Abstract
This study aimed to establish a high-performance liquid chromatography (HPLC) method to investigate the residues of patulin in apples, hawthorns, and their products. A total of 400 samples were collected from online shopping plats and supermarkets in China, including apples (n = 50), hawthorns (n = 50), and their products (apple juice, apple puree, apple jam, hawthorn juice, hawthorn chips, and hawthorn rolls, n = 300). In this experiment, this method had good linearity and a recovery of 82.3–94.4% for patulin. The limit of detection (LOD) was 0.2 µg/kg for liquid samples, while it was 0.3 µg/kg for solid and semi-fluid samples. The frequencies of patulin were 79.8% in 400 samples, and the patulin concentration is from 0.6 to 126.0 µg/kg. Two samples (0.5%) for patulin exceeded the regulatory limit (50 µg/kg) in 400 samples. The frequencies of patulin in kinds of samples were 32.0–98.0% (p < 0.05), and the percentage of samples exceeding the limit was not more than 2.0%. The frequencies of patulin in domestic samples were 83.0%, while they were 57.7% in imported samples. Two domestic samples (0.6%) contained patulin above the regulatory limit, while none of the imported samples exceeded the limit. Among the online and offline samples, the frequencies of patulin were 76.4 and 82.1%. Two online samples (1.0%) for patulin exceeded the regulatory limit, whereas none of the offline samples exceeded the limit. These results showed it is important to monitor regularly the content of patulin in apples, hawthorns, and their products to ensure consumer food safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apples and hawthorns are fruits with affluent nutritional value (Agriopoulou et al. 2020). Many of these fruits are processed into juice, cans, preserves, and other products. In daily life, individuals purchase these foods frequently from supermarkets, convenience stores, or online shopping platforms. With the increasing consumption of various fruits and their products, it is essential to consider their safety and the nutrients they provide for human health. Nevertheless, people only consider the freshness, shelf life, and presence of food additives while purchasing these foods (Alshannaq and Yu 2017). In recent years, food contaminant on food safety research has gradually brought into our view, which are mycotoxins (Aslam et al. 2021; Hassan et al. 2020; Ji et al. 2017; Sewram et al. 2000).
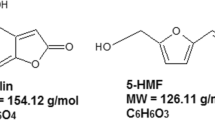
Mycotoxins are secondary metabolites produced by specific filamentous fungi under proper temperature and humidity conditions (Agriopoulou et al. 2020; Misihairabgwi et al. 2019; Xue and Zhang 2013). Common mycotoxins include aflatoxin, patulin, fumonisin, and so on (Nan et al. 2022). Patulin (Fig. 1), a heterocyclic unsaturated lactone, is one of the most vital mycotoxins, which is produced by Aspergillus spp, Penicillium spp, and Mycobacterium spp (Agriopoulou et al. 2020; Balázs and Schepers 2007). Patulin, as a fat-soluble molecule, has a strong ability to diffuse into the surrounding healthy pulp tissue (Mahato et al. 2021). Patulin is stable and resistant in high temperature and acidic condition. It can cause toxicity in vital organs, induce apoptosis, and lead to cytotoxicity by reactive oxygen species and endoplasmic reticulum stress (Assunção et al. 2016; Kumar et al. 2016; Sohrabi et al. 2021). Clinically, patulin has been associated with neurotoxicity, immunotoxicity, genotoxicity, and teratogenicity, as well as various acute and chronic diseases. Acute symptoms include convulsion, dyspnea, emesis, edema, ulcer, pulmonary congestion, gastrointestinal dilatation, intestinal bleeding, and kidney injury (Bacha et al. 2023). The International Agency for Research on Cancer (IARC) classifies patulin as category 3 (not carcinogenic to humans) (Malir et al. 2023; Ramalingam et al. 2019). Patulin mainly contaminates apples, hawthorns, and their products. It has been extensively studied in fruits and their processed products (Lien et al. 2020). When products such as fruit juice are produced from rotten fruit, it is difficult to eliminate patulin contamination through conventional food processing due to its stable chemical properties (Mahato et al. 2021; Saleh and Goktepe 2019). Therefore, patulin does not disappear in product processing but get into fruit products. It is essential to monitor and prevent the presence of patulin in food products. The maximum allowable level of patulin in food is set as 50 µg/kg in the national standard (China 2017), and the maximum short-term acceptable dietary intake (PMTDI) of patulin set by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) is 0.40 µg/kg body weight/day.
