Abstract
Chlorpyrifos is one of the most used insecticides in agro-ecosystems and is repeatedly applied due to the increase in pest resistance, which leads to environmental accumulation. The aim of this work was to evaluate the effect of chlorpyrifos on growth and aflatoxin B1 (AFB1) production by four Aspergillus section Flavi strains, under different water conditions—aW (0.93, 0.95 and 0.98)—on maize-based medium (MMEA) and maize grains supplied with 0.06 to 1.4 mmol/L of chlorpyrifos. MMEA plates were incubated at 18, 28, and 37 °C and plates with maize grains at 25 °C during 21 days. Chlorpyrifos stimulated the growth and AFB1 production of non-target organisms, such as Aspergillus section Flavi strains, both at low (0.06 mmol/L) and at high concentrations (1.4 mmol/L) on MMEA and maize grains. Stimulation occurred over a wide range of temperature and aw conditions. The toxin concentration produced by the two strains on MMEA at 18 °C increased when the concentration of chlorpyrifos also increased, being most significant at 0.6 mmol/L. In conclusion, the presence of chlorpyrifos should be considered as a factor, together with environmental conditions, for the development of effective production practices of maize grains, in order to avoid fungal growth and AFB1 production, to prevent both economic losses and risks to human and animal health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The agriculture in Argentina is based on extensive production of crops, vegetable, and fruits (Cabrini and Calcaterra 2016). The largest cultivated area corresponds to maize, soybean, sunflowers, peanuts, and wheat. Maize (Zea mays) is the main crop in the agricultural central regions of the provinces of Córdoba, Buenos Aires, and Santa Fe (Ministerio de Agricultura Presidencia de la Nación 2018), being one of the most important cereals for human diet and animal feed (Wu and Guclu 2013). Likewise, it is one of the most important products in the economy of many countries (Pechanova and Pechan 2015). In the last decades, the application of agronomic practices has caused an increase in maize yield (Chavas et al. 2014). Among these technologies, the implementation of several pesticides to prevent or control pests, diseases, and weeds reduced yield losses and guaranteed obtaining high-quality products (Cooper and Dobson 2007). Within the group of organophosphate (OP) pesticides, chlorpyrifos (CP) is one of the most used insecticides and is applied by foliar and soil application. CP is one of the main commercialized chlorinated organophosphate pesticides. It is a broad-spectrum insecticide, nematicide, and acaricide used for pest control on several crops (John and Shaike 2015). The doses recommended in maize fields range from 1.25 to 4 L/ha (Pina 2012) and is classified as a pesticide of level II (moderately dangerous) according to its toxicity risk (WHO 2016). The half-life of CP in soil varies from a few days to 4 years, greatly depending on application rate, the ecosystem type, soil microorganisms, and climatic conditions (John and Shaike 2015).
Recently, on January 2020, due to the high concerns to health issues on human and animals, the European Food Safety Authority (ESFA) has prohibited the market and application of CP and methyl-CP formulations within European Union (EU) above Commission Implementing Regulation (EU) 2020/17. This fact affected negatively those countries that are considered as a main market and consumer of CP and methyl-CP such as Spain, Argentina, Brazil, and China (ESFA 2019; Food Safety 2020). In the case of Argentina, CP insecticide has not been prohibited yet, but there are many regulations that restrict the use of these products to avoid the negative effects on humans and animals health (Res. MSN 456/2009). Pesticides are usually applied repeatedly leading to environmental accumulation. This fact leads to the contamination of the environment with potential threats to the sustainability of agricultural soils and their microbiota (Hua et al. 2009). On the other hand, pesticides can stimulate some soil microbes able to use these compounds as nutrient source, thus decontaminating environments by pesticide degradation (Salem et al. 2018).
