Abstract
Cys-loop receptors integrate a large family of pentameric ligand-gated ion channels that mediate fast ionotropic responses in vertebrates and invertebrates. Their vital role in converting neurotransmitter recognition into an electrical impulse makes these receptors essential for a great variety of physiological processes. In vertebrates, the Cys-loop receptor family includes the cation-selective channels, nicotinic acetylcholine and 5-hydroxytryptamine type 3 receptors, and the anion-selective channels, GABAA and glycine receptors, whereas in invertebrates, the repertoire is significantly larger. The free-living nematode Caenorhabditis elegans has the largest known Cys-loop receptor family as well as unique receptors that are absent in vertebrates and constitute attractive targets for anthelmintic drugs. Given the large number and variety of Cys-loop receptor subunits and the multiple possible ways of subunit assembly, C. elegans offers a large diversity of receptors although only a limited number of them have been characterized to date. C. elegans has emerged as a powerful model for the study of the nervous system and human diseases as well as a model for antiparasitic drug discovery. This nematode has also shown promise in the pharmaceutical industry search for new therapeutic compounds. C. elegans is therefore a powerful model organism to explore the biology and pharmacology of Cys-loop receptors and their potential as targets for novel therapeutic interventions. In this review, we provide a comprehensive overview of what is known about the function of C. elegans Cys-loop receptors from an electrophysiological perspective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cys-loop receptors integrate a wide family of pentameric ligand-gated ion channels (pLGIC) that mediate fast ionotropic responses in vertebrates and invertebrates. Their vital role in converting chemical recognition into an electrical impulse makes these receptors essential for physiological processes, including movement, memory, cognition, and plasticity. In addition to their functions as neurotransmitter-gated ion channels, Cys-loop receptors trigger and modulate different cell signalling pathways. They are present in the central and peripheric nervous systems, and in many types of non-neuronal cells, they are associated to a broad spectrum of disorders and are pharmacological targets for clinically relevant drugs (Lynagh and Pless 2014).
They were named as Cys-loop receptors because all subunits contain a conserved pair of disulfide-bonded cysteines separated by 13 residues. The discovery of orthologs in prokaryotes, which lack the double cysteines, has extended the Cys-loop family to the superfamily of pLGIC. Subunits of pLGICs are encoded by a gene family, derived from an ancestral gene shared with the prokaryotes (Tasneem et al. 2005). In mammals, the Cys-loop family consists of about 45 genes. This family has significantly expanded in nematodes (Jones and Sattelle 2008; Dent 2010; Beech and Neveu 2015). Different evolutionary and phylogenetic analyses for pLGIC and Cys-loop receptor families have been reported (Jones and Sattelle 2004, 2008; Tasneem et al. 2005; Morud et al. 2021; Jaiteh et al. 2016; Ortells 2016).
In vertebrates, the Cys-loop receptor family includes the cation-selective channels, nicotinic acetylcholine (nAChR) and 5-hydroxytryptamine type 3 receptors (5-HT3), and the anion-selective channels, glycine and gamma-aminobutyric acid type A (GABAA) receptors. In invertebrates, the repertoire is larger since in addition to different cation-permeable nAChRs and anion-permeable GABA receptors there are glutamate-gated anion channels (Jones and Sattelle 2008). Moreover, the free-living nematode Caenorhabditis elegans and other nematodes contain Cys-loop receptors not identified in vertebrates, including anionic channels gated by acetylcholine (ACh) or biogenic amines, serotonin, tyramine, and dopamine, as well as cationic channels activated by betaine (Ringstad et al. 2009; Beech and Neveu 2015). The functional and pharmacological properties of most of these nematode receptors remain largely unknown.
Cys-loop receptor subunits can arrange to form homomeric or heteromeric receptors, leading to a broad variety of receptor subtypes with different functional and pharmacological properties, localization, and physiological roles. The identification of novel pentameric arrangements, the determination of their functional properties and stoichiometries, and the discovery of selective ligands are all subjects of great interest.
Numerous physiological processes mediated by Cys-loop receptors rely on the rapid conversion of neurotransmitter binding into an electrical signal at the cell membrane. In vertebrates, nAChRs are involved in muscle contraction and in autonomic and central nervous system functions, including memory, attention, and cognition; 5-HT3 receptors regulate gut motility, secretion, emetic reflux, and visceral perception and play a modulatory role in synaptic signaling in brain; GABAA and glycine receptors constitute the primary inhibitory receptors in brain and spinal and brain cord, respectively (Changeux and Taly 2008; Gibbs and Chakrapani 2021; Lynagh and Pless 2014; Sigel and Steinmann 2012). In invertebrates, Cys-loop receptors also mediate key physiological processes, including movement, feeding, olfaction, and learning. Because of this, they are relevant as major targets of insecticides and anthelmintics.
The widely studied model organism, C. elegans, possesses one of the greatest Cys-loop receptor families in a single organism as well as unique receptors only present in nematodes. The large number of subunits and the multiple possible ways of subunit assembly give rise to a large diversity of receptors. However, only a low proportion of these receptors has been identified and characterized. In this review, we provide a comprehensive overview of what is known about the molecular function of C. elegans Cys-loop receptors from an electrophysiological perspective.
Cys-loop receptors: general structure and activation
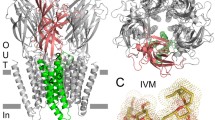
High-resolution 3D structures for several pLGICs have been solved (Hibbs and Gouaux 2011; Morales-Perez et al. 2016; Gharpure et al. 2019; Noviello et al. 2021). All models show that the five subunits are arranged pseudo-symmetrically around a central axis forming a receptor with defined structural modules: (i) the N-terminal extracellular domain (ECD), which carries the orthosteric binding sites; (ii) the transmembrane domain (TMD), composed of four α-helices from each subunit (M1-M4), which forms the ion pore; and (iii) the large intracellular domain (ICD), whose structure has been partially resolved (Fig. 1a). Between the ECD and the TMD, there is a structural transition zone, named as coupling region, essential for the functional link between agonist binding and channel opening (Bouzat et al. 2004). The ICD, comprising the region between M3 and M4, is the most variable region among Cys-loop receptors and plays a critical role in conductance, modulation, and interactions with intracellular proteins for anchoring and downstream signaling pathways (Bouzat and Sine 2018; Chrestia et al. 2023).
Cys-loop receptors: structure and activation. a Structural feature of a Cys-loop receptor. The model corresponds to the structure of the homopentameric GluClα from C. elegans (Protein Data Bank (PDB) 3RIF) in complex with the agonist glutamate and the allosteric agonist and modulator ivermectin (IVM) (Hibbs and Gouaux 2011). ECD, TMD, and ICD correspond to extracellular, transmembrane, and intracellular domains. Glu binds at interfaces between subunits in the ECD and IVM binds in the upper part of the TMD. For optimization of the receptor construct for crystallization, part of the ICD (Lys345-Lys402) was replaced with an Ala-Gly-Thr tripeptide. b Minimal model of Cys-loop activation mechanism. Cys-loop receptors can be found in three classes of conformational states: Closed (C), open (O), and desensitized (D)
Receptors can be found in three main classes of conformational states: closed, open, and desensitized (Fig. 1b). In the absence of agonists, the receptor is found mainly in a closed resting state. Neurotransmitters and orthosteric ligands bind to an ECD cavity at the interface of two adjacent subunits, resulting in the closure of a binding domain loop (loop C) around the agonist. This event triggers structural rearrangements at the ECD–TMD interface (coupling region) and ultimately the opening of the channel gate allowing ions, cations, or anions to flow through the channel (Hibbs and Gouaux 2011; Noviello et al. 2021). This chemoelectrical signaling mechanism underlies most rapid synaptic transmission in the nervous systems. Prolonged exposure to the agonist or high agonist concentrations leads to a desensitized state, which is a more stable non-conducting state.
C. elegans and Cys-loop receptors
C. elegans is a free-living, non-parasitic nematode that was introduced by Sydney Brenner to study biological processes (Brenner 1974). It has several advantages as a model organism, and it is a powerful tool for the pharmaceutical industry: It is a small (about 1 mm) and transparent roundworm very easy to manipulate and grow in the laboratory; it has two sexual forms, self-fertilizing hermaphrodites and males, and it can produce hundreds of offspring; it has a short life cycle of ~ 3 days from egg to adult worm with four larval stages (L1–L4) and a life span between 2 and 3 weeks, which facilitate the study of physiological and pathological processes; it can adopt a dauer state that allows the worms to survive harsh environments and live for months, and also allows freezing the worms at – 80 °C; it is very suitable for high-throughput drug screening; and it can be used to dissect the in vivo action of drugs, even if they modulate several targets. Also, the worm has a simple nervous system, the wiring diagram and cell lineage of the entire organism have been determined, it has a well-annotated genome, it allows to pursue both forward and reverse genetics, and it is a valuable resource for genetics, genomics, and systems biology (Sulston and Horvitz 1977; Kaletta and Hengartner 2006; Corsi et al. 2015).
A great percentage of the worm genes have a human ortholog, and the majority of human disease genes and human pathways are present in C. elegans (Culetto and Sattelle 2000; Kaletta and Hengartner 2006; Shaye and Greenwald 2011; Kim et al. 2018). Hence, C. elegans has many excellent advantages as an in vivo model for human diseases, such as neurodegenerative diseases (Alexander et al. 2014). In particular, human neurotransmission, receptors, and ion channels, including many Cys-loop receptors, are conserved in C. elegans. C. elegans is also widely used as a parasitic nematode model for anthelmintic drug discovery (Holden-Dye and Walker 2014; Burns et al. 2015; Sepúlveda-Crespo et al. 2020; Choudhary et al. 2022). It is a very effective and cost-efficient nematode model that overcomes the disadvantages of working with parasitic worms. It shares physiological and pharmacological features with parasitic nematodes, and it similarly responds to anthelmintic drugs (Holden-Dye and Walker 2007; Beech and Neveu 2015). Nematode Cys-loop receptors constitute important targets for antiparasitic drugs.
C. elegans possesses the largest known Cys-loop receptor family, with more than 102 LGIC subunit-encoding genes, that include 61 genes for nAChR subunits, 7 genes for GABAA subunits, 8 genes for aminergic receptor subunits, 6 genes for glutamate-activated chloride channel subunits, 12 genes for anionic nAChR subunits, and 8 genes comprising a diverse subgroup (Jones and Sattelle 2008; Hobert 2013; Hardege et al. 2023).