The research aims to study the current situation of patulin contained in foods to which consumers are exposed in daily life contaminated by patulin and help consumers understand the risks. A mounting number of researchers pay attention to patulin in foods and carry out more related research to promote food safety and protect human health (Cunha et al. 2014; Fujita et al. 2019). In this study, an HPLC method with high sensitivity, good repeatability, and high accuracy was established to determine the content of patulin. This study analyzed the levels of patulin in apples, hawthorns, and their products collected from online and offline sample points. The research results can provide a foundation for strengthening market supervision and reducing the possibility of food safety incidents for consumers.
Materials and methods
Materials and instruments
The standard of patulin (purity ≥ 99%) was purchased from Keli Technology Development Co Guangzhou Testing Center. Standard reference material for patulin quality control sample in apple juice (BWS0068-2016) was provided by Beijing Beiye Metrology Technology Research Institute (Beijing, China). Methanol, acetonitrile, and acetic acid were purchased from Acmes Biochemical Co., Ltd (Shanghai, China). Ethyl acetate was purchased from Macklin Biochemical CO., Ltd (Shanghai, China). Tetrahydrofuran was purchased from Tianjin Damao Chemical Reagent Factory (Tianjin, China). All reagents were chromatographic grade. Pectinase liquid was purchased from Fan Hong Trading Co., Ltd (Guangzhou, China).
Instruments
HPLC (Shimadzu LC-20AT, Japan) with diode array detection (DAD), T3 column (150 × 4.6 mm, 5 µm, Waters, The USA), C18 column (150 × 4.6 mm, 5 µm, Elite, The USA), C18 column (250 × 4.6 mm, 5 µm, Shim-pack VP-ODS C18, Shimadzu, Japan), Rotary evaporator (Yamato SINCE1889, Japan), Centrifuge (Eppendorf 5430R, Germany), and Intelligent homogenizer (NAI-ZNJJY-D, Shanghai, China). MycosepTM228 solid-phase purification column was purchased from Beijing Cluster Technology Co., Ltd. (Beijing, China).
Sample collection
A total of 400 samples were collected in online shopping platforms of China, and supermarkets in Jinan, Shandong Province, including 50 apples, 50 apple juice, 50 apple puree, 50 apple jam, 50 hawthorns, 50 hawthorn juice, 50 hawthorn chips, and 50 hawthorn rolls. They contained domestic and imported (from South Korea, Greece, Russia, Thailand, Spain, Japan, USA, and Thailand) products sold in China. They were kept in the refrigerator so that we can have a further analysis. The purchased apple and hawthorn samples were chosen free from debris, washed with water, shade-dried, and cut into small pieces with a sharp knife. A sample of about 100 g sample was thoroughly homogenized by an intelligent homogenizer. All samples of apple and hawthorn products were directly taken from original stored packages to homogenize by an intelligent homogenizer.
Sample preparation
Liquid samples
The homogenized sample was accurately weighed at 4 g (with an accuracy of 0.0001 g) and dropped into a 50-mL centrifuge tube. Then, 21 mL acetonitrile was added into the centrifuge tube and mixed thoroughly. The mixture was centrifuged at 4548 × g for 5 min. The next step is to purify it.
Solid and semi-fluid samples
The homogenized sample was accurately weighed at 1 g (with an accuracy of 0.0001 g), dropped in a 50-mL centrifuge tube, and mixed with 10 mL water and 150 µL pectinase. Then, the sample was placed all night long away from light at room temperature; after that, 10 mL ethyl acetate was added and well vortexed for 5 min. The mixture was centrifuged at 4548 × g for 5 min. Then, the ethyl acetate layer was removed to a conical flask. Ten milliliter of ethyl acetate was added again in order to extract further, and two ethyl acetate extracts were combined. The extract was concentrated to dry in a 40 °C water bath with a rotary evaporation apparatus. The residue was dissolved in 2 mL of 4% acetic acid solution, and then 8 mL acetonitrile was added to the mix. The next step is to purify.
Purification of samples
According to the instructions of the purification column, the extracted liquid prepared above was purified. The 1 mL initial purification solution was discarded, and the subsequent parts were collected. Then, a 3 mL purification solution was accurately absorbed with 20 µL 4% acetic acid added. The mixture of solution was nearly to be dry by blowing slowly with nitrogen at 40 °C. Then, the 4% acetic acid solution was added into the mixture solution to 1 mL at constant volume. After that, mix it for 30 s. Finally, the filtrate was collected in the injection bottle through a 0.22 µm filter membrane. The blank test was done in the same way.
