Abstract
Fumonisins are a group of mycotoxins commonly associated with corn-based products and require innovative alternatives to control exposure to its toxicity. The objective of this research was to determine the effect of amylose and resistant starch on fumonisin B1 (FB1) levels in extruded corn-based products as well as the toxin bioaccessibility upon digestion. Cornmeal contaminated with FB1 (1.5 µg/g) was extruded alone or combined with high-amylose corn starch (20%, w/w). FB1 was quantified both in the unextruded and extruded products by HPLC (high-performance liquid chromatography) fluorescence detector with pre-column derivatization. Samples were then subjected to an in vitro digestion model to evaluate the stability of the interaction between FB1 and the corn matrix extruded. The addition of high-amylose corn starch further reduced the detection of FB1 (74.9%), when compared with the effect of the extrusion alone (66.0%), confirming the binding of FB1 with the macromolecules or resistant starch. The bound fumonisin was stable upon simulated gastric digestion, and the duodenal bioaccessibility of free FB1 was lower than 35% when high-amylose corn starch ingredient was used in the product. Principal component analysis (PCA) showed that high-amylose corn starch and resistant starch content influenced the reduction of FB1 and its duodenal bioaccessibility. This study for the first time shows that addition of high-amylose corn starch during extrusion is an innovative strategy to reduce FB1 release under digestive conditions, therefore useful in mitigating the exposure to this mycotoxin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fumonisins are a group of mycotoxins distributed worldwide, and particularly associated with grain commodities, including maize (BIOMIN 2020). These fungal toxins are produced mainly by Fusarium verticillioides and Fusarium proliferatum species. Although different fumonisin types have been identified, FB1 is the most frequently found in food (Bordini et al. 2019). Exposure to fumonisin-contaminated foods may contribute to various adverse health outcomes, such as cancer and birth defects (WHO 2018). For this reason, the International Agency for Research on Cancer (IARC) has classified FB1 as probable carcinogenic for humans (group 2B) (IARC 2002). Therefore, strategies for mitigating fumonisin in corn products are of utmost importance.
A considerable proportion of corn products are commonly prepared by some heat treatment, so the use of a thermal approach can be an applicable strategy to reduce mycotoxin levels. Previous findings have shown a reduction of FB1 when corn is cooked by extrusion (Bullerman and Bianchini 2007). Extrusion applies high pressure, torque, and heat to raw ingredients, and this cooking process is the most versatile processing technology used by the food industry to produce snacks, breakfast cereals, and textured foodstuffs (Voss et al. 2017). The degree of FB1 reduction achieved is variable and depends on extrusion conditions and food matrix composition. Mechanism that promotes these reductions is not well understood, but it involves thermal decomposition or binding to food matrix components (Humpf and Voss 2004), forming “modified mycotoxins” (Rychlik et al. 2014). These mycotoxins normally remain undetected by analytical protocol for free mycotoxins (Humpf et al. 2019). The type of process and the composition of the food affect the modified mycotoxin levels present in the final product (Freire and Sant’Ana 2018).
The modified mycotoxin can be stable (Falavigna et al. 2012) or converted back to its free form in the digestive process and became available for intestinal absorption (Dall’Asta et al. 2010). Therefore, the total amount of an ingested contaminant does not always reflect the amount that is available to the body (Versantvoort et al. 2005). This concern has raised the need for in vitro studies to examine the fate of modified fumonisin by mimicking natural conditions during digestion.
Based on these aspects, it is hypothesized that fumonisins might bind to corn starch or resistant starch during extrusion and affect the FB1 bioaccessibility. And if so, this binding could influence the FB1 bioaccessibility. The purpose of this study was to evaluate whether the addition of high-amylose corn starch and formation of resistant starch during the extrusion of cornmeal reduces the amount of FB1 in extrudates and how it influences the mycotoxin in vitro bioaccessibility.
Material and methods
Cornmeal sample preparation
Commercial coarse cornmeal (Lincoln, NE, USA) and high-amylose corn starch (Hylon VII) were used in the study. Hylon VII ((Ingredion Incorporated, Westchester, IL, EUA) is an unmodified corn starch derived from high amylose corn. It contains approximately 70% amylose and is used in a variety of food applications.