Functional characterization has been performed on just over 50% of the genes within the Cys-loop pLGIC superfamily of C. elegans (Lees et al. 2012; Hardege et al. 2023). Thus, C. elegans offers a considerable diversity of receptors, many of which remain uncharacterized. The identification of Cys-loop receptors in C. elegans has been achieved by genetic techniques or by the prediction of subunit genes in genome data. Confirmation of the composition and properties of specific receptors has been facilitated by expression of subunits, ex vivo, mainly in Xenopus laevis oocytes or in mammalian cells (Lewis et al. 1980; Touroutine et al. 2005; Boulin et al. 2008; Degani-Katzav et al. 2016; Castro et al. 2020). There are limitations to the information obtained from heterologous systems since the expression may not include the native combination of subunits or the total accessory proteins required for assembly and formation of the mature receptors. Thus, electrophysiological studies from C. elegans preparations are relevant to decipher the channel properties of native receptors.
Patch-clamp recordings have been carried out on different worm preparations. One of these preparations consists of a dissection technique of adult worms that expose ventral muscles for electrophysiological recordings (Richmond and Jorgensen 1999). Another preparation consists of primary cultures of neurons and muscle cells (Christensen et al. 2002; Yuan et al. 2003; Rayes et al. 2007; Hernando and Bouzat 2014; Turani et al. 2018). In this preparation, embryonic cells, which are obtained from eggs, differentiate in vitro to neurons and muscle cells corresponding to the larva 1 developmental stage (Christensen et al. 2002). This culture represents an invaluable system for exploring molecular function and pharmacology of native receptors (Fig. 2) (Christensen et al. 2002; Yuan et al. 2003; Rayes et al. 2007; Hernando and Bouzat 2014; Turani et al. 2018). Cultures of cells corresponding to other larval stages have also been implemented for single-channel recordings (Zhang et al. 2011; Turani et al. 2018). Comparison of the properties of activation and drug modulation of a specific receptor between preparations from wild-type and mutant strains lacking a specific subunit has helped to decipher the receptor composition and the contribution of each subunit to its pharmacology and function (Richmond and Jorgensen 1999; Rayes et al. 2007; Hernando et al. 2012).
Scheme for electrophysiological recordings from L1 muscle cells. This in vitro culture technique allows to perform electrophysiological recordings from L1 muscle or neuronal cells. Gravid hermafrodyte adult worms are exposed to an alkaline hypochlorite solution and the released eggs are treated with chitinase. The embryonic cells are then isolated and cultured. Complete differentiation to the various cell types that comprise the newly hatched L1 larva are observed within 24 h (Christensen et al. 2002; Rayes et al. 2007; Hernando et al. 2012; 2014; Turani et al. 2018).These cells can be used for single-channel and macroscopic current recordings within at least one week. The whole-cell and single-channel currents correspond to L-AChRs from L1 muscle cells activated by 500 µM and 100 µM ACh, respectively. Created with BioRender.com
Nicotinic acetylcholine receptors (nAChRs)
C. elegans has an extensive and diverse nAChR subunit family, composed of at least 29 protein subunits, which represents the most substantial number of nAChR subunits reported for any organism (Jones and Sattelle 2004; Brown et al. 2006; Rand 2007; Treinin and Jin 2021). Why such a simple organism requires so many nAChR subunits remains intriguing.
The nAChR subunit main groups are designated based on the initial characterized subunit within each group. These include the DEG-3 group (comprising 8 members and linked to the degeneration of specific neurons), ACR-16 group (with 11 members and related to acetylcholine receptor function), UNC-29 group (with 3 members and associated with uncoordinated worm phenotype), UNC-38 group (comprising 4 members), and ACR-8 group (with 3 members) (Mongan et al. 1998; Jones et al. 2007; Hansen et al. 2022). nAChR subunits are classified as α-type, which contain a disulfide bridge involved in the binding of agonists, and non-α, which lack this motif. Receptors can be either heteromeric, composed of α and non-α subunits, or homomeric, composed of five identical α-type subunits. nAChRs are present in body wall and pharyngeal muscle and motor and sensory neurons and are involved in locomotion, feeding, and a variety of worm behaviors (Treinin and Jin 2021). Despite their key roles, the way that the different subunits co-assemble into pentameric arrangements and the biophysical properties of the majority of C. elegans nAChRs remains largely unknown. Only a few nAChRs have been characterized, particularly those involved in locomotion.
C. elegans muscle nAChRs
As in mammals, body wall muscle receives excitatory cholinergic innervation that activates nAChRs important for muscle contraction and movement. However, it also receives inhibitory, GABAergic, transmission through muscle GABAA receptors. A coordinated and fine balance between excitatory and inhibitory inputs onto body wall muscle enables the typical sinusoidal locomotion of C. elegans (Richmond and Jorgensen 1999). Thus, sustained activation of muscle nAChRs produces spastic paralysis and sustained activation of GABAA receptors produces flaccid paralysis. By means of these two opposite mechanisms, several drugs exert their anthelmintic effects.
C. elegans has two distinct types of muscle nAChRs that play a crucial role in movement, and these have received the most comprehensive examination: the levamisole-sensitive nAChR, L-AChR, and the nicotine-sensitive nAChR, N-AChR, which is levamisole-insensitive and nicotine-sensitive (Fig. 3a and b) (Richmond and Jorgensen 1999; Culetto et al. 2004; Touroutine et al. 2005).
Subunit composition and function of Cys-loop receptors in muscle cells. a L-AChR is composed of five different subunit but the disposition in the pentameric arrangement remains unknown. Macroscopic currents elicited by ACh (pipette potential -70 mV) and single-channel recordings elicited by ACh and the anthelmintic drugs levamisole and bephenium are shown (pipette potential, 100 mV). Channels are shown as upward deflections. Recordings were obtained from L1 muscle cells (Hernando et al. 2012). b N-AChR is a homopentameric receptor composed by ACR-16 subunits. Macroscopic currents elicited by ACh (pipette potential -70 mV) from L1 muscle cells obtained from a strain lacking the L-AChR receptor (unc29(e1072) revealed very small currents corresponding to N-AChR receptors (Hernando et al. 2012). c UNC-49 constitutes a GABAA-type receptor that is composed by UNC-B and UNC-49 C subunits. The stoichiometry remains unknown. Typical macroscopic currents elicited by GABA (pipette potential -70 mV) and single-channel recordings elicited by GABA, muscimol, and piperazine (PZE) (pipette potential 100 mV) obtained from L1 muscle cells are shown (Hernando and Bouzat 2014). d GluCl is a glutamate-gated chloride channel that is the main target of ivermectin. There are different GluCl subunits. Heteromeric GluClα1/β are expressed in mammalian cells, and typical currents elicited by glutamate are shown (pipette potential -60 mV) (Castro et al. 2020). e MOD-1 is a serotonin-activated chloride channel. It forms homomeric receptors in heterologous expression systems. Typical macroscopic responses to 5-HT are shown (pipette potential -50 mV) (Rodriguez Araujo et al. 2022)
Levamisole-sensitive nAChR (L-AChR)
L-AChR is the main nAChR involved in worm locomotion. Movement is profoundly impaired and uncoordinated in worms lacking L-AChRs. The presence of L-AChR in parasitic nematodes is particularly important since it is the target of anthelmintic drugs, such as levamisole, morantel, pyrantel, and bephenium, used to control human and animal worms’ infections. By acting as potent agonists of nematode L-AChRs, without being rapidly degraded, these drugs produce body wall muscle hypercontraction, paralysis, and ultimately death of nematodes. These anthelmintic drugs are highly selective for nematode nAChRs and are very low-efficacy agonists of vertebrate nAChRs (Martin et al. 1997; Rayes et al. 2004; Bartos et al. 2006; Turani et al. 2018).
The discovery of strains resistant to levamisole has allowed the identification of subunits that compose the L-AChR (Richmond and Jorgensen 1999). The systematic analysis of C. elegans levamisole-resistant mutant strains has shown that the α-type subunits, UNC-63, UNC-38, and LEV-8 subunits and the non-α type, UNC-29 and LEV-1, are main components of the adult L-AChR (Table 1) (Fleming et al. 1997; Culetto et al. 2004; Towers et al. 2005; Almedom et al. 2009). Boulin et al. (2008) reconstituted functional L-AChRs in X. laevis oocytes by co-expressing the five different L-AChR subunits together with three ancillary proteins. Macroscopic currents elicited by ACh of the reconstituted L-AChR receptor revealed that it is a cationic channel with high calcium permeability and that it shows very slow desensitization since currents do not decay during the ACh pulse (Boulin et al. 2008). Levamisole shows higher potency but reduced efficacy with respect to ACh, and nicotine does not activate the receptor but instead acts as an allosteric inhibitor (Table 1).
Single-channel recordings from C. elegans muscle cells corresponding to the L1 stage has provided detailed molecular information about the functional properties of the native single L-AChR channel. Single channels activated by ACh are readily detected in cell-attached patches from L1 muscle cells. Channel activity appears mainly as isolated brief openings of about 0.2–0.5 ms or in short bursts formed by two or three successive opening events (Fig. 3a). Channel events show a single conductance of about 30–35 pS (Rayes et al. 2007; Hernando et al. 2012). In contrast to vertebrate muscle nAChRs (Bouzat and Sine 2018), clusters corresponding to activation episodes of a single receptor are not detected at a broad ACh concentration range. In vertebrate muscle nAChRs, clusters include openings and closings of a single receptor and are separated by long closed periods in which the receptor is in the desensitized state (Bouzat and Mukhtasimova 2018). Thus, the lack of these prolonged closed periods is in accordance with the slow desensitization observed from macroscopic currents of L-AChRs. Single-channel currents in L1 muscle cells are also elicited by levamisole, pyrantel, morantel, and bephenium at the submicromolar range (Fig. 3a), thus confirming the actions of these anthelmintic drugs as potent agonists of L-AChRs (Rayes et al. 2007; Hernando et al. 2012; Turani et al. 2018).
Single-channel recordings from C. elegans L1 muscle cells derived from mutant strains lacking different nAChR subunits have allowed the identification of the native L-AChR subunit composition and the contribution of each subunit to channel function. No channel activity is detected in muscle cells derived from null mutants lacking the α-type subunits, UNC-38 and UNC-63, and the non-α type subunit, UNC-29. Thus, all three subunits are essential and assemble together in the pentameric arrangement. All these mutant worms show important uncoordinated behavior and levamisole-resistance. Recordings from a mutant strain carrying a mutation in the M4 segment of LEV-1 show a main population of lower amplitude channels and different activation pattern with respect to the wild-type (about 26 pS) (Rayes et al. 2007). The analysis revealed that LEV-1, a non-α subunit, is present in the native receptor; however, it can be replaced by other not yet identified subunit, leading to L-AChR channels with lower conductance and lower levamisole sensitivity than the wild-type L-AChR. Single-channel currents from a null mutant lacking LEV-8, an α-type subunit, show a different activation pattern compared to the wild-type. Channel activity of L-AChR lacking LEV-8 decreases significantly with time, indicating that this subunit plays an important role as a determinant of desensitization. Moreover, macroscopic current recordings show increased rate and extent of desensitization of the L-AChR lacking LEV-8. The recordings reveal that LEV-8 is not essential for functional receptors, but it is preferentially incorporated in the native L-AChR. In its absence, it can be replaced by another subunit. Thus, single-channel recordings indicate that L1 muscle expresses a main L-AChR type composed of five different subunits: UNC-38, UNC-63, UNC-29, LEV-1, and LEV-8. The disposition of these five subunits in a pentameric arrangement is still not known (Hernando et al. 2012).