Chromatographic conditions
In this study, the HPLC method was established for the determination of patulin in apples, hawthorns, and their products. The patulin separation was conducted by Shim-pack VP-ODS C18 column (250 × 4.6 mm, 5 µm) and a mixture of acetonitrile (A) and 0.8% tetrahydrofuran (B) as mobile phase at a flow rate of 0.8 mL/min with DAD detector. The gradient elution conditions were 95%B (0–11 min), 0%B (12 min), and 95%B (13–26.5 min). The column temperature was 40 °C. The wavelength was 276 nm, and the injection volume was 100 µL.
Method validation
We validated this method for five parts: linearity, LOD, the limit of quantification (LOQ), precision, and recovery. Linearity was assessed by analyzing standard solutions ranging from 1 to 100 ng/mL. The standard curve was established with the concentration of standard solution as the abscissa and the peak area as the ordinate. LOD and LOQ were determined by analyzing and calculating the baseline noise value and mean deviation from 12 consecutive measurements in blank solutions. Precision was evaluated by analyzing samples 6 times in the same method. Recovery was assessed by spiking samples with patulin standard solution at three levels (liquid samples: 20 µg/kg, 25 µg/kg, and 30 µg/kg; solid and semi-fluid samples: 80 µg/kg, 100 µg/kg, and 120 µg/kg), and each experiment was carried out in triplicate. We tested the spiked blank matrix sample after testing 10 samples to ensure the accuracy of the experiment. Meanwhile, the instrument method validation was examined by the standard reference material for the patulin quality control sample in apple juice (BWS0068-2016).
Statistical analysis
Samples with a concentration of patulin higher than the LOD were considered positive. For samples with a concentration below the LOD, a value of 1/2 LOD was considered for calculating the mean. The comparison of detection rate was analyzed by the chi-square test. The comparison of patulin content was analyzed by the variance test. When the variances were equal, one-way ANOVA was used for comparison between multiple groups, and the LSD test was used for pair-wise comparison. The Welch analysis of variance and the Games-Howell test were used when the variances were unequal. The analysis software was IBM SPSS27.0 (International Business Machines Corporation, America). Statistical significance was taken into consideration when the p value was < 0.05.
Results and discussion
Chromatographic condition establishment
According to the characteristics of patulin, it was determined that the liquid chromatographic detector for the determination of patulin was a DAD with a detection wavelength of 276 nm. Patulin solution was used as the standard to explore the chromatographic conditions:
The chromatographic column was Waters T3 (150 × 4.6 mm, 5 µm), with a column temperature of 40 °C, a flow rate of 0.8 mL/min, and a sample size of 100 µL. Acetonitrile and water were selected as the mobile phase, with equal elution (acetonitrile: water = 5:95). The chromatographic peak shape of the patulin standard solution was symmetrical, and the baseline was stable. However, different matrix components eluted together with patulin in samples. Gradually increasing in acetonitrile ratio resulted in the advance of patulin retention time. Meanwhile, the peak shape is better than before. Nevertheless, the patulin still eluted with impurities in samples. Attempting different gradient elution resulted in a tailing chromatographic peak for the standard solution. Therefore, we considered replacing the column.
The chromatographic column was replaced with Elite C18 (150 × 4.6 mm, 5 µm) while maintaining the column temperature of 40 °C, flow rate of 0.8 mL/min and sample size of 100 µL. Acetonitrile and water were still used as the mobile phases, but the peak shape under partial proportion became wider, and the peak area decreased which indicated that the sensitivity did not meet the requirements. Some samples could not be separated, and the peak shape gradually deteriorated without any improvement. Even when the mobile phase was replaced with acetonitrile and 0.02% formic acid, the chromatographic peak still exhibited tailing. Despite trying various gradient elution constantly, the target substance could not be qualitatively and quantitatively determined. The addition of the tetrahydrofuran in the water phase separated the patulin from the sample, but the peak shape forward protruded after changing the gradient. Considering the complexity of the sample matrix, the long chromatographic column was replaced for further adjustment.