The moisture content of the samples was adjusted to 22% and 29% (w/w) with the addition of distilled water, and samples were spiked with 1.5 µg g−1 of FB1 using a spray-nozzle bottle. The samples were kept in sealed plastic bags and stored at 4 °C for 24 h to allow moisture equilibration and homogeneous distribution of mycotoxins. Prior to the extrusion process, the products were allowed to reach ambient temperature (25 °C) and were mixed to ensure homogeneity. The moisture content was determined with a halogen moisture analyzer (MB27, Ohaus Corporation, Pine Brook NJ) set at 105 °C.
Extrusion process
Cornmeal samples were extruded in a laboratory-scale GR-8 single-screw extruder (C.W. Brabender Instruments, South Hackensack, NJ, USA) according to Jackson et al. (2011) with some modifications. The extruder screw had a compression ratio of 3:1 with a length-to-diameter ratio of 20:1. Feed rate of extruder was kept at a pace such that the feeding compartment was full at all times of operation. The temperature of the first feeding zone was set to 50 °C, whereas the transition and metering zones were set to 140 °C and 160 °C, respectively. Different screw speeds (160 and 210 rpm), moisture (22 and 29%), and Hylon VII concentration (0 and 20%) were used during the experiment.
For each sample, 800 g of cornmeal and cornmeal combined with Hylon VII were passed through the extruder. Work was done in duplicate. After stable extruder conditions were established, extrudates were collected and stabilized at room temperature (25 °C) for 24 h, ground to particle size less than 32 mesh, and sealed in polyethylene bags until further testing. Samples for fumonisin quantification were taken before (unextruded) and after the extrusion process (extruded).
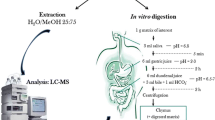
Bioaccessibility of FB1 in extruded products
The bioaccessibility assay was performed according to the in vitro digestion model developed by Versantvoort et al. (2004). Synthetic solutions containing the same components of the different portions of the digestive tract of monogastric animals were used and all digestive juices were kept at 37 ± 2 °C and pH determined before use. A detailed juice composition is shown in Table 1.
The simulated digestive process started by adding the simulated saliva formulation (6 mL) to the sample (4.5 g) and allowing a 5-min incubation at 37 ± 2 °C. The gastric digestion was simulated with the addition of gastric juice (12 mL), followed by a 2 h mixing at 55 rpm at 37 ± 2 °C using a shaking incubator (MaxQ 4000, Thermo Scientific, USA). The simulated duodenal digestion was accomplished with the simultaneous addition of the duodenal juice (12 mL), bile (6 mL), and 1 M bicarbonate solution (2 mL), followed by a 2 h mixing with the same conditions as described above. The pH determined in the chyme (supernatant) was in the range of 6.8 and 7.
In the simulated stomach and small intestine digestion process steps, the digestion tubes with the samples were centrifuged and two fractions were obtained: chyme (supernatant—digestible material) and the digested matrix fraction (pellet—non-digestible material). FB1 was quantified in the chyme, which allowed for bioaccessibility determination, according to Eq. 1.
where [FB1 chyme] = fumonisin B1 concentration of chyme in the stomach or small intestine. [FB1 extruded cornmeal] = fumonisin B1 concentration of extruded cornmeal.
Extraction and clean-up of FB1
Unextruded and extruded corn meals
A fumonisin B1 standard (Cayman Chemical, Ann Arbor, MI, USA) was used for sample spiking, and standard solution was prepared with acetonitrile/methanol (1:1, v/v) according to product information.
FB1 in unextruded and extruded corn meal were extracted according to FumoniTest™ HPLC method (Vicam) for corn. Extract was dried using a Reacti-Vap evaporating unit (18780, Pierce Chemical Company, Rockford, IL). Dried flasks were kept under refrigeration (4–8 °C) until analyzed.
Digestible fraction
The chyme collected from the in vitro digestion model was filtered, and then, 5 mL of each step (stomach or small intestine) were diluted in 5-mL 0.1% Tween-20/2.5% PEG (polyethylene glycol) PBS (phosphate-buffered saline) mixed well and then 5 mL passed through a FumoniTest™ immunoaffinity column (Vicam, Watertown, MA). The column was washed with 5 mL of PBS (#G1113, VICAM, Waters, USA) and sample was eluted into an amber flask by adding 2 mL of HPLC grade methanol (A452-4, Fisher scientific, Pittsburgh, PA, USA), and then, the extract was dried and kept under refrigeration (4–8 °C) until analysis.