L-AChR channel activity is also elicited by the anthelmintic bephenium (Turani et al. 2018) (Fig. 3a). The recordings from L1 muscle cells show that this anthelmintic drug is less potent than levamisole and ACh and that it also acts as an open-channel blocker at higher concentrations. Bephenium also activates mammalian muscle nAChRs, producing opening events that are briefer than those activated by ACh and that do not appear in activation episodes at a range of concentrations as shown for full agonists. The results indicate that bephenium is a very weak agonist of mammalian nAChRs as shown for other anthelmintics such as pyrantel and levamisole (Turani et al. 2018).
Single-channel currents elicited by levamisole have been also recorded from adult muscle preparations using a mutant worm strain that inhibits L-AChR aggregation at the neuromuscular junction (Qian et al. 2008). Channel activity of adult L-AChRs is similar to that described in L1 muscle cells. The lack of the LEV-8 subunit produces prolonged dwell times in the closed state without altering channel conductance and open durations, as described for L1 muscle cells. The lack of LEV-1 is accompanied by a reduction in the single-channel conductance and in the number of active channels (Qian et al. 2008). Thus, the biophysical properties of the L-AChR and the subunit contributions to channel function are similar at L1 and adult stages.
Anthelmintic treatment is crucial to control nematode infections affecting human, animal, and plant health. Since control is threatened by the emergence of drug resistant nematodes, there is a need to develop novel compounds. In this context, C. elegans represents a valuable organism for identifying pharmacological targets and compounds with anthelmintic activity. Plants provide a variety of phytochemicals, some of which show potential anthelmintic activity. Among these compounds, the plant terpenoids thymol, carvacrol, and eugenol induce rapid paralysis of worms. An in vivo screening of C. elegans strains carrying mutations in different receptors involved in worm locomotion revealed that two Cys-loop receptors—L-AChR and GABAA (UNC-49) receptor—are involved in the paralyzing effects of these terpenoids (Hernando et al. 2019). Terpenoids decrease macroscopic responses of L-AChRs and, at the single-channel level, reduce the frequency of opening events without affecting channel properties, thus stabilizing the receptor in a closed conformation. The observations are compatible with their actions as negative allosteric modulators (Hernando et al. 2019). Thus, terpenoids exert anthelmintic effects through the L-AChR by acting at a different site and by a different mechanism to the classical anthelmintic levamisole, which acts as an orthosteric agonist. Given the ever-increasing resistance of parasites to classical anthelmintic drugs, the use of terpenoids as a potential alternative or complementary anthelmintic strategy is worth exploring. This strategy could offer significant benefits in combating parasitic infections, particularly by reducing the infection burdens of soil-transmitted helminths.
C. elegans is used as a model of human diseases. Mutations in human muscle nAChR lead to congenital myasthenic syndromes (CMSs), due to reduced expression or kinetic changes. CMSs originated from changes in kinetics are classified in slow-channel CMSs, which show prolonged ACh-mediated postsynaptic responses and enhanced open probability of the muscle nAChR channel, and fast-channel CMSs, which show decreased responses and impaired opening and reduced open durations of muscle nAChRs (Engel et al. 2015). Interestingly, a C. elegans mutant strain carrying the UNC63-C151Y mutation that disrupts the Cys-loop motif of the essential UNC-63 subunit of L-AChRs has deficient muscle function reflected by impaired swimming. Single-channel recordings from L1 muscle cells from the mutant strain show a 100-fold reduced frequency of opening events and briefer channel openings of L-AChRs compared to wild-type worms. The changes in L-AChR kinetics recapitulate the kinetic changes found in patients with fast-channel congenital myasthenic syndromes (Jones et al. 2011). Thus, one the one hand, functional roles of key motifs, such as the Cys-loop, are conserved between human and C. elegans nAChRs, and on the other, C. elegans carrying mutations in nAChRs may offer a useful model to assist in the development of therapies for syndromes produced by altered function of human nAChRs.
Table 1 summarizes information on the expression and pharmacology of L-AChR and includes the comparison with human nAChRs.
Nicotine-sensitive AChR (N-AChR)
The N-AChR is a homomeric receptor composed of five ACR-16 subunits that expresses in body wall muscle of C. elegans and some parasitic nematode species (Raymond et al. 2000; Noonan and Beech 2022) (Fig. 3b, Table 1). Although loss-of-function mutation of ACR-16 greatly reduces ACh-dependent and evoked excitatory-muscle currents, the mutant animals do not show significant locomotion defects (Touroutine et al. 2005).
ACR-16 is the C. elegans nAChR with highest homology to the human α7 nAChR, a homomeric human receptor that is involved in cognition, attention, memory, and inflammation (Bouzat and Sine 2018) (Table 1). ACR-16 forms functional homomeric receptors in X. laevis oocytes (Ballivet et al. 1996; Raymond et al. 2000). As for human α7 nAChR, robust responses require the co-expression of RIC-3 ancillary protein to enhance expression in oocytes (Halevi 2002; Treinin and Jin 2021). Oocytes expressing either ACR-16 or α7 nAChR exhibit inward currents in response to ACh or nicotine. In both cases, nicotine is more potent than ACh, but whereas nicotine is a full agonist of α7, it is a partial agonist of ACR-16. Both receptors desensitize rapidly in the presence of ACh and nicotine (Bennett et al. 2012; Raymond et al. 2000). Although the pharmacology of ACR-16 is similar to that of α7 nAChR, its calcium permeability is lower (Ballivet et al. 1996; Bouzat and Sine 2018). Another pharmacological difference is that ACR-16 is relatively insensitive to methyllycaconitine and the snake toxin α-bungarotoxin, which are both potent antagonists of α7 nAChRs (Raymond et al. 2000; Bennett et al. 2012).
Whole-cell voltage-clamp recordings in adult C. elegans body wall muscle show significant inward currents evoked by pressure-applied nicotine (Almedom et al. 2009). In contrast, in L1 muscle cells, the contribution of N-AChR to the total ACh-elicited macroscopic current is insignificant; it mainly arises from the activation of L-AChRs. Macroscopic currents elicited by ACh from N-AChRs are detected in a very low percentage of cells and are significantly smaller than L-AChR currents (Fig. 3b). In addition to the reduced amplitude, N-AChR currents decay 40-fold faster and show insignificant steady-state currents, revealing more rapid and important desensitization than L-AChRs (Hernando et al. 2012). At the single-channel level, no channel activity elicited by nicotine has been detected from L1 muscle cells lacking L-AChRs (Rayes et al. 2007). The lack of detection of single N-AChR channels may be due to a very low channel conductance, very fast and stable desensitization, or very low expression at the L1 stage. Thus, the characterization of C. elegans single-ACR-16 channels is a pending issue.
Table 1 summarizes Information on the expression and pharmacology of N-AChR and includes the comparison with human nAChRs.
ACR-23
C. elegans ACR-23 (acetylcholine receptor) is a nAChR cationic channel that expresses in body wall muscles and in mechanosensory neurons and maintains basal levels of locomotion. ACR-23 may have an extra-synaptic location in C. elegans body wall muscles; therefore, its function could be to increase muscle excitability by slightly depolarizing the cells (Treinin and Jin 2021).
In heterologous expression in oocytes, ACR-23 functions as a homomeric non-selective cationic channel (Table 1). The interesting aspect of this receptor is that it is activated by betaine, which may be its endogenous neurotransmitter (Peden et al. 2013). Betaine is an amino acid derivative found in diverse organisms, from bacteria to plants and animals, with well-established functions as a methyl donor and osmolyte in all cells. Betaine has been shown to be synthesized in the nervous system of C. elegans, where it functions in the control of different behavioral states (Hardege et al. 2022). The endogenous ligand and biological function of ACR-23 remain to be established. Whereas some reports indicate that betaine is the only ligand that elicits ACR-23 currents (Peden et al. 2013), other studies postulate that choline, betaine, and the anthelmintic monepantel activate this receptor (Rufener et al. 2013). ACR-23 and its homologues in parasites are targets for the antiparasitic monepantel, which induces nematode paralysis (Peden et al. 2013; Rufener et al. 2013).
Table 1 summarizes information on the expression and pharmacology of ACR-23.
Neuronal nAChRs
There is a surprisingly large number of neuronal nAChR subunits, but just a few nAChRs have been characterized. There is still very limited information on how the subunits assemble and on the biophysical properties of the resulting receptors (See Treinin and Jin (2021) for a recent review). The characterization of the activation and pharmacological properties of these receptors has been mainly achieved by expressing subunits in heterologous systems, which, in turn, may differ from native receptors.
ACR-20 belongs to the same subunit group of ACR-23. It forms homomeric receptors in X. laevis oocytes (Table 1). Currents are elicited by betaine and choline, being betaine more potent than choline. Monepantel acts as a superagonist when applied to oocytes expressing ACR-20 as it elicits larger currents than saturating concentrations of choline or betaine (Baur et al. 2015).
The ACR-2 is a non-α subunit, present in cholinergic motor neurons (Jospin et al. 2009). Reconstitution of a functional ACh-activated ACR-2 containing channel in X. laevis requires expression of ACR-2, ACR-12, UNC-63, UNC-38, and ACR-3 subunits together with three auxiliary proteins (Jospin et al. 2009) (Table 1). The resulting receptor shows distinct pharmacology to that of the L-AChR although it shares two subunits.
DEG3/DES2 subunits form a neuronal nAChR expressed in sensory neurons (Table 1). The two subunits belong to the DEG-3 group that is nematode specific and includes also ACR-20 and ACR-23. In contrast to ACR-20 and ACR-23 that form homomeric receptors (Baur et al. 2015), DEG-3 cannot form a functional channel on its own (Treinin and Chalfie 1995). However, co-expression with DES-2 in oocytes, but not expression of each alone, leads to ACh-activated currents (Treinin et al. 1998; Yassin et al. 2001). The macroscopic current analysis shows that this receptor is highly permeable to calcium, and it is activated by betaine. ACh has relatively low affinity, and choline is more potent and efficacious than ACh (Yassin et al. 2001).