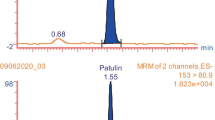
The chromatographic column was then replaced with a Shim-pack VP-ODS C18 column (250 × 4.6 mm, 5 µm) while keeping the column temperature, flow rate, and sample size. Based on the above conditions, it was initially believed that the peak tailing was caused by the slow flow rate. However, it is found that the tailing phenomenon is not improved by changing the flow rate. On the other hand, equal degree elution still failed to separate the target substance effectively. Gradient elution was investigated, as well as the proportion of tetrahydrofuran was constantly explored. Ultimately, a proportion of 0.8% tetrahydrofuran was determined to be the best, because it can lead to a good peak shape. The target substance in the sample could be determined qualitatively and quantitatively, with a retention time of 10.376 min (Fig. 2).
Method validation
Linearity, LOD, LOQ, and precision
Linear regression equation was Y = 508.24X − 12.229, R2 = 0.9999. It showed that the standard curve had a good fitting degree. The LOD was 0.2 µg/kg, and the LOQ was 0.6 µg/kg for liquid samples. The LOD was 0.3 µg/kg, and the LOQ was 1.1 µg/kg for solid and semi-fluid samples. It showed that the HPLC method had high sensitivity. The samples were determined 6 times by the above analysis method. The peak areas were 9782, 9811, 9671, 9869, 9639, and 9705. And the relative standard deviation (RSD) was 0.9%. It showed that the method had fine reproduction.
Accuracy
The accuracy of the analytical method was evaluated through a recovery test at three concentration levels (liquid samples: 20 µg/kg, 25 µg/kg, and 30 µg/kg; solid and semi-fluid samples: 80 µg/kg, 100 µg/kg, and 120 µg/kg), which resulted in a recovery of 93.0–94.3% in liquid samples and 82.3–94.4% in solid and semi-fluid samples. The RSD% ranged from 1.7 to 2.5% in liquid samples and from 1.3 to 4.4% in solid and semi-fluid samples. These results showed that the method had good accuracy. The recovery of patulin in apples, hawthorns, and their products tested three times is shown in Table 1. The recoveries of blank matrix samples ranged from 80.0 to 120.0%, indicating good stability during the experiment (Fig. 3).
The instrument method validation was examined by determining the standard reference material for the patulin quality control sample in apple juice (BWS0068-2016). The validation parameters of the method were listed in Table 2. It is obvious that the detecting results of standard reference material were within the standard range.
Occurrence of patulin in fruits and their products
Table 3 shows the findings on the patulin presence in 400 samples of apples, hawthorns, and their products. From the data, it was evident that 319 samples were found patulin-contaminated with a frequency level (79.8%) in 400 samples and an overall mean concentration of 4.5 µg/kg. It was worth noticing that 0.5% samples of 400 samples for patulin content surpassed the maximum permitted limit of 50 µg/kg set by the GB 2761–2017 (CFDA - China Food and Drug Administration 2017) and the European Union (EU - European Union 2023), reaching 130.0 µg/kg in a hawthorn roll sample.
In many studies, the detection of patulin in apples, hawthorns, and their products was common. In Poland, the patulin positive frequencies were 90% with an average concentration of 4.5 µg/kg in dietary supplements containing hawthorn, while natural dried hawthorns had an average concentration of 27.3 µg/kg (Przybylska et al. 2019). Similarly, a study monitoring apples, grapes, and their products in Pakistan found that 58.9% of apples and apple products contained patulin, with an average concentration of 49.8 µg/kg (Shabbir Hussain et al. 2020a, b). In our study, we also observed the high frequencies of patulin in apples, hawthorns, and their products. There were several reasons for the high frequencies of patulin in apples and hawthorns. On the one hand, fungi may contaminate apples and hawthorns during the growing and picking, leading to the frequencies of patulin. On the other hand, the climate variation increased the content of patulin during the storage and processing of apples, hawthorns, and their products. Furthermore, some retailers did not care about storage conditions, and sales managers lacked the pre-job training and knowledge for improving the shelf life of apples, hawthorns, and their products.
Occurrence of patulin in apples and their products
One hundred forty-four out of 200 (72.0%) apples and their products were found positive for patulin with a mean content of 3.4 ± 3.6 µg/kg and patulin content varied from 0.7 to 37.6 µg/kg. The frequencies of patulin in apples were the highest (98.0%), followed by apple puree (90.0%), apple jam (68.0%), and apple juice (32.0%). The patulin content in apple puree and apples was significantly higher than the levels in apple juice and apple jam (p < 0.05). The patulin content in apple juice was significantly the lowest compared with apples, apple puree, and apple jam (p < 0.05).
