subconjunctival injection. Controlled and sustained release as well as high tolerability, stability and safety of latanoprost were achieved. The results of these studies encouraged the development of similar systems for the treatment of glaucoma [65].
6 Application of nanoparticles in the treatment of specific eye diseases
6.1 Glaucoma
Glaucoma is a disease that leads to vision loss and even blindness. New drugs are expected to improve patients’ adherence and achieve higher bioavailability without creating any toxic effects, compared to eye drops. The main goal in the treatment of glaucoma is to reduce intraocular pressure and to maintain it for several months, but also to protect patients’ sight [66].
Nanoparticles that pass through the mucus, after the application on the surface of the eye, can improve drug efficacy in the treatment of glaucoma. PAMAM/PLGA nanosystem was developed for delivering brimonidine tartrate and timolol maleate. Nanoparticle size was around 258 nm with polydispersity index of 0,113–0,383 and zeta potential charge of 28,8 mV. It was proven to be non-toxic and not to induce inflammation. Prolonged retention time, increased drug bioavailability and controlled release were achieved, with both drugs slowly releasing for five weeks. Compared to eye drops, reduction of the intraocular pressure was maintained for four days and was higher 18%, using rabbits as animal models. Concentrations of both drugs were higher in the aqueous liquid (7,8%) and cornea (1,4%) [67].
Brimonidine tartrate-loaded chitosan nanoparticles, prepared with the sodium tripolyphosphate addition, may help decrease the dosing frequency by achieving postponed drug release in the treatment of glaucoma. The weight ratio of chitosan and sodium tripolyphosphate (4:1, 5:1, 6:1) was critical when discussing nanoparticle size (370,26 nm, 300,7 nm, 276 nm, respectively), polydispersity index (0,396, 0,383, 0,377, respectively), zeta potential charge (26,2 mV, 28,5 mV, 29,8 mV, respectively) and encapsulation efficacy (48,51%, 41,29%, 37,08%, respectively). Initial drug release lasted 45 min with 45% of a drug liberated, which was followed by a sustained release for four hours. This formulation was shown to be non-irritant and safe, according to the results of the Draize test. Intraocular pressure reduction was concluded to be a result of the interaction of chitosan with mucin and consequently the formation of a mucoadhesive coating, which helped prolong the retention time and achieve sustained drug release [68].
Methazolamide, which binds to calcium phosphate by adsorption to its inorganic core, extended the duration of reduced intraocular pressure (6 h for eye drops, 18 h for nanoparticles) and this may be useful in the local treatment of glaucoma. Rabbits were used as animal models. Nanoparticle size was 256,4 ± 31,1 nm with zeta potential charge of −30,4 ± 2,2 mV. Controlled drug release was achieved with 60% of a drug liberated in the first 30 min and 99,4% in the next four hours. The authors concluded that these nanoparticles depended on passive diffusion to go through the corneal eye barrier. Having extended retention time on the surface of the cornea would also increase methazolamide bioavailability. These systems were also very stable for six months [69].
The use of hyaluronic acid-modified chitosan nanoparticles filled with timolol maleate and dorzolamide hydrochloride can be promising for the treatment of glaucoma. Hyaluronic acid combined with chitosan, which is a polycationic polymer, improved mucoadhesive properties and provided controlled ocular drug delivery. Nanoparticle size was 319,5–767,7 nm with zeta potential charge of 15,4–33,3 mV and encapsulation efficacy for dorzolamide hydrochloride of 83,7–86,7% and for timolol maleate of 8,3–19,3%, based on the molar ratio of chitosan and hyaluronic acid. Release studies showed that 45–50% of a drug was liberated in the first hour, continuing with its slow, sustained release. The ocular irritation test in rabbits showed that these formulations were non-irritant. The reduction of intraocular pressure was greater (difference of 4–5 mmHg) compared to commercially available solution [70].
Dorzolamide hydrochloride- and methazolamide-loaded SLNs in the form of nanoemulsions reduced the dosing frequency and enhanced the patients’ adherence, in comparison to eye drops. Brimonidine tartrate-Eudragit nanoparticles were proved to prolong drug release in vitro and effectiveness of in vivo reduction of intraocular pressure. This could be used for open-angle glaucoma therapy. Brimonidine tartrate-nanoacids dissolved in the carbopolymer solution could decrease the frequency of dosing, thereby boost patients’ adherence. SLNs with their surface modified with phospholipids could be an effective approach of enhancing the bioavailability of timolol maleate in the eye. Acetazolamide-filled cationic nanoemulsions were proved to be a better option for the therapy of glaucoma in comparison to neutral-charged and anionic nanoemulsions. Nanoemulsions of dorzolamide hydrochloride exhibited substantial physical and chemical characteristics and were non-irritant. Nanoemulsions could be a very good way to treat glaucoma [71].