Table 1 summarizes information on the expression and pharmacology of the above mentioned nAChRs and includes the comparison with human nAChRs.
ACh-gated chloride channels
The large diversity of nAChRs in C. elegans with respect to vertebrates, in which nAChRs are permeable only to cations, is revealed by the existence of chloride-permeable nAChRs. This type of receptors includes, in turn, a broad variety of subtypes. To date, two different groups have been identified: the ACC (for ACh-gated chloride channel), comprising 8 different subunits (ACC-1 to 4 and LGC-46 to LGC-49) and the new LGC-57 group comprising 4 subunits (LGC-57, LGC-58, LGC-40, and LGC-39) (Hardege et al. 2023).
Thus, ACh in C. elegans contributes to both inhibitory and excitatory events in many neurons. ACC-1 and ACC-2 form homomeric channels when expressed in oocytes enabling detailed characterization. Both are activated by ACh in the micromolar range and chloride permeable. ACC-3 and ACC-4 do not form homomers, and co-expression studies provide support for interactions between these two subunits and ACC-1 or ACC-2 (Putrenko et al. 2005; Treinin and Jin 2021). Upon expression in oocytes, it was recently found that another subunit of the same group, LGC-49, forms a homomeric ACh-gated channel but does not show significant activation by choline (Hardege et al. 2023). The new identified ACh-gated chloride channel group, LGC-57, includes receptors with diverse ligand-binding properties. For LGC-40, LGC-57, and LGC-58, the primary ligand appears to be choline rather than ACh. LGC-39 is activated by both cholinergic and aminergic ligands and represents the first evidence of a truly polymodal channel (Hardege et al. 2023).
Overall, given the extensive family of nAChR subunits, the possibility of multiple subunit combinations, and the functional diversity of nAChRs, C. elegans provides an extremely rich system for understanding the cholinergic system and receptor function.
GABA-activated ion channels
GABA is an inhibitory neurotransmitter of vertebrate and invertebrate nervous systems. It is not the only inhibitory neurotransmitter in the Nematoda phylum since ACh-, monoamines-, and glutamate also activate chloride channels (Dent 2010; Hobert 2013; Putrenko et al. 2005).
The C. elegans genome contains at least seven predicted ionotropic GABA receptors (Hobert 2013). This rich repertoire of GABA receptors includes LGC receptors (ligand-gated ion channel: LGC-35, LGC-36, LGC-37, and LGC-38), EXP-1 (expulsion defective (defecation)), GAB-1 (GABA receptor subunit), and UNC-49 (uncoordinated). The abundance of GABA subunits leads to the formation of a broad range of receptor subtypes that vary significantly in terms of their functionality and pharmacology (Sigel and Steinmann 2012).
The UNC-49 receptor is present at the neuromuscular junction of nematodes and is unique to the phylum. It mediates the inhibition that leads to muscle relaxation allowing, in coordination with the muscle contraction elicited by L-AChR, the typical sinusoidal movement. The C. elegans muscle GABA receptor is encoded by the unc-49 gene, which is translated into three subunits: UNC-49A, UNC-49B, and UNC-49C. In adult C. elegans, the GABA receptor has been shown to be composed of UNC-49B and C subunits (Bamber et al. 2005) (Fig. 3c) (Table 1). The UNC-49B subunit confers synaptic localization and allows channel activation, whereas UNC-49C is a non-essential modulatory subunit that co-assembles with UNC-49B. The unc-49 null mutant exhibits the ‘‘shrinker’’ phenotype, owing to hypercontraction of the body wall muscles on both sides of the body. Worms become resistant to muscimol, which is a full agonist of vertebrate GABAA receptors. In wild-type worms, this drug relaxes all body wall muscles and causes lengthening of adult worms (Mclntire et al. 1993; Petzold et al. 2011).
Piperazine (PZE) is a GABA agonist of nematode GABAA receptors. It is an anthelmintic commercialized by nearly 70 years to treat Ascaris lumbricoides and Enterobius vermicularis infections in humans. Its mode of action has been studied in Ascaris suum and C. elegans, where its GABA-mimetic action causes a flaccid, reversible paralysis of body wall muscle of nematodes in the adult stage. C. elegans L1 stage is less sensitive to PZE than the adult stage because GABA receptors are not present in dorsal muscle and are only present in ventral muscle (Martin 1985; Hernando and Bouzat 2014). Muscimol is a full and potent agonist of C. elegans UNC-49 receptors, whereas it is less potent than GABA for A. suum and Haemonchus contortus receptors (Holden-Dye et al. 1989; Siddiqui et al. 2010). PZE has been shown to act as a low-efficacy agonist of GABA receptors of the parasitic nematodes A. suum (Martin 1985) and H. contortus (Brown et al. 2012) and C. elegans (Hernando and Bouzat 2014).
In L1 muscle cells, macroscopic currents elicited by GABA show rapid onset as well as rapid and full decay under the sustained pulse of agonist, indicating full desensitization (Hernando and Bouzat 2014). Single-channel currents of GABA receptors from L1 muscle cells show that GABA, muscimol, and PZE activate UNC-49 receptors (Fig. 3c). The proportion of patches that show detectable single-channel activity is very low at all agonist concentrations (< 15%). Single-channel openings exhibit brief open durations and amplitudes of about 2.5–3 pA (100 mV pipette potential). C. elegans UNC-49 channel activity was also reported in HEK cells transfected with UNC-49B and C cDNA subunits (Bamber et al. 1999). The estimated conductance was 37 pS for UNC-49B homomers and about 30 pS or UNC-49B/C heteromers. The comparison of the desensitization rate of macroscopic currents and the single-channel conductance determined in L1 cells with results from heterologously expressed UNC-49 receptors (Bamber et al. 1999) suggests that in L1 muscle cells the receptors may be UNC49B/C heteromers.
Terpenoids produce a rapid paralysis of C. elegans acting through L-AChR and UNC-49 GABA receptor at the neuromuscular junction. As described for L-AChRs, whole-cell recordings from L1 cells demonstrate that terpenoids decrease macroscopic responses of UNC-49 receptors to GABA, acting as inhibitors (Hernando et al. 2019). Medicinal plants provide an alternative source of potential anthelmintic compounds, and UNC-49 becomes an attractive target to test new compounds and formulations. UNC-49 is distinct to vertebrate GABA receptors, and it shows an interesting pharmacological profile, which emphasizes its use as an anthelmintic target (S. Choudhary et al. 2022; Cochrane et al. 2022). Thus, the molecular and pharmacological characterization of these nematode receptors will have important implications for the development of novel anthelmintic drugs as well as for our understanding of GABA receptor pharmacology.
Table 1 summarizes information on the expression and pharmacology of C. elegans UNC-49 receptors and the comparison with human receptors.
Glutamate-activated chloride channels (GluCl)
As other invertebrates, C. elegans contains a unique type of glutamate-gated chloride channels (GluCl) (Cully et al. 1994; Jones and Sattelle 2008). GluCls are of considerable medical and economical importance because they are targets of macrocyclic lactones, such as ivermectin (IVM), which are the most widely used antiparasitic drugs (Chen and Kubo 2018). IVM is used in veterinary for gastrointestinal roundworms, lungworms, grubs, and sucking lice and mange mites and in humans for treating filarial diseases (Campbell 2012).
There are at least six C. elegans genes encoding GluCl subunits: avr-14 (GluClα3 subunit), avr-15 (GluClα2), glc-1 (GluClα1), glc-2 (GluClβ), glc-3 (GluClα4), and glc-4 (Cully et al. 1994, 1996; J. A. Dent et al. 2000; Horoszok et al. 2001; Vassilatis et al. 1997). Of these, all except glc-2 encode α-type GluCls; glc-2 is the lone β-type (Degani-Katzav et al. 2016). The parasitic nematode H. contortus has two other glc genes that are not present in C. elegans, glc-5, and glc-6 (Glendinning et al. 2011). Throughout the phylum Nematoda, the GluCl subunits that exhibit the highest degree of conservation are AVR-14, GLC-2, GLC3, and GLC-4 (Lamassiaude et al. 2022). Except for GLC-4, all GluCl subunits can form functional homomeric receptors when expressed in X. laevis oocytes. Although GluCl subunits can form homomeric or heteromeric receptors in heterologous expression systems, the composition of the native receptors remains mostly unknown. Physiological functions associated with GluCl receptors include pharyngeal pumping, which is required for feeding and maintaining hydrostatic pressure, and regulation of locomotion, olfactory, and temperature responses (Jones and Sattelle 2008).
The homomeric GluClα was the first eukaryotic Cys-loop receptor whose X-ray structure was determined (Fig. 1a) (Hibbs and Gouaux 2011). The X-ray structure was solved in complex with the allosteric agonist IVM, the endogenous neurotransmitter L-glutamate, and the open-channel blocker picrotoxin. The structure revealed information about the five binding sites of IVM, located at subunit interfaces on the periphery of the TM domains and proximal to the extracellular side of the membrane, the site for the orthosteric agonist L-glutamate, at subunit interfaces in the ECD, and the location of picrotoxin in the ion channel (Hibbs and Gouaux 2011).
Heterologous expression studies have shown that both GluClα1 (GLC-1) and GluClβ (GLC-2) subunits form functional homomeric receptors, the first responding to IVM and the latter to glutamate (Li et al. 2002; Vassilatis et al. 1997). GluClα1/β heteropentamers respond to both IVM and glutamate (Dent et al. 1997; Degani-Katzav et al. 2016) (Table 1). GluClα1/β also forms functional receptors when expressed in mammalian cells. Currents elicited by rapid application of 3 mM glutamate decay in the presence of the agonist due to desensitization, and the magnitude of the currents increases linearly with the voltage, indicating an ohmic behavior with no significant rectification (Fig. 3d) (Castro et al. 2020).
Macroscopic and single-channel recordings of GluCl carrying different mutations and expressed in CHO cells show that the heteromeric GluClα1/β contains three α subunits and two β subunits arranged in an anticlockwise β-α-β-α-α manner as viewed from the extracellular side, with two Glu-binding sites located at the β( +)/α( −) subunit interfaces. The α( +)/α( −) interface creates a third Glu-binding site that becomes functional upon a conformational change induced by a mutation in the IVM-binding pocket (Degani-Katzav et al. 2016).