Method validation
The validation was performed according to the European Commission (EC 2006) and guidelines from the Brazilian Health Regulatory Agency (ANVISA 2017b). The linearity, determination, and correlation coefficient, as well as the limit of detection (LOD) and quantification (LOQ) were determined. The instrument LOD and LOQ were obtained considering three and ten times the signal-to-baseline (noise) ratio, respectively. The LOD and LOQ methods were estimated considering the dilution in the extraction method.
The analytical curve was constructed using FB1 standard solutions with increasing concentrations, from 0.025 to 1 µg/mL in triplicate. The accuracy (recovery) was evaluated spiking a known blank sample with a FB1 standard (0.25 µg/g) in triplicate. The precision (repeatability) was evaluated with recovery determination. The matrix effect (ME) of both unextruded and extruded cornmeal was evaluated according to Malachová et al. (2014).
In order to evaluate the FB1 recovery during the bioaccessibility assay, unspiked unextruded corn meal (control blank) was extruded and digested. The supernatant (chyme) was spiked with FB1 at levels equivalent to tenfold the LOQ in triplicate to evaluate the analytical method in terms of accuracy and precision. The ME of the chyme fraction was evaluated according to Malachová et al. (2014).
FB1 quantification
The AOAC (Association of Official Agricultural Chemists) official method no 995.15 (AOAC 2000), based on Shephard et al. (1990) was used for quantification of FB1. The samples were derivatized prior to HPLC injection. FB1 was quantified using an HPLC UltiMate 3000 Series (Dionex, Sunnyvale, CA), with a fluorescence detector at an excitation of 335 nm and emission of 440 nm. Separation was done on a Nova-Pak C18 column (4 µm, 3.9 × 150 mm, WAT086344, Waters Corporation, Milford, MA) at 25 °C. The mobile phase was prepared with acetonitrile and water (50:50, v/v) and the pH adjusted at 2.45 with acetic acid (HPLC grade, Fisher Scientific, Fair Lawn, NJ). Ultrapure water (> 18.2 MΩ/cm resistivity) was obtained using a Milli-Q® SP Reagent Plus water system and acetonitrile was sourced from Fisher Scientific, Fair Lawn, NJ. The flow rate was set to 0.5 mL/min, with 20 µL of reconstituted sample per injection.
The OPA-MCE reagent was prepared dissolving 40 mg ortho-phthalaldehyde (OPA) (Sigma-Aldrich, St. Louis, MO, USA) in 1 mL methanol. OPA was later diluted with 5 mL of 0.1 M sodium borate decahydrate (Sigma-Aldrich, St. Louis, MO, USA) adjusted to pH 9.5. Fifty microliters of 2-mercaptoethanol (MCE) (Sigma-Aldrich, St. Louis, MO, USA) was incorporated to complete the derivatization solution. The solution was well mixed with a vortex and stored in dark conditions for up to 1 week at 8 °C in an amber vial (Motta and Scott 2007). The dry extract was dissolved in 0.5 mL water/acetonitrile (70:30, v/v) and the extract was filtered. One hundred microliters of the filtered extract was transferred to a clean vial and 100 µL of OPA-MCE reagent was added, followed by mixing using a vortex and allowing for a 2-min reaction prior to quantification.
Resistant and digestible starch
Resistant and digestible starch contents of unextruded and extruded cornmeal were quantified according to the AOAC method 996.11 modified by Walter et al. (2008) in triplicate. The determination of the resistant starch after hydrolysis was performed by quantifying the glucose released by a glucose oxidase assay (GAGO-20 Sigma), converting readings to starch with a factor of 0.9.
Statistical analysis
Analysis of variance was carried out using Software Statistica 6.0, followed by Tukey mean difference test. Differences with a probability value of p < 0.05 were considered significant. A principal component analysis (PCA) was performed in Past software (folk.uio.no/ohammer/past) to establish correlations among FB1 reduction and bioaccessibility, and variables’ association with extrusion, resistant starch, digestible starch, and Hylon VII content.