6.2 Corneal diseases
SLNs developed with Compritol and stearic acid were confirmed to be a great ocular drug delivery system, taking into account smaller nanoparticle size, nanoparticle stability and physiologically tolerable components. Flurbiprofen-incorporated PLGA nanospheres improved drug concentration in the eye. Cyclosporine-A-filled or fluorescein-labeled nanoparticles consisting of Miglyol 840 and Precirol ATO 5 as liquid lipids were also developed. Indomethacin-chitosan nanoemulsions showed better healing of the corneal chemical ulcers in the internal ocular tissue, thus increasing the efficacy of drug delivery. Fluorescent nanoparticles with a positive charge displayed excellent penetration and permeation capability in the internal ocular tissue. Baicalin-SLN could carry larger drug amounts and reduce eye irritation. High baicalin concentration was detected in the aqueous liquid of the eye. Polymeric suspensions of nanoparticles filled with diclofenac sodium showed prolonged release and non-irritating effects on the cornea and the iris and therefore could be a suitable drug carrier. PLGA-coated-PEG nanospheres which incorporated flurbiprofen penetrated through the corneal epithelium via the transcellular pathways. Fluorescent (fluoresceinamine) hyaluronic acid/chitosan nanoparticles were resuspended in nanobuffer and then also proved to be biocompatible [71].
6.3 Corneal neovascularization
Corneal neovascularization is a condition that compromises vision and is linked to eye surface conditions. Plasmids delivered by nanoparticles (size of 270,2 nm) that exhibit Flt23k (anti-vascular endothelial-growth factor (VEGF) intraceptor, that blocks VEGF and reduces leukocyte infiltration and macrophage migration), applied via subconjunctival injection in mice as animal models, had significant effects on the reduction of corneal neovascularization and lymphangiogenesis, especially when they were taken with steroids (triamcinolone). The graft survival rate in two months was about 20% without steroids and 91,6% with steroids [72].
Nanoparticles with a core-frame system filled with VEGF were effective when it came to the new blood vessel formation and were used in the studies regarding anti-inflammatory effects and antiangiogenesis. Celastrol-filled PEG-block-polycaprolactone polymeric nanomicelles (size of 48 nm) were planned to boost the solubility of celastrol and significantly inhibit the induction of neovascularization of the cornea. They evinced drug release in two phases: initial fast phase followed by a slow and prolonged one. These nanosystems reduced the area of corneal neovascularization by inhibiting leukocyte infiltration, macrophage migration and VEGF expression in rats, from 7,71 mm2 to 2,29 mm2 [73].
6.4 Autoimmune uveitis
Autoimmune uveitis is a condition that attacks the posterior eye parts. A more effective drug delivery way was used in the treatment of autoimmune uveitis but with less adverse reactions. Nanoparticles allow nonsteroidal anti-inflammatory drugs (e.g. indomethacin) to reach the internal ocular structures, via the transmucosal pathways [74].
Therapeutic effects of betamethasone phosphate encapsulated with biodegradable PEG-block-PLA copolymers and PLA homopolymers (size of 120 nm) in rats in which autoimmune uveoretinitis was induced by S-antigen peptide were the reduction of clinical outcomes in one day with maintenance for two weeks (40% reduction of inflammation) and the decrease of inflammatory cytokine levels in the retina. Betamethasone phosphate was concentrated in rats’ retina from 24 to 72 h. This treatment prolonged blood circulating while also providing delayed betamethasone phosphate release [75].
Nanoparticles prepared by sialyl-Lewis X conjugated liposomes (size of 103 nm) in the targeted drug delivery systems using mice as animal models showed selective targeting of autoimmune uveoretinitis. Accumulation took place in five minutes and was inhibited by the anti-E-selectin antibody. This could lead to new therapeutic strategies in the treatment of intraocular inflammation, because these systems showed two times the increase of dexamethasone concentration in the retina (6,67 ± 0,32 ng/mg) [76].
6.5 Age-related macular degeneration
PEGylated liposome-protamine-hyaluronic acid nanoparticles filled with siRNA will be a promising type of treatment for AMD. Cerium oxide in intravitreal injection inhibited the appearance of reactive oxygen species and the production of neovascular lesions in intraretinal and subretinal layers when incorporated into nanoparticles. This could be used as a potential treatment of neurodegenerative diseases such as AMD and diabetic retinopathy [71].