All functional GluCl homomeric receptors are sensitive to IVM, except GLC-2. It is noteworthy that the GLC-2 in C. elegans can co-assemble with either GLC-1 or AVR-15 to produce two distinct heteromeric GluCl subtypes that are sensitive to IVM and exhibit different pharmacological properties. Thus, GLC-2 plays a pivotal role in heteromeric GluCl composition (Cully et al. 1994; Vassilatis et al. 1997; Lamassiaude et al. 2022). Also, a heteromeric GLC-2/GLC-3 GluCl expressed in Xenopus oocytes has been recently characterized and shows distinctive pharmacological characteristics, emphasizing the potential role of heteromeric GluCls in the nematodes’ sensitivity to macrocyclic lactones (Lamassiaude et al. 2022).
Expression of GluCl receptors in heterologous systems combined with electrophysiological studies provides a powerful tool for discovering new mechanisms of anthelmintic action. In this respect, the compound dibenzo[b,e]oxepin-11(6H)-one (doxepinone) was shown to induce paralysis and reduce the swimming and pharyngeal pumping rates of C. elegans, indicating a marked anthelmintic activity (Castro et al. 2020). The in vivo screening of selected strains carrying mutations in different Cys-loop subunit genes showed that a triple mutant strain lacking avr-14, avr-15, and glc-1 genes of GluCl subunits is resistant to doxepinone effects, indicating that GluCl is involved in doxepinone effects. To unravel the molecular mechanism, whole-cell currents from GluClα1/β expressed in mammalian cells were exposed to the compound. Doxepinone does not activate GluCls but instead produces a significant decrease of the decay time constant and the net charge of glutamate-elicited currents, indicating that it allosterically inhibits GluCl. This mechanism is different to that of IVM, indicating that different modulations of GluCls can result in anthelmintic effects (Castro et al. 2020).
Table 1 summarizes information on the expression and pharmacology of different pentameric arrangements of GluCl subunits and includes comparison with human Cys-loop receptors.
Monoamine-gated Cys-loop receptors
C. elegans and nematodes possess Cys-loop receptors that respond to monoamines, including serotonin, tyramine, dopamine, octopamine. Some of the characterized receptors include LGC-55, a tyramine-gated chloride channel; LGC-53, a dopamine-gated chloride channel (Pirri et al. 2009; Ringstad et al. 2009); and the recently deorphanized chloride channels that respond to dopamine and tyramine when expressed in oocytes, LGC-54, LGC-52, and GGR-3 (Morud et al. 2021).
Serotonin activates two different Cys-loop receptors, MOD-1, a chloride ion channel (Ranganathan et al. 2000), and LGC-50, a recently identified cation channel that is required for serotonin-dependent pathogen avoidance learning and functions in interneurons critical for this process (Morud et al. 2021).
MOD-1 expresses in C. elegans neurons and muscles that control behaviors, such as locomotion, egg laying, feeding, pharyngeal pumping, decision making, and aversive learning (Churgin et al. 2017). Interestingly, MOD-1 is present in vertebrate and plant parasitic nematodes, and it is therefore emerging as an attractive anthelmintic target since it is not present in vertebrates (Beech et al. 2013; Crisford et al. 2020).
While MOD-1 and vertebrate 5-HT3 share the ability to respond to 5-HT, they differ in their function in that 5-HT3 is a non-selective cation channel, whereas MOD-1 is a chloride channel (Dent 2006). As an anionic Cys-loop receptor, MOD-1 shows about 30% identity with vertebrate GABA and glycine receptors. It is important to note that no specific glycine-gated channels have been identified in C. elegans.
MOD-1 can be expressed in oocytes and mammalian cells (Ranganathan et al. 2000; Rodriguez Araujo et al. 2022) (Table 1). Macroscopic current recordings show that MOD-1 responses are rapidly elicited by 5-HT, decay in the presence of the agonist due to desensitization, and recover rapidly from desensitization in the absence of agonist (Fig. 3e). Currents are of similar amplitudes at positive and negative membrane potentials, indicating no significant rectification (Rodriguez Araujo et al. 2022). Concentration–response curves from macroscopic currents reveal EC50 for 5-HT of about 1 µM, which is in the same order as that of human and mouse 5-HT3A receptors (Corradi and Bouzat 2014). However, MOD-1 responds very differently to 5-HT3A partial agonists. In this regard, tryptamine is a significantly more efficacious and potent agonist of MOD-1 than of 5-HT3A and 2-Me-5HT, which efficaciously activates 5-HT3A receptors, cannot activate MOD-1. Thus, the agonist selectivity differs between these two 5-HT-activated Cys-loop receptors. Also, although they share 5-HT as their endogenous neurotransmitter, they differ in how it interacts at the binding site (Mu et al. 2003). Moreover, studies in oocytes show that high concentrations of granisetron and ondansetron, both of which are potent antagonists of the 5-HT3A receptors, do not affect the action of 5-HT on MOD-1 channels (Ranganathan et al. 2000). MOD-1 also differs from 5-HT3 in the fact that it is not activated by the allosteric agonist thymol (Rodriguez Araujo et al. 2022).
The differential selectivity between vertebrate 5-HT3A and MOD-1 can be exploited for the development of novel anthelmintic drugs. Regarding this, it was shown that tryptamine, which is a very poor agonist of vertebrate 5-HT3A receptors, reduces worm motility. Hence, tryptamine-derived agents may be promising compounds for further antiparasitic drug research (Rodriguez Araujo et al. 2022).
Macroscopic currents of MOD-1 expressed in mammalian cells or oocytes have allowed the identification of novel allosteric inhibitors, such as the metabotropic 5-HT receptor antagonists mianserin and methiothepin as well as the UNC-49 agonist piperazine (PZE) (Ranganathan et al. 2000; Rodriguez Araujo et al. 2022). Piperazine-derived ligands could be explored as anthelmintic drugs, and the inhibition of MOD-1 by PZE is a novel mechanism that acts synergically to its classical anthelmintic action as agonist of GABA receptors.
Ion selectivity is of importance since it determines whether receptor activation produces an excitatory or inhibitory response. MOD-1 carries the determinants that govern anion selectivity in Cys-loop receptors, which are located at the pore-forming M2 segment as first described in glycine receptors (Keramidas et al. 2000). In MOD-1, the triple mutant in the pore-forming M2 segment (proline insertion, Ala to Glu substitution at the central ring, and Thr to Val at the hydrophobic ring) converts the selectivity of MOD-1 from anionic to cationic, resulting in a highly K+-selective channel. Moreover, charge reversal at the central ring alone (A270E) is sufficient to convert MOD-1 to cation permeable (Menard et al. 2005). Thus, the main determinants of ion charge selectivity in pLGICs are conserved between vertebrate and invertebrate receptors.
Table 1 summarizes information on the native expression and pharmacology of MOD-1.
Conclusions
The free-living nematode C. elegans has emerged as an organism model for the study of the nervous system and human diseases as well as a model for antiparasitic drug discovery. It is also an attractive platform in the pharmaceutical industry for the search of new therapeutic compounds. Surprisingly, C. elegans has more than 100 different Cys-loop receptor subunit genes, more than double the number present in the human genome. It has the largest and diverse known family of Cys-loop receptors, some of which are only present in invertebrates and others are exclusive of nematodes. Only a limited number of them have been characterized to date, and several remain without a known ligand or function. In addition to multiple cationic nAChRs and GABAA-like receptors, this family includes anionic channels gated by glutamate, ACh, dopamine, and serotonin as well as receptors activated by other ligands not present in vertebrates, such as tyramine and betaine. Thus, C. elegans constitutes an ideal organism to explore the biology and pharmacology of Cys-loop receptors and their potential as targets for novel therapeutic interventions. The understanding of the physiological roles and molecular function of this diverse receptor family is still in its infancy. Future work will allow to identify many new or conserved features of this large and diverse family of receptors.
Data availability
The data that support the findings of this study are available within the article.