Patulin can cause food-borne diseases and serious damage to human organ systems (Chen et al. 2022; Diao et al. 2022). In our study, we found a high content of patulin in apple puree. More importantly, apple puree was mostly marketed as complementary foods for infants and young children. Hence, it was crucial to monitor the patulin content in apple puree. At the same time, the patulin content in apples was high in our study. The high positive of patulin in apples may be due to the perishable nature of apples. Because of the high content of patulin in apple puree, the possibility of using apples containing patulin as raw material should be considered. Therefore, the production of apple puree should prioritize safety by reducing the patulin content, including using fresh fruit and keeping a clean and safe production environment. It was also necessary to strengthen patulin supervision in apples to minimize the harm of corresponding products from the source. The clarification, filtration, and enzyme treatment can significantly reduce the content of patulin during juice processing (Mahato et al. 2021). In our study, the content of patulin in apple juice was the lowest compared to apples, apple puree, and apple jam. Additionally, patulin was the one of important indexes to judge the quality and safety of apple juice (Tangni et al. 2023). The stricter patulin supervision and production technology in apple juice resulted in lower patulin content compared to other products.
Occurrence of patulin in hawthorns and their products
As presented in Table 3, patulin frequency levels in hawthorns and their product samples were 87.5%, with a mean content of 5.5 ± 10.5 µg/kg. The patulin content varied from 0.6 to 126.0 µg/kg. Two samples of hawthorns and their products surpassed the maximum regulatory limit of 50 µg/kg. The frequencies of patulin were 98.0% in hawthorn chips, followed by hawthorns at 94.0%, hawthorn juice at 84.0%, and hawthorn rolls at 74.0%. A hawthorn chip and a hawthorn roll both surpassed the limit levels of 50 µg/kg. The content in hawthorn chips was the highest, significantly higher than the levels in hawthorn juice and hawthorns (p < 0.05).
Besides apples and their products, hawthorns and their products were also susceptible to patulin. Hawthorns were collected generally by shaking or hitting them to make them fall to the ground. However, the storage period of hawthorns was very short after falling. If we did not process in time, hawthorns were easy to mildew. In addition, hawthorns had a large purchase volume in a short period of time, leading to a storage environment with poor ventilation and high temperature. More importantly, hawthorns were rich in water and soluble nutrients, making them prone to rot (Ji et al. 2019). It was found that 4 out of 43 hawthorn juice samples exceeded the Chinese legislative requirement of 50 µg/kg in the study by Li et al. (2007). Similarly, Hussain et al. (2020a, b) found that 27.3% of fruit samples contained patulin levels beyond the maximum regulatory limit. In our study, we found that 2 samples of hawthorn products exceeded the regulatory limit, and the patulin concentration in hawthorn chips and hawthorn rolls was higher than the levels in hawthorns. The excessive content of patulin in hawthorn products may be due to the use of hawthorns with high levels of patulin as raw material. Furthermore, the process of hawthorn products was complex and time consuming, and improper operation can also lead to an increase in patulin during the production process. The processing products were placed for a long time or the environment was humid, so it was difficult to ensure that products did not product patulin during the placement process that indicated the need to strengthen the supervision of production process to ensure food safety. Moreover, raw material should be refrigerated in time, minimized fruit damage, reducing patulin production. At the same time, the various processes should be regularly checked to avoid the accumulation of patulin content.
Occurrence of patulin in domestic and imported samples
As shown in Table 4, the frequencies of patulin in domestic samples were 83.0%, while they were 57.7% in imported samples. The percentage of domestic samples that exceeded the maximum level of patulin was 2 out of 348 (0.6%), while none of the imported samples exceeded the maximum level of patulin. These results indicated that both domestic and imported samples were detected with patulin, and the patulin frequencies in domestic samples were significantly higher than the proportions in imported samples (p < 0.05). The mean content in domestic samples was higher than that of the levels in imported samples, but the difference was not statistically significant (p > 0.05), indicating that it may be due to sample size. In any case, it was necessary to regularly monitor for patulin levels in domestic and imported samples to ensure the safety of consumers.
Occurrence of patulin in samples collected from different sampling points
In Table 5, the contamination of online samples with patulin was 76.4%, and 1.0% of samples had a patulin content above the safe limit of 50 µg/kg. However, the contamination levels in offline supermarkets were 82.4%, all within the safe limit. The widest detection range of patulin was observed in online shopping platform, ranging from 0.6 to 126.0 µg/kg. As can be seen from Table 6, the patulin concentration of online samples in apples, hawthorns, and hawthorn chips was higher than the concentration of offline samples (p < 0.05).