Mice model was used in the research of the ceramide part in the retinal deterioration. This is a proapoptotic sphingolipid, therefore the possibility of reducing the photoreceptor loss by interrupting its signaling pathways was also an important dilemma. While the photoreceptor loss was at its maximum, ceramide concentration in the retina enhanced. Intraocular injection of SLNs filled with myriocin stabilized ceramide concentration in the retina and hence prevented further photoreceptor loss. This kind of prolonged treatment delayed the progress of retinitis pigmentosa. SLNs could facilitate the continuous, non-invasive retinitis pigmentosa therapy, especially with hydrophobic drugs and allow a higher survival rate of photoreceptors, preserve their morphology and the retina’s ability to respond to light [77].
6.6 Choroidal neovascularization
Choroidal neovascularization originating from AMD could be treated by injecting nanoparticles of biodegradable polymers. Intravitreal injection of basic fibroblast-growth factor-impregnated nanoparticles prevented the degeneration of photoreceptors by inhibiting apoptosis in the rats’ retina. Intravitreal injections were suitable for the treatment of CNV [71].
Tabular overview of different treatment applications of nanoparticles with incorporated substances and related sources is presented in Table 2.
7 Conclusion
In the past few decades, nanoparticles have received considerable attention in the research of the drug distribution pathways and scientists have sought to prepare drug-filled nanoparticles for the transfer to anterior and posterior parts of the eye. Conventional drug delivery formulations have proven their efficacy in the therapy of anterior eye part diseases, but are less powerful in the therapy of posterior eye part diseases, even after frequent dosing. Therefore, nanoparticles represent candidates for ocular use, because they do not irritate the eye due to their small size. Avoiding frequent use of drugs can also be achieved this way, because of the prolonged release of a drug from nanoparticles. Nanoparticles have also been successfully researched for drug delivery to the posterior eye segment, and that is a very significant discovery. Application of nanoparticles in the ocular delivery of drugs allows overcoming existing barriers when it comes to formulating dosage forms, which then allows prolonged retention of a drug in the eye with the use of smaller doses and provides better drug effects and greater patients’ compliance. Nanoparticles can be used in the therapy of different eye diseases and, with adequate formulations, their permeability characteristics for targeted delivery of drugs to the posterior parts of the eye can be exploited. Studies that have been carried out so far relate to the use of nanoparticles for the drug delivery to the eye on in vivo and in vitro models of rabbits and mice and further studies are needed to demonstrate the possibility of human application.
Some of the most recent research carried out regarding the application of nanoparticles in ocular drug delivery systems are discussed in the following content.
Nanoscale-dispersed eye ointments (NDEO) were developed for dry eye condition treatment, using mice models. A mixture of lipid liquids (medium-chain triglycerides; MCT) and semi-solid lipids (lanolin or petrolatum) was dispersed in the polyvinyl pyrrolidone solution. MCT nanoemulsion was formed with that lipid mixture captured in it. The particle size of 100 nm allowed that lipid mixture to stick to and stay inside the conjunctiva. The advantages of this formulation are better stability when stored at 4 °C, no cytotoxicity signs to the RPE, a positive correlation with a higher concentration of lipid mixture and moisturizing and lubricating effects, when compared to artificial polymer-based tears. This nanosystem was proved to be more effective for the ocular delivery of lipophilic drugs, especially because the occurrence of eyelid adhesion and blurred vision were reduced [78].
Cubosomes were developed as liquid nanocrystalline structures, consisting of the amphiphilic lipids with the carbon chain. Compared to liposomes, they had higher physicochemical stability, which was caused by electric repulsions. Their microstructure was similar to the structure of the cell membrane, which provided enhanced drug permeation, while their loading-ability was attributed to the high-surface lipid bilayer. They were investigated in the treatment of glaucoma, using rabbit models. They were made up of pilocarpine nitrate with Poloxamer 407 and glycerol monoolein. Compared to eye drops, cubosomes demonstrated higher transcorneal flux (about two times), prolonged retention time (about two times) and a more effective reduction of intraocular pressure (about 50%). Cubosomes were also made up of timolol maleate with Poloxamer 407 and glycerol monoolein, also with rabbit models. They demonstrated higher permeation through the cornea (about 3,5 times), prolonged retention time (about two times) and a more effective reduction of intraocular pressure (about 36%) [79,80,81].
Gene therapy is a promising alternative for the treatment of different eye conditions. Chitosan-DNA nanoparticles had the appropriate size (98,2 ± 4,4 nm) and positive zeta potential charge (44,1 ± 3,5 mV), therefore they were adequate to be used in this type of treatment [82]. Oligodeoxynucleotides could be incorporated into PLGA nanoparticles and transported to the cornea due to the polymer erosion. Hybrid nanoparticles composed of cationic gelatin and polyanions, such as chondroitin sulphate and dextran sulphate, reduced the in vitro toxicity, providing both safety and effectiveness [83]. Nanosystems could also be formed as hyaluronic acid-chitosan nanoparticles as gene therapy carriers [84].