References
Abongwa M, Marjanovic DS, Tipton JG, Zheng F, Martin RJ, Trailovic SM, Robertson AP (2018) Monepantel is a non-competitive antagonist of nicotinic acetylcholine receptors from Ascaris suum and Oesophagostomum dentatum. Int J Parasitol Drugs Drug Resist 8(1):36–42. https://doi.org/10.1016/j.ijpddr.2017.12.001
Alexander AG, Marfil V, Li C (2014) Use of Caenorhabditis elegans as a model to study Alzheimers disease and other neurodegenerative diseases. Front Genet 5:279. https://doi.org/10.3389/fgene.2014.00279
Almedom RB, Liewald JF, Hernando G, Schultheis C, Rayes D, Pan J, Schedletzky T, Hutter H, Bouzat C, Gottschalk A (2009) An ER-resident membrane protein complex regulates nicotinic acetylcholine receptor subunit composition at the synapse. EMBO J 28(17):2636–2649. https://doi.org/10.1038/emboj.2009.204
Ballivet M, Alliod C, Bertrand S, Bertrand D (1996) Nicotinic acetylcholine receptors in the nematode Caenorhabditis elegans. J Mol Biol 258(2):261–269. https://doi.org/10.1006/jmbi.1996.0248
Bamber BA, Beg AA, Twyman RE, Jorgensen EM (1999) The Caenorhabditis elegans unc-49 locus encodes multiple subunits of a heteromultimeric GABA receptor. J Neurosci Off J Soc Neurosci 19(13):5348–5359. https://doi.org/10.1523/JNEUROSCI.19-13-05348.1999
Bamber BA, Richmond JE, Otto JF, Jorgensen EM (2005) The composition of the GABA receptor at the Caenorhabditis elegans neuromuscular junction. Br J Pharmacol 144(4):502–509. https://doi.org/10.1038/sj.bjp.0706052
Bartos M, Rayes D, Bouzat C (2006) Molecular determinants of pyrantel selectivity in nicotinic receptors. Mol Pharmacol 70(4):1307–1318. https://doi.org/10.1124/mol.106.026336
Baur R, Beech R, Sigel E, Rufener L (2015) Monepantel irreversibly binds to and opens Haemonchus contortus MPTL-1 and Caenorhabditis elegans ACR-20 receptors. Mol Pharmacol 87(1):96–102. https://doi.org/10.1124/mol.114.095653
Baylis HA, Matsuda K, Squire MD, Fleming JT, Harvey RJ, Darlison MG, Barnard EA, Sattelle DB (1997) ACR-3, a Caenorhabditis elegans nicotinic acetylcholine receptor subunit. Molecular cloning and functional expression. Recept Channels 5(3–4):149–158. http://www.ncbi.nlm.nih.gov/pubmed/9606719
Beech RN, Callanan MK, Rao VTS, Dawe GB, Forrester SG (2013) Characterization of cys-loop receptor genes involved in inhibitory amine neurotransmission in parasitic and free living nematodes. Parasitol Int 62(6):599–605. https://doi.org/10.1016/j.parint.2013.03.010
Beech RN, Neveu C (2015) The evolution of pentameric ligand-gated ion-channels and the changing family of anthelmintic drug targets. Parasitology 142(2):303–317. https://doi.org/10.1017/S003118201400170X
Bennett HM, Lees K, Harper KM, Jones AK, Sattelle DB, Wonnacott S, Wolstenholme AJ (2012) Xenopus laevis RIC-3 enhances the functional expression of the C. elegans homomeric nicotinic receptor, ACR-16, in Xenopus oocytes. J Neurochem 123(6):911–918. https://doi.org/10.1111/jnc.12013
Blanchard A, Guégnard F, Charvet CL, Crisford A, Courtot E, Sauvé C, Harmache A, Duguet T, O’Connor V, Castagnone-Sereno P, Reaves B, Wolstenholme AJ, Beech RN, Holden-Dye L, Neveu C (2018) Deciphering the molecular determinants of cholinergic anthelmintic sensitivity in nematodes: when novel functional validation approaches highlight major differences between the model Caenorhabditis elegans and parasitic species. PLOS Pathog 14(5):e1006996. https://doi.org/10.1371/journal.ppat.1006996
Boulin T, Gielen M, Richmond JE, Williams DC, Paoletti P, Bessereau J-L (2008) Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc Natl Acad Sci 105(47):18590–18595. https://doi.org/10.1073/pnas.0806933105
Bouzat C, Gumilar F, Spitzmaul G, Wang H-L, Rayes D, Hansen SB, Taylor P, Sine SM (2004) Coupling of agonist binding to channel gating in an ACh-binding protein linked to an ion channel. Nature 430(7002):896–900. https://doi.org/10.1038/nature02753
Bouzat C, Mukhtasimova N (2018) The nicotinic acetylcholine receptor as a molecular machine for neuromuscular transmission. Curr Opin Physio 4:40–48. https://doi.org/10.1016/j.cophys.2018.04.008
Bouzat C, Sine SM (2018) Nicotinic acetylcholine receptors at the single-channel level. Br J Pharmacol 175(11):1789–1804. https://doi.org/10.1111/bph.13770
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77(1):71–94. https://doi.org/10.1093/genetics/77.1.71
Brown DDR, Siddiqui SZ, Kaji MD, Forrester SG (2012) Pharmacological characterization of the Haemonchus contortus GABA-gated chloride channel, Hco-UNC-49: Modulation by macrocyclic lactone anthelmintics and a receptor for piperazine. Vet Parasitol 185(2–4):201–209. https://doi.org/10.1016/j.vetpar.2011.10.006
Brown LA, Jones AK, Buckingham SD, Mee CJ, Sattelle DB (2006) Contributions from Caenorhabditis elegans functional genetics to antiparasitic drug target identification and validation: nicotinic acetylcholine receptors, a case study. Int J Parasitol 36(6):617–624. https://doi.org/10.1016/j.ijpara.2006.01.016
Burns AR, Luciani GM, Musso G, Bagg R, Yeo M, Zhang Y, Rajendran L, Glavin J, Hunter R, Redman E, Stasiuk S, Schertzberg M, Angus McQuibban G, Caffrey CR, Cutler SR, Tyers M, Giaever G, Nislow C, Fraser AG, Roy PJ (2015) Caenorhabditis elegans is a useful model for anthelmintic discovery. Nat Commun 6(1):7485. https://doi.org/10.1038/ncomms8485
Campbell WC (2012) History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents. Curr Pharm Biotechnol 13(6):853–865. https://doi.org/10.2174/138920112800399095
Castro MJ, Turani O, Faraoni MB, Gerbino D, Bouzat C (2020) A new antagonist of Caenorhabditis elegans glutamate-activated chloride channels with anthelmintic activity. Front Neurosci 14:879. https://doi.org/10.3389/fnins.2020.00879
Changeux J-P, Taly A (2008) Nicotinic receptors, allosteric proteins and medicine. Trends Mol Med 14(3):93–102. https://doi.org/10.1016/j.molmed.2008.01.001
Chen I-S, Kubo Y (2018) Ivermectin and its target molecules: shared and unique modulation mechanisms of ion channels and receptors by ivermectin. J Physiol 596(10):1833–1845. https://doi.org/10.1113/JP275236
Choudhary N, Khatik GL, Choudhary S, Singh G, Suttee A (2021) In vitro anthelmintic activity of Chenopodium album and in-silico prediction of mechanistic role on Eisenia foetida. Heliyon 7(1):e05917. https://doi.org/10.1016/j.heliyon.2021.e05917
Choudhary S, Kashyap SS, Martin RJ, Robertson AP (2022) Advances in our understanding of nematode ion channels as potential anthelmintic targets. Int J Parasitol Drugs Drug Resist 18:52–86. https://doi.org/10.1016/j.ijpddr.2021.12.001
Chrestia JF, Turani O, Araujo NR, Hernando G, Esandi MDC, Bouzat C (2023) Regulation of nicotinic acetylcholine receptors by post-translational modifications. Pharmacol Res 190:106712. https://doi.org/10.1016/j.phrs.2023.106712
Christensen M, Estevez A, Yin X, Fox R, Morrison R, McDonnell M, Gleason C, Miller DM, Strange K (2002) A primary culture system for functional analysis of C. elegans neurons and muscle cells. Neuron 33(4):503–514. https://doi.org/10.1016/S0896-6273(02)00591-3
Churgin MA, McCloskey RJ, Peters E, Fang-Yen C (2017) Antagonistic serotonergic and octopaminergic neural circuits mediate food-dependent locomotory behavior in Caenorhabditis elegans. J Neurosci 37(33):7811–7823. https://doi.org/10.1523/JNEUROSCI.2636-16.2017
Cochrane E, Foster J, Khatami MH, de Haan HW, Forrester SG (2022) Characterization of adjacent charged residues near the agonist binding site of the nematode UNC-49 GABA receptor. Mol Biochem Parasitol 252:111521. https://doi.org/10.1016/j.molbiopara.2022.111521
Corradi J, Bouzat C (2014) Unraveling mechanisms underlying partial agonism in 5-HT3A receptors. J Neurosci. 34(50):16865–16876. https://doi.org/10.1523/JNEUROSCI.1970-14.2014
Corsi AK, Wightman B, Chalfie M (2015) A transparent window into biology: a primer on Caenorhabditis elegans. Genetics 200(2):387–407. https://doi.org/10.1534/genetics.115.176099
Crisford A, Calahorro F, Ludlow E, Marvin JMC, Hibbard JK, Lilley CJ, Kearn J, Keefe F, Johnson P, Harmer R, Urwin PE, O’Connor V, Holden-Dye L (2020) Identification and characterisation of serotonin signalling in the potato cyst nematode Globodera pallida reveals new targets for crop protection. PLOS Pathogens 16(10):e1008884. https://doi.org/10.1371/journal.ppat.1008884
Culetto E, Baylis HA, Richmond JE, Jones AK, Fleming JT, Squire MD, Lewis JA, Sattelle DB (2004) The Caenorhabditis elegans unc-63 gene encodes a levamisole-sensitive nicotinic acetylcholine receptor α subunit. J Biol Chem 279(41):42476–42483. https://doi.org/10.1074/jbc.M404370200
Culetto E, Sattelle DB (2000) A role for Caenorhabditis elegans in understanding the function and interactions of human disease genes. Hum Mol Genet 9(6):869–877. https://doi.org/10.1093/hmg/9.6.869
Cully DF, Paress PS, Liu KK, Schaeffer JM, Arena JP (1996) Identification of a Drosophila melanogaster glutamate-gated chloride channel sensitive to the antiparasitic agent avermectin. J Biol Chem 271(33):20187–20191. https://doi.org/10.1074/jbc.271.33.20187
Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LHT, Schaeffer JM, Arena JP (1994) Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature 371(6499):707–711. https://doi.org/10.1038/371707a0
Degani-Katzav N, Gortler R, Gorodetzki L, Paas Y (2016) Subunit stoichiometry and arrangement in a heteromeric glutamate-gated chloride channel. Proc Natl Acad Sci 113(5):E644-E653. https://doi.org/10.1073/pnas.1423753113
Dent JA (2006) Evidence for a diverse Cys-loop ligand-gated ion channel superfamily in early bilateria. J Mol Evol 62(5):523–535. https://doi.org/10.1007/s00239-005-0018-2
Dent JA, Davis MW, Avery L (1997) avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. EMBO J 16(19):5867–5879. https://doi.org/10.1093/emboj/16.19.5867
Dent JA, Smith MM, Vassilatis DK, Avery L (2000) The genetics of ivermectin resistance in Caenorhabditis elegans. Proc Natl Acad Sci 97(6):2674–2679. https://doi.org/10.1073/pnas.97.6.2674