Results and discussion
Analytical method performance
The analytical curve exhibited linearity of 0.025 to 1.0 μg/mL and correlation coefficient greater than 0.99. The LOQ of the instrument and method (0.025 μg/mL and 0.025 μg/g) were satisfactory for the determination of FB1, being well below the recommended maximum levels for fumonisins in degermed dry-milled corn products in Brazil, which is 1.5 μg/g (ANVISA 2017a) and in the USA, which is 2.0 μg/g (USFDA 2001).
The recovery values of FB1 for unextruded, extruded cornmeal, and duodenal chyme were 90.9%, 99.7%, and 91.5%, respectively. These percentages were within the criteria approved by the European Regulatory Committee, which recommends concentrations lower than or equal to 0.5 μg/g recoveries with a range from 60 to 120% (EC 2006).
The repeatability was evaluated by the relative standard deviation (RSD) and the values were 8.2%, 12.3%, and 15.4% for unextruded, extruded cornmeal, and chyme, respectively. All RSD values were lower than 20%, which is accepted limits for the concentrations used in a repeatability test (EC 2006) and are therefore also in agreement with the results of the recovery. The matrix effects for unextruded (13.9%), extruded (− 19.9%), and chyme (− 16.6%) indicate that the solvent curve can be used to determine FB1 levels with reliability (ME < +− 20%) (SANTE 2017).
Fumonisin reduction with extrusion process
Extrusion cooking resulted in reductions of detectable FB1 (Table 2). The addition of Hylon VII further reduced detectable FB1 (75%), when compared with the effect of the extrusion alone (66%), indicating the possible association of fumonisin with this macromolecule. The extrusion parameters (moisture content and screw speed) did not seem to affect the reduction of FB1.
Some authors showed that the extrusion processing efficacy can be further improved by the addition of reducing sugars (Jackson et al. 2011) and sodium chloride (Castells et al. 2009). The reduction in fumonisin in some of these cases was probably due to bound forms promoted by chemical interactions or reactions between the amino group of FB1 and glucose or other reducing sugar. The result is the formation of N-(deoxy-D-fructos-1-yl)-FB1 and N-carboxymethyl-FB1 (Howard et al. 1998).
Park et al. (2004) detected protein-bound FB1 in corn flakes and corn-based breakfast cereals. Previous research had shown that this toxin is able to bind to proteins via their two tricarballylic acid side chains (Seefelder et al. 2003).
These results reinforce the hypothesis that thermal processes applied to food can form modified fumonisins, including fumonisins covalently bound to starch, proteins, and reducing sugars (Falavigna et al. 2012). To date, there is no information about the reduction of FB1 in corn-based products with addition of high-amylose corn starch in extrusion process. The higher reduction verified in this study with this ingredient can be explained by the association of fumonisin with this particular macromolecule. A study performed in model systems indicated the formation of covalent bounds between carboxyl groups of fumonisins and a hydroxyl group present in the starch molecule (Seefelder et al. 2003). Moreover, the FB1 may also be physically entrapped into the macromolecular structure of starch (Bryła et al. 2014). Even though the entrapping mechanism is still not clear from a physicochemical point of view, the existing data indicates that biopolymers such as amylose and amylopectin can form inclusion complexes with fumonisin (Kovač et al. 2018).
The apparent reduction shown by extrusion can be partly reversible under some conditions (Kovač et al. 2018). Therefore, there is a need for bioassays when evaluating the efficacy of the extrusion process on mycotoxin reduction. In response to this knowledge gap, bioaccessibility assays can be used to investigate whether modified fumonisins are released during digestion in the gastrointestinal tract.
FB1 content in the gastric and duodenal fluid fractions
For a more accurate risk assessment related to hazard exposure, it is important to evaluate whether mycotoxins are bioaccessible to be absorbed and to act on the different organs or tissues of the human body. Bioaccessibility is defined as the fraction of a compound that is released from the food matrix in the gastrointestinal tract and thus becomes available for intestinal absorption (Versantvoort et al. 2005). Knowledge about the toxicological relevance of modified mycotoxins is still modest. In order to evaluate a more realistic exposure to FB1 through the consumption of extruded corn-based products, an in vitro bioaccessibility component was included in the present study.