Leucaena leucocephala galactomannan (LLG) was used in the development of polymeric nanoparticles (size of 199,7 ± 9,84 nm, polydispersity index of 0,342, zeta potential charge of −26,5 mV and encapsulation efficacy of 75,5 ± 3,74%) of bioadhesive properties filled with dorzolamide hydrochloride. Amine and carboxymethyl derivatives of LLG were used for their preparation. As a result, transcorneal permeation studies and release studies displayed higher permeation through the cornea, compared to the conventional formulation (87,88% and 65,54%, respectively). These nanoparticles reduced the intraocular pressure for a longer period than conventional formulation (23,97% and 22,61%, respectively) [85].
Redox autoregenerative nanoparticles coated with PEG-PLGA were also developed. An animal model used in this study was rats with induced diabetic cataracts. These nanoparticles were applied by subconjunctival injection. As an antioxidant, they can protect epithelial cells from oxidative stress, because they can eliminate reactive oxygen species. They can also suppress α-crystallin glycation, because of their role as a glycation inhibitor. The lens is therefore kept transparent. In conclusion, these nanosystems could be a great alternative for the treatment of diabetic retinopathy and diabetic cataracts [86].
Prednisolone acetate was incorporated into the nanoparticles-loaded gel. This system was prepared by the method of complexation of sodium deoxycholate and chitosan (5:1), loaded with this poorly water-soluble drug. An animal model used in this study was guinea pigs. When the molar ratio of chitosan and sodium deoxycholate was lower, nanoparticle size decreased from 976 ± 43 nm to 480 ± 28 nm and polydispersity index increased from 1285 to 1396. When polyvinyl alcohol was included in the preparation, smaller nanoparticles were formed: 462 ± 19 nm with polydispersity index of 0,942 and 321 ± 22 nm with polydispersity index of 0,454, for the preparation of this nanosystem without and with polyvinyl alcohol, respectively. The interaction between sodium deoxycholate and chitosan was detected, but not between these components and prednisolone. Drug release had biphasic characteristics, with an average of 98,6% of a drug released in 24 h [87].
Surfactant-based nanovesicles were incorporated into mucoadhesive nanogel for the ocular delivery of acetazolamide. The molar ratio of sorbitan monostearate and sodium deoxycholate was 80:20, with the particle size of 202,9 nm, zeta potential charge of −38,1 mV and encapsulation efficacy of 90,2%. Nanogel composed of 1,5% chitosan showed optimal rheological behavior, adhesion time and viscosity (thixotropic characteristics, 290 min and 118,246 cP, respectively). Drug release was controlled, with 12% of acetazolamide released in the first hour, 52,9% in the following five hours with the cumulative release of 64,9%. After six hours, 38,3% of a drug was released from the nanogel. In comparison to acetazolamide suspension, the results of the ocular irritation test showed minimal irritation. Nanogel also prolonged the effect of the reduced intraocular pressure, compared to acetazolamide tablets [88].
Lutein ester is a poorly water-soluble carotenoid fatty acid ester. It is used in the prevention and treatment of AMD. Lutein ester nanoparticles were prepared to enhance its bioavailability and ocular delivery. Deionized water was used as an antisolvent, Poloxamer 188 as a surfactant and tetrahydrofuran as a solvent. The size of nanoparticles containing lutein ester was around 164,1 ± 4,3 nm. The dissolution rate and the solubility of lutein ester in these nanoparticles were 9,65 and 12,75 times higher compared to the raw ester, respectively. Its bioavailability increased 1,41 times when incorporated into nanoparticles compared to the raw ester, as well as the antioxidant capacity and scavenging performance (3,31 times higher). When rats were used as an animal model, lutein concentration was higher in rats treated with lutein ester nanoparticles compared to its concentration in rats treated with the raw ester, with the maximum increase of 2,34 times [89].
All of the above-mentioned recent studies showed promising results, but further research has to be carried out to realize how to prevail all of the risk factors of the use of nanoparticles in ocular drug delivery, but also how to make the most of all of their assets.
References
Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58(11):1131–5.
Lee VH, Robinson JR. Topical ocular drug delivery: recent developments and future challenges. J Ocul Pharmacol Ther. 1986;2(1):67–108.
Kaur IP, Kanwar M. Ocular preparations: the formulation approach. Drug Dev Ind Pharm. 2002;28(5):473–93.
Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: an overview. World J Pharmacol. 2013;2(2):47–64.
Runkle EA, Antonetti DA. The blood-retinal barrier: structure and functional significance. Methods Mol Biol. 2011;686:133–48.
Mannermaa E, Vellonen KS, Urtti A. Drug transport in corneal epithelium and blood-retina barrier: emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev. 2006;58(11):1136–63.
Vandamme TF. Microemulsions as ocular drug delivery systems: recent developments and future challenges. Prog Retin Eye Res. 2002;21(1):15–34.
Baranowski P, Karolewicz B, Gajda M, Pluta J. Ophthalmic drug dosage forms: characterisation and research methods. Sci World J. 2014;2014(7):861–904.
Reimondez-Troitiño S, Csaba N, Alonso MJ, De La Fuente M. Nanotherapies for the treatment of ocular diseases. Eur J Pharm Biopharm. 2015;95(1):279–93.
Cholkar K, Patel A, Vadlapudi DA, Mitra AK. Novel nanomicellar formulation approaches for anterior and posterior segment ocular drug delivery. Recent Pat Nanomed. 2012;2(2):82–95.
Herrero-Vanrell R, De La Torre MV, Andres-Guerrero V, Barbosa-Alfaro D, Molina-Martinez IT, Bravo-Osuna I. Nano- and microtechnologies for ophthalmic administration, an overview. J Drug Deliv Sci Tec. 2013;23(2):75–102.
Bu HZ, Gukasyan HJ, Goulet L, Lou XJ, Xiang C, Koudriakova T. Ocular disposition, pharmacokinetics, efficacy and safety of nanoparticle-formulated ophthalmic drugs. Curr Drug Metab. 2007;8(2):91–107.
Civiale C, Licciardi M, Cavallaro G, Giammona G, Mazzone MG. Polyhydroxyethylaspartamide-based micelles for ocular drug delivery. Int J Pharm. 2009;378(1–2):177–86.
Patravale VB, Date AA, Kulkarni RM. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol. 2004;56(7):827–40.
Kassem MA, Rahman AA, Ghorab MM, Ahmed MB, Khalil RM. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int J Pharm. 2007;340(1–2):126–33.
Vandamme TF, Brobeck L. Poly (amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J Control Release. 2005;102(1):23–38.
Yavuz B, Bozdag-Pehlivan S, Unlu N. Dendrimeric systems and their applications in ocular drug delivery. Sci World J. 2013;2013(7):732–40.
Müller RH, Junghanns JU. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomedicine. 2008;3(3):295–310.
Sahoo SK, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today. 2008;13(3–4):144–51.
Paolicelli P, Prego C, Sánchez A, Alonso MJ. Surface-modified PLGA-based nanoparticles that can efficiently associate and deliver virus-like particles. Nanomedicine. 2010;5(6):843–53.
Kondiah P, Choonara Y, Kondiah P, Marimuthu T, Kumar P, du Toit L, et al. Nanocomposites for therapeutic application in multiple sclerosis. In: Inamuddin I, Asiri A, Mohammad A, editors. Applications of nanocomposite materials in drug delivery. 1st ed. Amsterdam: Elsevier; 2018. p. 391–408.
Pignatello R, Bucolo C, Spedalieri G, Maltese A, Puglisi G. Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials. 2002;23(15):3247–55.
Giannavola C, Bucolo C, Maltese A, Paolino D, Vandelli MA, Puglisi G, et al. Influence of preparation conditions on acyclovir-loaded nanospheres. Pharm Res. 2003;20(4):584–90.
Hühn D, Kantner K, Geidel C, Brandholt S, de Cock I, Soenen S, et al. Polymer-coated nanoparticles interacting with proteins and cells: focusing on the sign of the net charge. ACS Nano. 2013;7(4):3253–63.
Tatur S, Maccarini M, Barker R, Nelson A, Fragneto G. Effect of functionalized gold nanoparticles on floating lipid bilayers. Langmuir. 2013;29(22):6606–14.
Pimienta C, Lenaerts V, Cadieux C, Raymond P, Juhasz J, Simard MA, et al. Mucoadhesion of hydroxypropylmethacrylate nanoparticles to rat intestinal ileal segments in vitro. Pharm Res. 1990;7(1):49–53.
Amrite AC, Kompella UB. Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J Pharm Pharmacol. 2005;57(12):1555–63.
Zhang L, Li Y, Zhang C, Wang Y, Song C. Pharmacokinetics and tolerance study of intravitreal injection of dexamethasone-loaded nanoparticles in rabbits. Int J Nanomedicine. 2009;4(1):175–83.