Dent LA (2010) Murine nematode immunology in Australasia. Parasite Immunol 32(8):560–571. https://doi.org/10.1111/j.1365-3024.2010.01233.x
Engel AG, Shen X-M, Selcen D, Sine SM (2015) Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol 14(5):461. https://doi.org/10.1016/S1474-4422(15)00010-1
Etter A, Cully DF, Liu KK, Reiss B, Vassilatis DK, Schaeffer JM, Arena JP (1999) Picrotoxin blockade of invertebrate glutamate-gated chloride channels: subunit dependence and evidence for binding within the pore. J Neurochem 72(1):318–326. https://doi.org/10.1111/jnc.1999.72.1.318
Fleming JT, Squire MD, Barnes TM, Tornoe C, Matsuda K, Ahnn J, Fire A, Sulston JE, Barnard EA, Sattelle DB, Lewis JA (1997) Caenorhabditis elegans levamisole resistance genes lev-1, unc-29, and unc-38 encode functional nicotinic acetylcholine receptor subunits. J Neurosci Off J Soc Neurosci 17(15):5843–5857. https://doi.org/10.1523/JNEUROSCI.17-15-05843.1997
Francis MM, Evans SP, Jensen M, Madsen DM, Mancuso J, Norman KR, Maricq AV (2005) The Ror receptor tyrosine kinase CAM-1 is required for acr-16-mediated synaptic transmission at the c. elegans neuromuscular junction. Neuron 46(4):581–594. https://doi.org/10.1016/j.neuron.2005.04.010
Gharpure A, Teng J, Zhuang Y, Noviello CM, Walsh RM, Cabuco R, Howard RJ, Zaveri NT, Lindahl E, Hibbs RE (2019) Agonist selectivity and ion permeation in the α3β4 ganglionic nicotinic receptor. Neuron 104(3):501-511.e6. https://doi.org/10.1016/j.neuron.2019.07.030
Gibbs E, Chakrapani S (2021) Structure, function and physiology of 5-hydroxytryptamine receptors subtype 3. Subcell Biochem 96:373–408. https://doi.org/10.1007/978-3-030-58971-4_11
Glendinning SK, Buckingham SD, Sattelle DB, Wonnacott S, Wolstenholme AJ (2011) Glutamate-gated chloride channels of Haemonchus contortus restore drug sensitivity to ivermectin resistant Caenorhabditis elegans. PLoS ONE 6(7):e22390. https://doi.org/10.1371/journal.pone.0022390
Halevi S (2002) The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J 21(5):1012–1020. https://doi.org/10.1093/emboj/21.5.1012
Hansen TVA, Sager H, Toutain CE, Courtot E, Neveu C, Charvet CL (2022) The Caenorhabditis elegans DEG-3/DES-2 channel is a betaine-gated receptor insensitive to monepantel. Molecules 27(1):312. https://doi.org/10.3390/molecules27010312
Hardege I, Morud J, Courtney A, Schafer WR (2023) A novel and functionally diverse class of acetylcholine-gated ion channels. J Neurosci 43(7):1111–1124. https://doi.org/10.1523/JNEUROSCI.1516-22.2022
Hardege I, Morud J, Yu J, Wilson TS, Schroeder FC, Schafer WR (2022) Neuronally produced betaine acts via a ligand-gated ion channel to control behavioral states. Proc Natl Acad Sci 119(48):e2201783119. https://doi.org/10.1073/pnas.2201783119
Hernando G, Bergé I, Rayes D, Bouzat C (2012) Contribution of subunits to Caenorhabditis elegans levamisole-sensitive nicotinic receptor function. Mol Pharmacol 82(3):550–560. https://doi.org/10.1124/mol.112.079962
Hernando G, Bouzat C (2014) Caenorhabditis elegans neuromuscular junction: GABA receptors and ivermectin action. PLoS ONE 9(4):e95072. https://doi.org/10.1371/journal.pone.0095072
Hernando G, Turani O, Bouzat C (2019) Caenorhabditis elegans muscle Cys-loop receptors as novel targets of terpenoids with potential anthelmintic activity. PLOS Negl Trop Dis 13(11):e0007895. https://doi.org/10.1371/journal.pntd.0007895
Hibbs RE, Gouaux E (2011) Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474(7349):54–60. https://doi.org/10.1038/nature10139
Hobert O (2013) The neuronal genome of Caenorhabditis elegans. WormBook, ed. The C. elegans Research Community, WormBook. https://doi.org/10.1895/wormbook.1.161.1
Holden-Dye L, Krogsgaard-Larsen P, Nielsen L, Walker RJ (1989) GABA receptors on the somatic muscle cells of the parasitic nematode, Ascaris suum: stereoselectivity indicates similarity to a GABAA-type agonist recognition site. Br J Pharmacol 98(3):841–850. https://doi.org/10.1111/j.1476-5381.1989.tb14613.x
Holden-Dye L, Walker RJ (2007) Anthelmintic drugs. WormBook: The Online Review of C. Elegans Biology WormBook, ed. The C. elegans Research Community, WormBook. https://doi.org/10.1895/wormbook.1.143.1
Holden-Dye L, Walker RJ (2014) Anthelmintic drugs and nematicides: studies in Caenorhabditis elegans. WormBook, ed. The C. elegans Research Community, WormBook, https://doi.org/10.1895/wormbook.1.143.2
Horoszok L, Raymond V, Sattelle DB, Wolstenholme AJ (2001) GLC-3: a novel fipronil and BIDN-sensitive, but picrotoxinin-insensitive, L-glutamate-gated chloride channel subunit from Caenorhabditis elegans. Br J Pharmacol 132(6):1247–1254. https://doi.org/10.1038/sj.bjp.0703937
Jaiteh M, Taly A, Hénin J (2016) Evolution of pentameric ligand-gated ion channels: pro-loop receptors. PloS One 11(3):e0151934. https://doi.org/10.1371/journal.pone.0151934
Jones AK, Davis P, Hodgkin J, Sattelle DB (2007) The nicotinic acetylcholine receptor gene family of the nematode Caenorhabditis elegans: an update on nomenclature. Invertebr Neurosci 7(2):129–131. https://doi.org/10.1007/s10158-007-0049-z
Jones AK, Rayes D, Al-Diwani A, Maynard TPR, Jones R, Hernando G, Buckingham SD, Bouzat C, Sattelle DB (2011) A Cys-loop mutation in the caenorhabditis elegans nicotinic receptor subunit UNC-63 impairs but does not abolish channel function. J Biol Chem 286(4):2550–2558. https://doi.org/10.1074/jbc.M110.177238
Jones AK, Sattelle DB (2004) Functional genomics of the nicotinic acetylcholine receptor gene family of the nematode, Caenorhabditis elegans. Bioessays 26(1):39–49. https://doi.org/10.1002/bies.10377
Jones AK, Sattelle DB (2008) The Cys-loop ligand-gated ion channel gene superfamily of the nematode, Caenorhabditis elegans. Invert Neurosci 8(1):41–47. https://doi.org/10.1007/s10158-008-0068-4
Jospin M, Qi YB, Stawicki TM, Boulin T, Schuske KR, Horvitz HR, Bessereau J-L, Jorgensen EM, Jin Y (2009) A neuronal acetylcholine receptor regulates the balance of muscle excitation and inhibition in Caenorhabditis elegans. PLoS Biol 7(12):e1000265. https://doi.org/10.1371/journal.pbio.1000265
Kalamida D, Poulas K, Avramopoulou V, Fostieri E, Lagoumintzis G, Lazaridis K, Sideri A, Zouridakis M, Tzartos SJ (2007) Muscle and neuronal nicotinic acetylcholine receptors. FEBS J 274(15):3799–3845. https://doi.org/10.1111/j.1742-4658.2007.05935.x
Kaletta T, Hengartner MO (2006) Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov 5(5):387–399. https://doi.org/10.1038/nrd2031
Keramidas A, Moorhouse AJ, French CR, Schofield PR, Barry PH (2000) M2 pore mutations convert the glycine receptor channel from being anion- to cation-selective. Biophys J 79(1):247–259. https://doi.org/10.1016/S0006-3495(00)76287-4
Kim W, Underwood RS, Greenwald I, Shaye DD (2018) OrthoList 2: a new comparative genomic analysis of human and Caenorhabditis elegans genes. Genetics 210(2):445–461. https://doi.org/10.1534/genetics.118.301307
Lamassiaude N, Courtot E, Corset A, Charvet CL, Neveu C (2022) Pharmacological characterization of novel heteromeric GluCl subtypes from Caenorhabditis elegans and parasitic nematodes. Br J Pharmacol 179(6):1264–1279. https://doi.org/10.1111/bph.15703
Lasala M, Fabiani C, Corradi J, Antollini S, Bouzat C (2019) Molecular modulation of human α7 nicotinic receptor by amyloid-β peptides. Front Cell Neurosci 13:37. https://doi.org/10.3389/fncel.2019.00037
Lees K, Sluder A, Shannan N, Hammerland L, Sattelle D (2012) Ligand-gated ion channels as targets for anthelmintic drugs: past, current, and future perspectives. In Parasitic Helminths (pp 1–21). Wiley-VCH Verlag GmbH and Co. KGaA. https://doi.org/10.1002/9783527652969.ch1
Lewis JA, Wu CH, Berg H, Levine JH (1980) The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics 95(4):905–928. https://doi.org/10.1093/genetics/95.4.905
Li P, Slimko EM, Lester HA (2002) Selective elimination of glutamate activation and introduction of fluorescent proteins into a Caenorhabditis elegans chloride channel. FEBS Lett 528(1–3):77–82. https://doi.org/10.1016/S0014-5793(02)03245-3
Luo Y, Balle T (2022) GABA A receptors as targets for anaesthetics and analgesics and promising candidates to help treat coronavirus infections: a mini-review. Basic Clin Pharmacol Toxicol 131(6):443–451. https://doi.org/10.1111/bcpt.13798
Lynagh T, Pless SA (2014) Principles of agonist recognition in Cys-loop receptors. Front Physiol 5:160. https://doi.org/10.3389/fphys.2014.00160
Lynch JW (2009) Native glycine receptor subtypes and their physiological roles. Neuropharmacology 56(1):303–309. https://doi.org/10.1016/j.neuropharm.2008.07.034
Martin RJ (1985) γ-Aminobutyric acid- and piperazine-activated single-channel currents from Ascaris suum body muscle. Br J Pharmacol 84(2):445–461. https://doi.org/10.1111/j.1476-5381.1985.tb12929.x
Martin RJ, Robertson AP, Bjorn H (1997) Target sites of anthelmintics. Parasitology 114 Suppl:S111–24. http://www.ncbi.nlm.nih.gov/pubmed/9309773
Mclntire SL, Jorgensen E, Kaplan J, Horvitz HR (1993) The GABAergic nervous system of Caenorhabditis elegans. Nature 364(6435):337–341. https://doi.org/10.1038/364337a0
Menard C, Horvitz HR, Cannon S (2005) Chimeric mutations in the M2 segment of the 5-hydroxytryptamine-gated chloride channel MOD-1 define a minimal determinant of anion/cation permeability. J Biol Chem 280(30):27502–27507. https://doi.org/10.1074/jbc.M501624200
Mongan NP, Baylis HA, Adcock C, Smith GR, Sansom MS, Sattelle DB (1998) An extensive and diverse gene family of nicotinic acetylcholine receptor alpha subunits in Caenorhabditis elegans. Receptors Channels 6(Issue 3):213–228
Morales-Perez CL, Noviello CM, Hibbs RE (2016) Manipulation of subunit stoichiometry in heteromeric membrane proteins. Structure 24(5):797–805. https://doi.org/10.1016/j.str.2016.03.004
Morud J, Hardege I, Liu H, Wu T, Choi M-K, Basu S, Zhang Y, Schafer WR (2021) Deorphanization of novel biogenic amine-gated ion channels identifies a new serotonin receptor for learning. Curr Biol 31(19):4282-4292.e6. https://doi.org/10.1016/j.cub.2021.07.036