Patulin was a natural contaminant found in apple-based products and other fruits, and its production was influenced by the temperature at storage facilities. Studies had shown that the patulin content could increase with increasing temperature (Barad et al. 2016). Moreover, apples and hawthorns purchased online were often sent to customers in cardboard boxes, which could cause the temperature to rise over time. Combining our results with previous studies, we reasonably speculated that temperature had a notable influence on online fruits. In addition, storage time could also affect the content of patulin in fruit and their products. Compared with offline samples, the transportation time of online samples was longer slightly, which would increase the patulin content in fruits. Studies showed that fruit rot was often along with mycotoxin contamination. Mycotoxins remained in fruits even if the decay had been removed and could spread to healthy tissues (Li et al. 2022). Some online small factories failed to timely remove rotten fruit to save costs, and some have poor production environments. Both factors could lead to an increase in the patulin content in fruits and their products.
Above all, it was an important measure to protect food safety by preventing or purifying fungal toxins in food. We could prevent the production of mycotoxins by freezing and ultraviolet treatment (Deberghes et al. 1995; Li et al. 2020). Storage environmental conditions greatly affected fungi and their mycotoxins. It was also necessary to prevent the production of mycotoxins through adequately drying the moisture to less than 10% (Pose et al. 2010). Besides, mature fruits must be collected in sacks or containers free of contamination. Any overripe, damaged, or fallen fruits onto the soil must be discarded and eliminated. More importantly, with the increasing demand and willingness for online food shopping, major platforms should enhance supervision to prohibit unqualified products from flowing into the market and prevent the occurrence of food safety incidents that affect the health of consumers.
Conclusion
An HPLC method with high sensitivity, good repeatability, and high accuracy for the detection of patulin in apples, hawthorns, and their products was established and verified, which was applied to the 400 samples analysis. The levels of patulin were higher in hawthorn samples compared to apple samples. The highest positivity and mean concentration were found in hawthorn chips. The frequencies and content of patulin were higher in domestic samples compared to imported samples. Two samples, obtained from online shopping platforms for hawthorn samples, exceeded the maximum limit level. Therefore, stricter regulatory strategies should be formulated to monitor fruits and their products during the picking and processing stages. At the same time, the technology to reduce patulin production in food is needed. Our study was useful for people including producers, processors, and supervisors to prevent and control patulin in fruits and their products before placing them on the market or exporting.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Agriopoulou S, Stamatelopoulou E, Varzakas T (2020) Advances in occurrence, importance, and mycotoxin control strategies: prevention and detoxification in foods. Foods 9(2):137. https://doi.org/10.3390/foods9020137
Alshannaq A, Yu JH (2017) Occurrence, toxicity, and analysis of major mycotoxins in food. Int J Environ Res Public Health 14(6):632. https://doi.org/10.3390/ijerph14060632
Aslam K, Iqbal SZ, Razis AFA, Usman S, Ali NB (2021) Patulin contamination of citrus fruits from Punjab and northern Pakistan and estimation of associated dietary intake. Int J Environ Res Public Health 18(5):2270. https://doi.org/10.3390/ijerph18052270
Assunção R, Alvito P, Kleiveland CR, Lea TE (2016) Characterization of in vitro effects of patulin on intestinal epithelial and immune cells. Toxicol Lett 250–251:47–56. https://doi.org/10.1016/j.toxlet.2016.04.007