In humans, digestion is a sequential process that begins in the mouth, where food is chewed and mixed with saliva, rich in amylases, that helps in breaking down polysaccharides. In the stomach, the food constituents continue to break in the presence of hydrochloric acid; in this condition, the protein degradation to lower weight peptides is facilitated. The third part of the process is in the small intestine, where enzymes and fluids are added to the chyme that act on the digestion of starch, lipids, and other food components. At the end of the process, the absorption of nutrients (Silverthorn 2017), including free mycotoxins, by the cells of the intestinal epithelium takes place (Benito and Miller 1998).
After the absorption, the contaminant will be metabolized and then may cause the toxic effect in the body. The food matrix mainly affects the bioaccessibility, whereas absorption and metabolism depend more on the toxin-specific properties and on the animal physiology. Therefore, the food matrix is expected to have less influence on absorption and metabolism (Brandon et al. 2006). This study does not take into account the large intestine, as absorption of mycotoxins takes mainly place in the small intestine (Gonzáles-Arias et al. 2013). Table 3 shows the results regarding the simulated gastric and duodenal bioaccessibility expressed as FB1 concentration and as percentage related to the initial FB1 concentration in extruded samples.
Bioaccessibility values for both gastric and duodenal fractions were lower than 100% indicating that any potential modified fumonisin formed during the extrusion process was stable under the in vitro digestion. This suggested that digestive enzymes were not able to cleave the covalent bonds in these toxin-matrix conjugates. Falavigna et al. (2012) also showed that FB1 conjugates formed with reducing sugars, proteins, and starch are stable under in vitro digestion. From a chemical standpoint, FB1 bound to glucose by an amino group is a secondary amine, which is largely stable upon heating as well as under acidic and alkaline conditions (Falavigna et al. 2012).
A maximum of 54% of the FB1 present in extruded samples achieved the intestine unmodified, available for the absorption by the cells of the intestinal epithelium (Table 4 ). Other studies support these findings, showing a low bioaccessibility of FB1 in chyme on corn flakes (37–64%) where hydrolyzed FB1 was not detected (Motta and Scott 2007, 2009). The low duodenal bioaccessibility of FB1 in the present study may also be a consequence of the ability of FB1 to bind to bile salts (Prelusky et al. 1994; Mahfoud et al. 2002).
The food matrix and preparation process can affect the FB1 reduction and bioaccessibility. Therefore, a correlation analysis with the different variables within the extrusion process becomes of importance to identify which parameter could contribute to the reduction of FB1, as well as its bioaccessibility.
Influence of extrusion parameters on FB1 content and bioaccessibility
In order to evaluate the association among extrusion conditions, resistant starch and FB1 reduction, and bioaccessibility, a multivariate analysis was employed (Fig. 1).
The principal component (PC) 1 (56.9%) and PC2 (20.8%) together explained 77.7% of the results’ variation. The resistant starch and Hylon VII variables influenced the reduction of FB1 (oval with solid lines). It can be observed, as pointed out by the highlighted regions in the graph (Fig. 1), that the gastric bioaccessibility of FB1 showed a significant (p < 0.05) positive correlation with the moisture (R = 0.49) and a negative correlation with screw speed (R = −0.49), shown in the rectangle with solid lines.
The moisture content (0.72) positively influenced the duodenal bioaccessibility; however, the screw speed (−0.72), Hylon VII content (−0.65), resistant starch (−0.73), and FB1 reduction (−0.65) showed a significant negative correlation (circle with dotted lines) with the bioaccessibility. Therefore, the higher the content of Hylon VII, resistant starch, and the reduction of FB1 after the extrusion cooking, the lower its bioaccessibility in the small intestine.
This study showed for the first time the positive effect on food safety associated with extrusion of FB1-contaminated corn in the presence of Hylon VII, as well as the stability of modified fumonisins under simulated digestive conditions. This research highlights that the food matrix in which mycotoxins may be present can have an effect on their bioavailability, as complex and diverse reactions occur during thermal processes during food production. These interactions between mycotoxin and food matrix components will likely result in different levels of free toxins available for absorption in the intestinal tract.
These results show that adding high-amylose corn starch during extrusion is an innovative strategy to reduce the FB1 release under digestive conditions and therefore useful in mitigating the exposure to this mycotoxin. Therefore, more specific studies should be undertaken in order to clarify potential toxic effects associated with these modified mycotoxins.