The agricultural soil is the main source of inoculum of Aspergillus section Flavi (Nesci and Etcheverry 2002; Carranza et al. 2016b; Benito et al. 2018). Aspergillus flavus and A. parasiticus are opportunistic and saprophytic fungi that infect foods and feeds. They are one of the most widely studied fungal species because of the capacity of some strains to produce aflatoxins (AFs) (Alvarenga et al. 2017). Among them, aflatoxin B1 (AFB1) is the most frequent and potent toxin and was classified by the International Agency for Research on Cancer as belonging to the group I carcinogens (IARC 2002). In warm and humid subtropical and tropical conditions, maize is susceptible to infection by A. flavus and A. parasiticus. This infection occurs especially via insect damage and during colonization. The colonization of grains and the production of AFs may occur after crop maturation and/or harvest (Williams et al. 2011; Bhatnagar-Mathur et al. 2015). Therefore, they can significantly damage grains and affect milling quality, seed germination, and nutritional value; thus producing economic losses as had been shown for other commodities (Dayo and Oluwaniyi 2015).
The growth of fungi and the accumulation of mycotoxins in foods and feeds are influenced, for example, by water activity (aW), temperature, pH, substrate, and time of incubation. In addition, the presence of xenobiotic compounds in agricultural environments also influences fungal development. The main environmental determinants for growth of aflatoxigenic producing species and for AF production are aW and temperature (Magan et al. 2003; Magan and Aldred 2007). Pre-harvest, harvesting and drying, and post-harvest phases need to be efficiently managed to avoid fungal spoilage and the potential contamination with AFB1 (Mandeel 2005; Bhatnagar-Mathur et al. 2015). At the present time, CP is one of the main insecticides used in maize crops but it can affect the growth of fungi and AF production on maize grains. In the previous studies (Carranza et al. 2016a), CP tolerance was evaluated on non-toxigenic Aspergillus section Flavi strains isolated from agricultural soils. These strains have the ability to resist and degrade high doses of CP, using the insecticide as phosphorous, nitrogen, and carbon source. In addition, the degradation studies showed that the A. oryzae strain had the ability to degrade CP under optimal environmental conditions for growth (Carranza et al. 2016a). Previous reports done by Gareis and Ceynowa (1994) informed an increase of nivalenol (NIV) mycotoxin produced by Fusarium culmorum on the presence of the fungicide Matador on winter wheat. However, there is a lack of information on the effect of CP on growth and AFB1 production by non-target organisms such as aflatoxigenic Aspergillus section Flavi strains. Therefore, the objective of this work was to evaluate the effect of CP on the lag phase, growth rate, and production of AFB1 by Aspergillus section Flavi strains isolated from agricultural soils, under different aW and temperature conditions on maize-based medium and on maize grains.
Materials and methods
Solid medium assay
Fungal strains
Four Aspergillus strains were evaluated: A. parasiticus (NRRL2999 and AP55) and A. flavus (AF56 and AF63). These strains were isolated from maize soil samples exposed to pesticides (Benito et al. 2018) and were identified by classic taxonomy and molecular methods according to the methodology by Klich (2002), Pildain et al. (2005), and Samson et al. (2010, 2014). The nucleotide sequences for the ß-tubulin and calmodulin gene of A. flavus AF56 (accession numbers: MH743101- MH743108), A. flavus AF63 (accession numbers: MH743102- MH743108), and A. parasiticus AP55 (accession numbers: MH743103- MH743104) strains were deposited in GenBank. In addition, AF production was also assessed (Geisen 1996). The strains are kept in the culture collection at the Department of Microbiology and Immunology, National University of Río Cuarto, Córdoba, Argentina.
Culture medium and CP application
Maize meal extract agar at 3% (w/v) (MMEA) was used, and the aW of the medium was adjusted to 0.98, 0.95, and 0.93 with glycerol with the aim to simulate different environmental water availabilities in natural conditions to which the grain may be exposed and AFs could be produced (Barberis et al. 2009). The media were sterilized (120 °C for 20 min), cooled to 50 °C, and added with the CP solution before pouring into Petri plates. CP was obtained from a commercial formulation (Hor-tal®, Buenos Aires, Argentina). Stock solution of CP (1 mol/L) was prepared, and then, working solutions were done by appropriate dilutions in sterile distilled water. CP was applied to the sterilized media to obtain different concentrations (0.06, 0.014, 0.3, 0.6, and 1.4 mmol/L). Lower concentrations represent the doses usually used in fields for pest control, while the highest concentration tested (1.4 mmol/L) represents the contamination possibly present in areas where pesticides were spilled. In addition, control plates at each aW value and without CP were prepared; and each condition was prepared in triplicate.