Bacha SAS, Li Y, Nie J, Xu G, Han L, Farooq S (2023) Comprehensive review on patulin and alternaria toxins in fruit and derived products. Front Plant Sci 14:1139757. https://doi.org/10.3389/fpls.2023.1139757
Balázs E, Schepers JS (2007) The mycotoxin threat to food safety. Int J Food Microbiol 119(1–2):1–2. https://doi.org/10.1016/j.ijfoodmicro.2007.07.018
Barad S, Sionov E, Prusky D (2016) Role of patulin in post-harvest diseases. Fungal Biol Rev 30(1):24–32. https://doi.org/10.1016/j.fbr.2016.02.001
CFDA - China Food and Drug Administration (2017) GB 2761–2017 Limits of mycotoxins in food under the national standard for food safety (China). Available from: http://down.foodmate.net/standard/yulan.php?itemid=50747
Chen L, Sun L, Zhang R, Liao N, Qi X, Chen J (2022) Surveillance for foodborne disease outbreaks in Zhejiang province, China, 2015–2020. BMC Public Health 22(1):135. https://doi.org/10.1186/s12889-022-12568-4
Cunha SC, Faria MA, Pereira VL, Oliveira TM, Lima AC, Pinto E (2014) Patulin assessment and fungi identification in organic and conventional fruits and derived products. Food Control 44:185–190. https://doi.org/10.1016/j.foodcont.2014.03.043
Deberghes P, Betbeder AM, Boisard F, Blanc R, Delaby JF, Krivobok S, Steiman R, Seigle-Murandi F, Creppy E (1995) Detoxification of ochratoxin A, a food contaminant: prevention of growth of Aspergillus ochraceus and its production of ochratoxin A. Mycotoxin Res 11(1):37–47. https://doi.org/10.1007/bf03192060
Diao E, Ma K, Qian S, Zhang H, Xie P, Mao R, Song H (2022) Removal of patulin by thiol-compounds: a review. Toxicon 205:31–37. https://doi.org/10.1016/j.toxicon.2021.11.010
EU - European Union (2023) Commission regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing regulation (EC) no 1881/2006 (Text with EEA relevance) OJ L 119,5.5:103-157 Last consolidated version available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R0915
Fujita M, Yamamoto Y, Watanabe S, Sugawara T, Wakabayashi K, Tahara Y, Horie N, Fujimoto K, Kusakari K, Kurokawa Y, Kawakami T, Kojima K, Kojima H, Ono A, Katsuoka Y, Tanabe H, Yokoyama H, Kasahara T (2019) Cause of and countermeasures for oxidation of the cysteine-derived reagent used in the amino acid derivative reactivity assay. J Appl Toxicol 39(2):191–208. https://doi.org/10.1002/jat.3707
Hassan NH, Othman H, Abdul Malek NR, Zulkurnain M, Saad B, Wong YF (2020) Simultaneous quantitative assessment of ochratoxin A, patulin, 5-hydroxymethylfurfural, and bisphenol A in fruit drinks using HPLC with diode array-fluorimetric detection. Foods 9(11):1633. https://doi.org/10.3390/foods9111633
Hussain S, Asi MR, Iqbal M, Akhtar M, Imran M, Ariño A (2020a) Surveillance of patulin in apple, grapes, juices and value-added products for sale in Pakistan. Foods 9(12):1744. https://doi.org/10.3390/foods9121744
Hussain S, Asi MR, Iqbal M, Khalid N, Wajih-Ul-Hassan S, Arino A (2020b) Patulin mycotoxin in mango and orange fruits, juices, pulps, and jams marketed in Pakistan. Toxins (Basel) 12(1):52. https://doi.org/10.3390/toxins12010052
Ji X, Li R, Yang H, Qi P, Xiao Y, Qian M (2017) Occurrence of patulin in various fruit products and dietary exposure assessment for consumers in China. Food Control 78:100–107. https://doi.org/10.1016/j.foodcont.2017.02.044
Ji XF, Yang H, Wang JM, Ni XL, Zhang YH, Zhang MR (2019) Simultaneous determination of 8 mycotoxins in dried hawthorn products by ultra performance liquid chromato-graphy-tandem mass spectrometry. Qual Saf Ag Prod (01):39–44. https://doi.org/CNKI:SUN:NYZL.0.2019-01-010
Kumar P, Mahato DK, Kamle M, Mohanta TK, Kang SG (2016) Aflatoxins: a global concern for food safety, human health and their management. Front Microbiol 7:2170. https://doi.org/10.3389/fmicb.2016.02170
Li B, Chen Y, Zhang Z, Qin G, Chen T, Tian S (2020) Molecular basis and regulation of pathogenicity and patulin biosynthesis in Penicillium expansum. Compr Rev Food Sci Food Saf 19(6):3416–3438. https://doi.org/10.1111/1541-4337.12612
Li F, Zhao S, Chin L, Li Y, Wu D, Zhao X, Han C, Zhang H, Ji R (2007) Determination of patulin in apple and hawthorn beverages by solid-phase filtration column and liquid chromatography. J AOAC Int 90(1):167–172. https://www.ncbi.nlm.nih.gov/pubmed/17373448