References
ANVISA- National Health Surveillance Agency (2017a). RDC Resolution No. 138, of February 8, 2017. Maximum tolerated limits (MLT) for mycotoxins in food. Available from: http://portal.anvisa.gov.br/documents/10181/3219534/RDC_138_2017_.pdf/b36e60b0-5112-43dc-9142-932f502fc46b?version=1.0
ANVISA- National Health Surveillance Agency (2017b) Resolution of the Collegiate Board-RDC No. 166, of July 24, 2017 Provides for the validation of analytical methods and provides other measures. Available from: http://portal.anvisa.gov.br/documents/10181/2721567/RDC_166_2017_COMP.pdf/d5fb92b3-6c6b-4130-8670-4e3263763401
AOAC - Association of Official Analytical Chemistry (2000) Official Methods of Analysis of Association of Official Analytical Chemists. Edited by Cunniff (Gaithersburg: AOAC Internacional), chapter 49, pp. 1-51
Benito P, Miller D (1998) Iron absorption and bioavailability: and updated review. Nutr Res 18:581–603
Biomin (2020) Pesquisa Mundial de Micotoxinas: Impacto em 2020. Available from:https://www.biomin.net/br/science-hub/pesquisa-mundial-de-micotoxinas-impacto-em-2020/
Bordini JG, Ono MA, Garcia GT, Vizoni É, Amador IR, Hirozawa MT, Ono EYS (2019) Transgenic versus conventional corn: fate of fumonisins during industrial dry milling. Mycotoxin Res 35:169–176
Brandon EF, Oomen AG, Rompelberg CJ, Versantvoort CH, Van Engelen JG, Sips AJ (2006) Consumer product in vitro digestion model: bioaccessibility of contaminants and its application in risk assessment. Regul Toxicol Pharm 44:161–171
Bryła M, Roszko M, Szymczyk K, Jedrzejczak R, Słowik EB, Obiedziński MW (2014) Effect of baking on reduction of free and hidden fumonisins in gluten-free bread. J Agric Food Chem 62:10341–10347
Bullerman LB, Bianchini A (2007) Stability of mycotoxins during food processing. Int J Food Microbiol 119:140–146
Castells M, Ramos AJ, Sanchis V, Marín S (2009) Reduction of fumonisin B1 in extruded corn breakfast cereals with salt, malt and sugar in their formulation. Food Addit Contam 26:512–517
Dall’Asta C, Falavigna C, Galaverna G, Dossena A, Marchelli R, (2010) In vitro digestion assay for determination of hidden fumonisins in maize. J Agric Food Chem 58:12042–12047
EC – European Commission (2006) Commission regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off J Eur Union L 70/12-34 Last consolidated version available from: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32006R0401
Falavigna C, Cirlini M, Galaverna G, Dall’Asta C (2012) Masked fumonisins in processed food: co-occurrence of hidden and bound forms and their stability under digestive conditions. World Mycotoxin J 5:325–334
Freire L, Sant’Ana AS (2018) Modified mycotoxins: an updated review on their formation, detection, occurrence, and toxic effects. Food Chem Toxicol 111:189–205
Gonzáles-Arias CA, Marín S, Sanchis V, Ramos AJ (2013) Mycotoxin bioaccessibility/absorption assessment using in vitro digestion models: a review. World Mycotoxin J 6:167–184
Howard PC, Churchwell MI, Couch LH, Marques MM, Doerge DR (1998) Formation of N-(carboxymethyl) fumonisin B1, following the reaction of fumonisin B1 with reducing sugars. J Agric Food Chem 46:3546–3557
Humpf H, Rychlik M, Cramer B (2019) Modified Mycotoxins: A New Challenge In: Encyclopedia of Food Chemistry 393–400.