Koo H, Moon H, Han H, Na JH, Huh MS, Park JH, et al. The movement of self-assembled amphiphilic polymeric nanoparticles in the vitreous and retina after intravitreal injection. Biomaterials. 2012;33(12):3485–93.
Kim H, Robinson SB, Csaky KG. Investigating the movement of intravitreal human serum albumin nanoparticles in the vitreous and retina. Pharm Res. 2009;26(2):329–37.
Muhamad I, Selvakumaran S, Lazim N. Designing polymeric nanoparticles for targeted drug delivery system. In: Seifalian A, de Mel A, Kalaskar D, editors. Nanomedicine. Manchester: One Central Press; 2014. p. 287–313.
Souto EB, Doktorovova S, Gonzalez-Mira E, Egea MA, Garcia ML. Feasibility of lipid nanoparticles for ocular delivery of anti-inflammatory drugs. Curr Eye Res. 2010;35(7):537–52.
Almeida H, Amaral MH, Lobão P, Silva AC, Loboa JMS. Applications of polymeric and lipid nanoparticles in ophthalmic pharmaceutical formulations: present and future considerations. Journal of Pharmacy & Pharmaceutical Sciences. 2014;17(3):278–93.
Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54(S1):131–55.
Üner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomedicine. 2007;2(3):289–300.
Hirlekar R, Garse H, Kadam V. Solid lipid nanoparticles and nanostructured lipid carriers: a review. Current Drug Therapy. 2011;6(4):240–50.
Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Advanced Pharmaceutical Bulletin. 2015;5(3):305–13.
Okur NÜ, Gökçe EH. Lipid nanoparticles for ocular drug delivery. International Journal of Ophthalmic Research. 2015;1(3):77–82.
Kao HJ, Lo YL, Lin HR, Yu SP. Characterization of pilocarpine-loaded chitosan/carbopol nanoparticles. J Pharm Pharmacol. 2006;58(2):179–86.
Yuan X, Yuan Y, Jiang W, Liu J, Tian E, Shun H, et al. Preparation of rapamycin-loaded chitosan/PLA nanoparticles for immunosuppression in corneal transplantation. Int J Pharm. 2008;349(1–2):241–8.
Motwani SK, Chopra S, Talegaonkar S, Kohli K, Ahmad FJ, Khar RK. Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: formulation, optimisation and in vitro characterisation. Eur J Pharm Biopharm. 2008;68(3):513–25.
Ibrahim H, El-Leithy I, Makky A. Mucoadhesive nanoparticles as carrier systems for prolonged ocular delivery of gatifloxacin/prednisolone bitherapy. Mol Pharm. 2010;7(2):576–85.
Mahmoud A, El-Feky G, Kamel R, Awad G. Chitosan/sulphobutylether-β-cyclodextrin nanoparticles as a potential approach for ocular drug delivery. Int J Pharm. 2011;413(1–2):229–36.
Nagarwal RC, Kumar R, Pandit JK. Chitosan-coated sodium alginate-chitosan nanoparticles loaded with 5-FU for ocular delivery: in vitro characterization and in vivo study in rabbit eye. Eur J Pharm Sci. 2012;47(4):678–85.
Bhatta RS, Chandasana H, Chhonker YS, Rathi C, Kumar D, Mitra K, et al. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: in vitro and pharmacokinetics studies. Int J Pharm. 2012;432(1–2):105–12.
Gupta H, Aqil M, Khar R, Ali A, Bhatnagar A, Mittal G. Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomedicine. 2010;6(2):324–33.
Gupta H, Aqil M, Khar R, Ali A, Bhatnagar A, Mittal G. Nanoparticles laden in situ gel for sustained ocular drug delivery. Journal of Pharmacy and Bioallied Sciences. 2013;5(2):162–5.
Gupta H, Aqil M, Khar R, Ali A, Bhatnagar A, Mittal G. Nanoparticles laden in situ gel of levofloxacin for enhanced ocular retention. Drug Delivery. 2013;20(7):306–9.
Cavalli R, Gasco MR, Chetoni P, Burgalassi S, Saettone MF. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int J Pharm. 2002;238(1–2):241–5.
Attama AA, Reichl S, Muller-Goymann CC. Diclofenac sodium delivery to the eye: in vitro evaluation of novel solid lipid nanoparticle formulation using human cornea construct. Int J Pharm. 2008;355(1–2):307–13.
Gökçe EH, Sandri G, Bonferoni MC, Rossi S, Ferrai F, Güneri T, et al. Cyclosporine-A-loaded SLNs: evaluation of cellular uptake and corneal cytotoxicity. Int J Pharm. 2008;364(1):76–86.