Li H, Zhang Y, Gao C, Gao Q, Cheng Y, Zhao M, Guan J (2022) Mycotoxin production and the relationship between microbial diversity and mycotoxins in Pyrus bretschneideri Rehd cv. Huangguan Pear. Toxins (Basel) 14(10):699. https://doi.org/10.3390/toxins14100699
Lien KW, Ling MP, Pan MH (2020) Probabilistic risk assessment of patulin in imported apple juice and apple-containing beverages in Taiwan. J Sci Food Agric 100(13):4776–4781. https://doi.org/10.1002/jsfa.10536
Mahato DK, Kamle M, Sharma B, Pandhi S, Devi S, Dhawan K, Selvakumar R, Mishra D, Kumar A, Arora S, Singh NA, Kumar P (2021) Patulin in food: a mycotoxin concern for human health and its management strategies. Toxicon 198:12–23. https://doi.org/10.1016/j.toxicon.2021.04.027
Malir F, Pickova D, Toman J, Grosse Y, Ostry V (2023) Hazard characterisation for significant mycotoxins in food. Mycotoxin Res 39(2):81–93. https://doi.org/10.1007/s12550-023-00478-2
Misihairabgwi JM, Ezekiel CN, Sulyok M, Shephard GS, Krska R (2019) Mycotoxin contamination of foods in Southern Africa: a 10-year review (2007–2016). Crit Rev Food Sci Nutr 59(1):43–58. https://doi.org/10.1080/10408398.2017.1357003
Nan M, Xue H, Bi Y (2022) Contamination, detection and control of mycotoxins in fruits and vegetables. Toxins 14(5):309. https://doi.org/10.3390/toxins14050309
Pose G, Patriarca A, Kyanko V, Pardo A, Fernandez Pinto V (2010) Water activity and temperature effects on mycotoxin production by Alternaria alternata on a synthetic tomato medium. Int J Food Microbiol 142(3):348–353. https://doi.org/10.1016/j.ijfoodmicro.2010.07.017
Przybylska A, Bazylak G, Kosicki R, Altyn I, Twaruzek M, Grajewski J, Soltys-Lelek A (2019) Advantageous extraction, cleanup, and UHPLC-MS/MS detection of patulin mycotoxin in dietary supplements and herbal blends containing hawberry from Crataegus spp. J Anal Methods Chem 2019:1–13. https://doi.org/10.1155/2019/2159097
Ramalingam S, Bahuguna A, Kim M (2019) The effects of mycotoxin patulin on cells and cellular components. Trends Food Sci Technol 83:99–113. https://doi.org/10.1016/j.tifs.2018.10.010
Saleh I, Goktepe I (2019) The characteristics, occurrence, and toxicological effects of patulin. Food Chem Toxicol 129:301–311. https://doi.org/10.1016/j.fct.2019.04.036
Sewram V, Nair JJ, Nieuwoudt TW, Leggott NL, Shephard GS (2000) Determination of patulin in apple juice by high-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J Chromatogr A 897(1–2):365–374. https://doi.org/10.1016/s0021-9673(00)00830-x
Sohrabi H, Arbabzadeh O, Khaaki P, Khataee A, Majidi MR, Orooji Y (2021) Patulin and Trichothecene: characteristics, occurrence, toxic effects and detection capabilities via clinical, analytical and nanostructured electrochemical sensing/biosensing assays in foodstuffs. Crit Rev Food Sci Nutr 62(20):5540–5568. https://doi.org/10.1080/10408398.2021.1887077
Tangni EK, Masquelier J, Van Hoeck E (2023) Analysis of patulin in apple products marketed in Belgium: intra-laboratory validation study and occurrence. Toxins (Basel) 15(6):368. https://doi.org/10.3390/toxins15060368
Xue J, Zhang W (2013) Understanding China’s food safety problem: an analysis of 2387 incidents of acute foodborne illness. Food Control 30(1):311–317. https://doi.org/10.1016/j.foodcont.2012.07.024
Acknowledgements
This work was supported by the China National Center for Food Safety Risk Assessment for the project on validation and analysis of multicomponent mycotoxin detection methods in various fruits and vegetables and their products (no. 20211068). We are grateful to the School of Public Health, Cheeloo College of Medicine, Shandong University and Professor Ping Liu for providing all necessary facilities and encouragement in the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Xu, W., Liu, R. et al. Determination and analysis of patulin in apples, hawthorns, and their products by high-performance liquid chromatography. Mycotoxin Res 40, 235–244 (2024). https://doi.org/10.1007/s12550-024-00522-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-024-00522-9