Humpf HU, Voss K (2004) Effects of thermal food processing on the chemical structure and toxicity of fumonisin mycotoxins. Mol Nutr Food Res 48:255–269
IARC- International Agency for Research on Cancer (2002) Monographs on the Evaluation of Carcinogenic Risks To Humans. Some tradicional herbal medicines, some mycotoxins, naphthalene and styrene, v. 82, 2002. Available from: http://monographs.iarc.fr/ENG/Monographs/vol83/mono83-1.pdf
Jackson LS, Jablonski J, Bullerman LB, Bianchini A, Hanna MA, Voss KA, Ryu D (2011) Reduction of fumonisin B1 in corn grits by twin-screw extrusion. J Food Sci 76:T150–T155
Kovač M, Šubarić D, Bulaić M, Kovač T, Šarkanj B (2018) Yesterday masked, today modified; what do mycotoxins bring next? Arch Ind Hyg Toxicol 69:196–214
Mahfoud R, Maresca M, Santelli M, Pfohl-Leszkowicz A, Puigserver A, Fantini J (2002) pH-dependent interaction of fumonisin B1 with cholesterol: physicochemical and molecular modeling studies at the air−water interface. J Agric Food Chem 50:327–331
Malachová A, Sulyok M, Beltrán E, Berthiller F, Krska R (2014) Optimization and validation of a quantitative liquid chromatography-tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J Chromatogr A 1362:145–156
Motta EL, Scott PM (2007) Effect of in vitro digestion on fumonisin B 1 in corn flakes. Mycotoxin Res 23:166–172
Motta EL, Scott PM (2009) Bioaccessibility of total bound fumonisin from corn flakes. Mycotoxin Res 25:229–232
Park JW, Scott PM, Lau B-Y, Lewis D (2004) Analysis of heat-processed corn foods for fumonisins and bound fumonisins. Food Addit Contam 21:1168–1178
Prelusky DB, Trenholm HL, Savard ME (1994) Pharmacokinetic fate of 14C-labelled fumonisin B1 in Swine. Nat Toxins 2:73–80
Rychlik M, Humpf HU, Marko D, Dänicke S, Mally A, Berthiller F, Lorenz N (2014) Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res 30:197–205
SANTE (2017) Guidance document on analytical quality control and method validation procedures for pesticide residues and analysis in food and feed
Seefelder W, Knecht A, Humpf H-U (2003) Bound fumonisin B1: Analysis of fumonisin-B1 glyco and amino acid conjugates by liquid chromatography− electrospray ionization− tandem mass spectrometry. J Agric Food Chem 51:5567–5573
Shephard GS, Sydenham EW, Thiel PG, Gelderblom WCA (1990) Quantitative determination of fumonisin B1 and B by high-performance liquid chromatography with fluorescence detection. J Liq Chromatogr 13:2077–2087
Silverthorn DU (2017) Fisiologia humana Uma abordagem integrada, 7th edn. Artmed Editora Ltda, Porto Alegre
USFDA (2001) Guidance for Industry: Fumonisin Levels in Human Foods and Animal Feeds, FDA-2013-S-0610. Accessed 20 Aug 2019
Versantvoort CHM, Oomen AG, Van de Kamp E, Rompelberg CJM, Sips AJAM (2005) Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem Toxicol 43:31–40
Versantvoort CHM, Van de Kamp E, Rompelberg CJM (2004) Development and applicability of an in vitro digestion model in assessing the bioaccessibility of contaminants from food RIVM report 320102002/2004 320102
Vicam AWB FumoniTest and FumoniTest WB. Instruction Manual
Voss K, Ryu D, Jackson L, Riley R, Gelineau-van Waes J (2017) Reduction of fumonisin toxicity by extrusion and nixtamalization (alkaline cooking). J Agric Food Chem 65:7088–7096
Walter M, Silva LP, Perdomo DMX (2008) Available and resistant starch in foods: adaptation of the AOAC method 996.11 Aliment Nutr 16:39–43
WHO - World Health Organization (2018) Food Safety Digest. Fumonisins. Available from: https://www.who.int/foodsafety/FSDigest_Fumonisins_EN.pdf?ua=1
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. This project is based on research that was partially supported by the Nebraska Agricultural Experiment Station with funding from the Hatch Multistate Research capacity funding program “Marketing and Delivery of Quality Grains, Oilseeds, Bioproducts and Coproduct; Accession Number 1017645” from the USDA National Institute of Food and Agriculture.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Kelly Cristina Massarolo, José Rodrigo Mendoza, and Tushar Verma. The first draft of the manuscript was written by Kelly Cristina Massarolo and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Massarolo, K.C., Mendoza, J.R., Verma, T. et al. Stability of fumonisin B1 and its bioaccessibility in extruded corn-based products. Mycotoxin Res 37, 161–168 (2021). https://doi.org/10.1007/s12550-021-00426-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-021-00426-y