Mu T-W, Lester HA, Dougherty DA (2003) Different binding orientations for the same agonist at homologous receptors: a lock and key or a simple wedge? J Am Chem Soc 125(23):6850–6851. https://doi.org/10.1021/ja0348086
Noonan JD, Beech RN (2022) Reconstitution of an N-AChR from Brugia malayi, an evolved change in acetylcholine receptor accessory protein requirements in filarial parasites. PLOS Pathog 18(11):e1010962. https://doi.org/10.1371/journal.ppat.1010962
Noviello CM, Gharpure A, Mukhtasimova N, Cabuco R, Baxter L, Borek D, Sine SM, Hibbs RE (2021) Structure and gating mechanism of the α7 nicotinic acetylcholine receptor. Cell 184(8):2121-2134.e13. https://doi.org/10.1016/j.cell.2021.02.049
Ortells M (2016) Structure and function development during evolution of pentameric ligand gated ion channels. Neurotransmitter 3:e1273. https://doi.org/10.14800/nt.1273
Peden AS, Mac P, Fei YJ, Castro C, Jiang G, Murfitt KJ, Miska EA, Griffin JL, Ganapathy V, Jorgensen EM (2013) Betaine acts on a ligand-gated ion channel in the nervous system of the nematode C. elegans. Nat Neurosci 16(12):1794–1801. https://doi.org/10.1038/NN.3575
Petrash HA, Philbrook A, Haburcak M, Barbagallo B, Francis MM (2013) ACR-12 ionotropic acetylcholine receptor complexes regulate inhibitory motor neuron activity in Caenorhabditis elegans. J Neurosci 33(13):5524–5532. https://doi.org/10.1523/JNEUROSCI.4384-12.2013
Petzold BC, Park S-J, Ponce P, Roozeboom C, Powell C, Goodman MB, Pruitt BL (2011) Caenorhabditis elegans body mechanics are regulated by body wall muscle tone. Biophys J 100(8):1977–1985. https://doi.org/10.1016/j.bpj.2011.02.035
Pirri JK, McPherson AD, Donnelly JL, Francis MM, Alkema MJ (2009) A tyramine-gated chloride channel coordinates distinct motor programs of a Caenorhabditis elegans escape response. Neuron 62(4):526–538. https://doi.org/10.1016/j.neuron.2009.04.013
Putrenko I, Zakikhani M, Dent JA (2005) A family of acetylcholine-gated chloride channel subunits in Caenorhabditis elegans. J Biol Chem 280(8):6392–6398. https://doi.org/10.1074/jbc.M412644200
Qian H, Robertson AP, Powell-Coffman JA, Martin RJ (2008) Levamisole resistance resolved at the single-channel level in Caenorhabditis elegans. FASEB J 22(9):3247–3254. https://doi.org/10.1096/fj.08-110502
Rand JB (2007) Acetylcholine. WormBook, ed. The C. elegans Research Community, WormBook, https://doi.org/10.1895/wormbook.1.131.1
Ranganathan R, Cannon SC, Horvitz HR (2000) MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature 408(6811):470–475. https://doi.org/10.1038/35044083
Rayes D, De Rosa MJ, Bartos M, Bouzat C (2004) Molecular basis of the differential sensitivity of nematode and mammalian muscle to the anthelmintic agent levamisole. J Biol Chem 279(35):36372–36381. https://doi.org/10.1074/jbc.M403096200
Rayes D, Flamini M, Hernando G, Bouzat C (2007) Activation of single nicotinic receptor channels from Caenorhabditis elegans muscle. Mol Pharmacol 71(5):1407–1415. https://doi.org/10.1124/mol.106.033514
Raymond V, Mongan NP, Sattelle DB (2000) Anthelmintic actions on homomer-forming nicotinic acetylcholine receptor subunits: chicken α7 and ACR-16 from the nematode Caenorhabditis elegans. Neuroscience 101(3):785–791. https://doi.org/10.1016/S0306-4522(00)00279-7
Richmond JE, Jorgensen EM (1999) One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat Neurosci 2(9):791–797. https://doi.org/10.1038/12160
Ringstad N, Abe N, Horvitz HR (2009) Ligand-gated chloride channels are receptors for biogenic amines in C. elegans. Science 325(5936):96–100. https://doi.org/10.1126/science.1169243
Rodriguez Araujo N, Hernando G, Corradi J, Bouzat C (2022) The nematode serotonin-gated chloride channel MOD-1: a novel target for anthelmintic therapy. J Biol Chem 298(9):102356. https://doi.org/10.1016/j.jbc.2022.102356
Rufener L, Bedoni N, Baur R, Rey S, Glauser DA, Bouvier J, Beech R, Sigel E, Puoti A (2013) acr-23 encodes a monepantel-sensitive channel in Caenorhabditis elegans. PLoS Pathogens 9(8):e1003524. https://doi.org/10.1371/journal.ppat.1003524
Sallard E, Letourneur D, Legendre P (2021) Electrophysiology of ionotropic GABA receptors. Cell Mol Life Sci 78(13):5341–5370. https://doi.org/10.1007/s00018-021-03846-2
San Martín VP, Sazo A, Utreras E, Moraga-Cid G, Yévenes GE (2022) Glycine receptor subtypes and their roles in nociception and chronic Pain. Front Mol Neurosci 15:848642. https://doi.org/10.3389/fnmol.2022.848642
Sepúlveda-Crespo D, Reguera RM, Rojo-Vázquez F, Balaña-Fouce R, Martínez-Valladares M (2020) Drug discovery technologies: Caenorhabditis elegans as a model for anthelmintic therapeutics. Med Res Rev 40(5):1715–1753. https://doi.org/10.1002/med.21668
Shaye DD, Greenwald I (2011) OrthoList: a compendium of C. elegans genes with human orthologs. PLoS ONE 6(5):e20085. https://doi.org/10.1371/journal.pone.0020085
Siddiqui SZ, Brown DDR, Rao VTS, Forrester SG (2010) An UNC-49 GABA receptor subunit from the parasitic nematode Haemonchus contortus is associated with enhanced GABA sensitivity in nematode heteromeric channels. J Neurochem 113(5):1113–1122. https://doi.org/10.1111/J.1471-4159.2010.06651.X
Sigel E, Steinmann ME (2012) Structure, function, and modulation of GABAA receptors. J Biol Chem 287(48):40224–40231. https://doi.org/10.1074/jbc.R112.386664
Squire MD, Tornøe C, Baylis HA, Fleming JT, Barnard EA, Sattelle DB (1995) Molecular cloning and functional co-expression of a Caenorhabditis elegans nicotinic acetylcholine receptor subunit (acr-2). Receptors Channels 3(2):107–115. http://www.ncbi.nlm.nih.gov/pubmed/8581398
Sulston JE, Horvitz HR (1977) Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 56(1):110–156. https://doi.org/10.1016/0012-1606(77)90158-0
Tasneem A, Iyer LM, Jakobsson E, Aravind L (2005) Identification of the prokaryotic ligand-gated ion channels and their implications for the mechanisms and origins of animal Cys-loop ion channels. Genome Biol 6(1):R4
Touroutine D, Fox RM, Von Stetina SE, Burdina A, Miller DM, Richmond JE (2005) acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. J Biol Chem 280(29):27013–27021. https://doi.org/10.1074/jbc.M502818200
Towers PR, Edwards B, Richmond JE, Sattelle DB (2005) The Caenorhabditis elegans lev-8 gene encodes a novel type of nicotinic acetylcholine receptor alpha subunit. J Neurochem 93(1):1–9. https://doi.org/10.1111/j.1471-4159.2004.02951.x
Treinin M, Chalfie M (1995) A mutated acetylcholine receptor subunit causes neuronal degeneration in C. elegans. Neuron 14(4):871–877. https://doi.org/10.1016/0896-6273(95)90231-7
Treinin M, Gillo B, Liebman L, Chalfie M (1998) Two functionally dependent acetylcholine subunits are encoded in a single Caenorhabditis elegans operon. Proc Natl Acad Sci 95(26):15492–15495. https://doi.org/10.1073/pnas.95.26.15492
Treinin M, Jin Y (2021) Cholinergic transmission in C. elegans: functions, diversity, and maturation of ACh-activated ion channels. J Neurochem 158(6):1274–1291. https://doi.org/10.1111/jnc.15164
Turani O, Hernando G, Corradi J, Bouzat C (2018) Activation of Caenorhabditis elegans levamisole-sensitive and mammalian nicotinic receptors by the antiparasitic bephenium. Mol Pharmacol 94(5):1270–1279. https://doi.org/10.1124/mol.118.113357
Vassilatis DK, Elliston KO, Paress PS, Hamelin M, Arena JP, Schaeffer JM, Van der Ploeg LHT, Cully DF (1997) Evolutionary relationship of the ligand-gated ion channels and the avermectin-sensitive, glutamate-gated chloride channels. J Mol Evol 44(5):501–508. https://doi.org/10.1007/PL00006174
Wang M, Witvliet D, Wu M, Kang L, Shao Z (2021) Temperature regulates synaptic subcellular specificity mediated by inhibitory glutamate signaling. PLOS Genetics 17(1):e1009295. https://doi.org/10.1371/journal.pgen.1009295
Yassin L, Gillo B, Kahan T, Halevi S, Eshel M, Treinin M (2001) Characterization of the DEG-3/DES-2 receptor: a nicotinic acetylcholine receptor that mutates to cause neuronal degeneration. Mol Cell Neurosci 17(3):589–599. https://doi.org/10.1006/mcne.2000.0944
Yates DM, Wolstenholme AJ (2004) An ivermectin-sensitive glutamate-gated chloride channel subunit from Dirofilaria immitis. Int J Parasitol 34(9):1075–1081. https://doi.org/10.1016/j.ijpara.2004.04.010
Yuan A, Santi CM, Wei A, Wang Z-W, Pollak K, Nonet M, Kaczmarek L, Crowder CM, Salkoff L (2003) The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron 37(5):765–773. https://doi.org/10.1016/S0896-6273(03)00096-5
Zhang S, Banerjee D, Kuhn JR (2011) Isolation and culture of larval cells from C. elegans. PLoS ONE 6(4):e19505. https://doi.org/10.1371/journal.pone.0019505
Zoli M, Pistillo F, Gotti C (2015) Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology 96(PB):302–311. https://doi.org/10.1016/j.neuropharm.2014.11.003
Acknowledgements
This work was supported by grants from Universidad Nacional del Sur (PGI 24/B298 to CB), Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación, Argentina (PICT 2017-1170 and PICT 2020-00936 to CB; PICT 2019 01751 to GH), and Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina (CONICET, PIP11220200102356). We thank the Caenorhabditis Genetics Center (CGC), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440), and WormBase.
Funding
This work was supported by grants from Universidad Nacional del Sur (PGI 24/B298 to CB), Agencia Nacional de Promoción Científica y Tecnológica (PICT 2017–1170 and PICT 2020–00936 to CB; PICT 2019 01751 to GH), and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP11220200102356).
Author information
Authors and Affiliations
Contributions
CB, GH, OT, and NRA wrote the paper.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hernando, G., Turani, O., Rodriguez Araujo, N. et al. The diverse family of Cys-loop receptors in Caenorhabditis elegans: insights from electrophysiological studies. Biophys Rev 15, 733–750 (2023). https://doi.org/10.1007/s12551-023-01080-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-023-01080-7