Araújo J, Gonzalez-Mira E, Egea MA, Garcia ML, Souto EB. Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int J Pharm. 2010;393(1–2):167–75.
Liu Z, Zhang X, Wu H, Li J, Shu L, Liu R, et al. Preparation and evaluation of solid lipid nanoparticles of baicalin for ocular drug delivery system in vitro and in vivo. Drug Dev Ind Pharm. 2011;37(4):475–81.
Gonzalez-Mira E, Egea MA, Souto EB, Calpena AC, Garcia ML. Optimizing flurbiprofen-loaded NLC by central composite factorial design for ocular delivery. Nanotechnology. 2011;22(4):45–57.
Hao J, Fang X, Zhou Y, Wang J, Guo F, Li F, et al. Development and optimization of solid lipid nanoparticle formulation for ophthalmic delivery of chloramphenicol using a box-Behnken design. Int J Nanomedicine. 2011;6(1):683–92.
Liu R, Liu Z, Zhang C, Zhang B. Nanostructured lipid carriers as novel ophthalmic delivery system for mangiferin: improving in vivo ocular bioavailability. J Pharm Sci. 2012;101(10):3833–44.
Hippalgaonkar K, Adelli GR, Hippalgaonkar K, Repka MA, Majumdar S. Indomethacin-loaded solid lipid nanoparticles for ocular delivery: development, characterization, and in vitro evaluation. J Ocul Pharmacol Ther. 2013;29(2):216–28.
Zhang W, Li X, Ye T, Chen F, Yu S, Chen J, et al. Nanostructured lipid carrier surface modified with Eudragit RS 100 and its potential ophthalmic functions. Int J Nanomedicine. 2014;9(1):4305–15.
Üstundag-Okur N, Gökçe EH, Bozbıyık DI, Egrilmez S, Ozer O, Ertan G. Preparation and in vitro-in vivo evaluation of ofloxacin-loaded ophthalmic nanostructured lipid carriers modified with chitosan oligosaccharide lactate for the treatment of bacterial keratitis. Eur J Pharm Sci. 2014;63(1):204–15.
Üstundag-Okur N, Gökçe EH, Bozbıyık DI, Egrilmez S, Ertan G, Ozer O. Novel nanostructured lipid carrier-based inserts for controlled ocular drug delivery: evaluation of corneal bioavailability and treatment efficacy in bacterial keratitis. Expert Opinion on Drug Delivery. 2015;12(11):1791–807.
Vega E, Egea MA, Valls O, Espina M, Garcia ML. Flurbiprofen-loaded biodegradable nanoparticles for ophthalmic administration. J Pharm Sci. 2006;95(11):2393–405.
Zhang Z, He Z, Liang R, Ma Y, Huang W, Jiang R, et al. Fabrication of a micellar supramolecular hydrogel for ocular drug delivery. Biomacromolecules. 2016;17(3):798–807.
Mun EA, Morrison PW, Williams AC, Khutoryanskiy VV. On the barrier properties of the cornea: a microscopy study of the penetration of fluorescently labeled nanoparticles, polymers, and sodium fluorescein. Mol Pharm. 2014;11(10):3556–64.
Chew EY, Glassman AR, Beck RW, Bressler NM, Fish GE, Ferris FL. Ocular side effects associated with peribulbar injections of triamcinolone acetonide for diabetic macular edema. Retina. 2011;31(1):284–9.
Natarajan JV, Darwitan A, Barathi VA, Ang M, Htoon HM, Boey F, et al. Sustained drug release in nanomedicine: a long-acting nanocarrier-based formulation for glaucoma. ACS Nano. 2014;8(1):419–29.
Lavik E, Kuehn MH, Kwon YH. Novel drug delivery systems for glaucoma. Eye. 2011;25(5):578–86.
Yang H, Tyagi P, Kadam RS, Holden CA, Kompella UB. Hybrid dendrimer hydrogel/PLGA nanoparticle platform sustains drug delivery for one week and antiglaucoma effects for four days following one-time topical administration. ACS Nano. 2012;6(9):7595–606.
Singh KH, Shinde UA. Chitosan nanoparticles for controlled delivery of brimonidine tartrate to the ocular membrane. Die Pharmazie. 2011;66(8):594–9.
Chen R, Qian Y, Li R, Zhang Q, Liu D, Wang M, et al. Methazolamide calcium phosphate nanoparticles in an ocular delivery system. Yakugaku Zasshi. 2010;130(3):419–24.
Wadhwa S, Paliwal R, Paliwal SR, Vyas SP. Hyaluronic acid-modified chitosan nanoparticles for effective management of glaucoma: development, characterization, and evaluation. J Drug Target. 2010;18(4):292–302.
Zhou HY, Hao JL, Wang S, Zheng Y, Zhang WS. Nanoparticles in the ocular drug delivery. International Journal of Ophthalmology. 2013;6(3):390–6.
Cho YK, Uehara H, Young JR, Tyagi P, Kompella UB, Zhang X, et al. Flt23k nanoparticles offer additive benefit in graft survival and anti-angiogenic effects when combined with triamcinolone. Investig Ophthalmol Vis Sci. 2012;53(4):2328–36.
Li Z, Yao L, Li J, Zhang W, Wu X, Liu Y, et al. Celastrol nanoparticles inhibit corneal neovascularization induced by suturing in rats. Int J Nanomedicine. 2012;7(1):1163–73.
Gomes-Bittencourt M, Sepah YJ, Do DV, Agbedia O, Akhtar A, Liu H, et al. New treatment options for noninfectious uveitis. Dev Ophthalmol. 2012;51(1):134–61.
Sakai T, Ishihara T, Higaki M, Akiyama G, Tsuneoka H. Therapeutic effect of stealth-type polymeric nanoparticles with encapsulated betamethasone phosphate on experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2011;52(3):1516–21.
Hashida N, Ohguro N, Yamazaki N, Arakawa Y, Oiki E, Mashimo H, et al. High-efficacy site-directed drug delivery system using sialyl-Lewis X conjugated liposome. Exp Eye Res. 2008;86(1):138–49.
Strettoi E, Gargini C, Novelli E, Sala G, Piano I, Gasco P, et al. Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. Proc Natl Acad Sci. 2010;107(43):18706–11.
Zhang W, Wang Y, Lee B, Liu C, Wei G, Lu W. A novel nanoscale-dispersed eye ointment for the treatment of dry eye disease. Nanotechnology. 2014;25(12):125101.
Huang J, Peng T, Li Y, Zhan Z, Zeng Y, Huang Y, et al. Ocular cubosome drug delivery system for timolol maleate:pPreparation, characterization, cytotoxicity, ex vivo, and in vivo evaluation. AAPS PharmSciTech. 2017;18(8):2919–26.
Karami Z, Hamidi M. Cubosomes: remarkable drug delivery potential. Drug Discov Today. 2016;21(5):789–801.
Li J, Wu L, Wu W, Wang B, Wang Z, Xin H, et al. A potential carrier based on liquid crystal nanoparticles for ophthalmic delivery of pilocarpine nitrate. Int J Pharm. 2013;455(1–2):75–84.
Klausner EA, Zhang Z, Chapman RL, Multack RF, Volin MV. Ultrapure chitosan oligomers as carriers for corneal gene transfer. Biomaterials. 2010;31(7):1814–20.
Konat Zorzi G, Contreras-Ruiz L, Párraga JE, López-García A, Romero Bello R, Diebold Y, et al. Expression of MUC5AC in ocular surface epithelial cells using cationized gelatin nanoparticles. Mol Pharm. 2011;8(5):1783–8.
De La Fuente M, Seijo B, Alonso MJ. Novel hyaluronic acid-chitosan nanoparticles for ocular gene therapy. Investig Ophthalmol Vis Sci. 2008;49(5):2016–24.
Mittal N, Kaur G. Leucaena leucocephala (Lam.) galactomannan nanoparticles: optimization and characterization for ocular delivery in glaucoma treatment. Int J Biol Macromol. 2019;139(1):1252–62.
Zhou Y, Li L, Li S, Li S, Zhao M, Zhou Q, et al. Autoregenerative redox nanoparticles as an antioxidant and glycation inhibitor for palliation of diabetic cataracts. Nanoscale. 2019;11(27):13126–38.
Hanafy AF, Abdalla AM, Guda TK, Gabr KE, Royall PG, Alqurshi A. Ocular anti-inflammatory activity of prednisolone acetate loaded chitosan-deoxycholate self-assembled nanoparticles. Int J Nanomedicine. 2019;14(1):3679–89.
Abdel-Rashid RS, Helal DA, Omar MM, El Sisi AM. Nanogel loaded with surfactant based nanovesicles for enhanced ocular delivery of acetazolamide. Int J Nanomedicine. 2019;14(1):2973–83.
Wu M, Feng Z, Deng Y, Zhong C, Liu Y, Liu J, et al. Liquid antisolvent precipitation: an effective method for ocular targeting of lutein esters. Int J Nanomedicine. 2019;14(1):2667–81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Omerović, N., Vranić, E. Application of nanoparticles in ocular drug delivery systems. Health Technol. 10, 61–78 (2020). https://doi.org/10.1007/s12553-019-00381-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12553-019-00381-w