Then, the aW of representative plates for each treatment was checked at the beginning and the end of the assay by detection of any change in the aW level (AquaLab Series 3, Decagon Devices, Inc., WA, USA).
Inoculation and incubation conditions
The media for each treatment were centrally needle-inoculated with a suspension of fungal spores from 7-day-old cultures on malt extract agar medium (MEA). Inoculated Petri plates of the same aW were sealed in closed containers to avoid changes in their water content. The plates were incubated at 18, 28, and 37 °C for 21 days, temperatures within the range that can occur during maize growth and allow production of AFs. All the experiments were repeated twice.
Determination of growth parameters
Two measures of colony diameter from each plate were performed daily. From these data, the radius of each colony was calculated and plotted versus time. Then, a linear regression was applied to obtain the growth and estimate the growth rate (mm/d). The lag phase (h) prior to growth was also determined (Barberis et al. 2010). Number of growth and lag phase analyses = three aW × three temperatures × six treatments (five CP concentrations and one control) × four strains × three replicates.
Natural substrate assay
Fungal strains
Two strains, AP55 and AF63, were evaluated in maize grains since they showed the best growth parameters on the MMEA assay.
Substrate
Irradiated maize grains (10–12 kGy) with retained germinative capacity were used. The grains were checked for the absence of fungal and AF contamination and were kept at 4 °C until use. The initial aW of maize grains was determined (AquaLab 3 Decagon Devices, Inc. city, WA, USA). The assay was performed with a known quantity of maize grains in sterile flasks; then, different volumes of CP were added to obtain the final concentrations used (0.06, 0.14, 0.6, and 1.4 mmol/L). Maize grains were re-hydrated and conditioned to 0.98, 0.95, and 0.93 aW using a absorption curve. All aW values were verified as described before. Then, single layers of grains were carefully placed on sterile plastic Petri plates (9 cm).
Inoculation and incubation
Maize grains were inoculated centrally with 2 μL of a spore suspension from a 7-day-old culture growing on MEA. Inoculated maize plates with the same aW were sealed in plastic containers to avoid changes in water content. Each container had beakers with a NaCl/water solution, to maintain constant relativity humidity. Three replicate plates per treatment and the corresponding control without CP were made. All plates were incubated at 25 °C for 21 days; and all the experiments were repeated twice. This temperature represents the average value within the range that can occur during maize growth.
Determination of growth parameters
The estimation of growth rate and lag phase was done according to the description named before.
Determination of AFB1 in culture media and maize grains
With regard to culture media, the methodology proposed by Geisen (1996) with some modifications was used in this study. Plugs of MMEA cultures (1 × 1 cm) were taken at 7, 14, and 21 days and transferred to microtubes and 500 μl of chloroform was then added. The mixture was centrifuged at 450 g for 20 min. The chloroform extract was dried under nitrogen gas. The dried extract was dissolved in acetonitrile/water (9:1, v/v) and then derivatized with trifluoroacetic acid/acetic acid/ water (20:10:70 v/v/v).
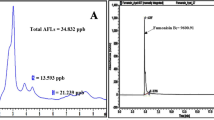
On the other hand, at 7, 14, and 21 days, maize grains contained in each plate (controls and treatments) were removed, dried, and ground, and AFB1 was extracted following the methodology proposed by the Official Method of Analysis with some modifications (AOAC 1995). Grains (5 g) were extracted with 25 mL methanol/water (60: 40 v/v), 15 mL hexane, and 0.5 g of NaCl. The mixture was shaken for 30 min and filtered (Microclar, Buenos Aires, Argentina). Two extractions were performed with chloroform on 10 mL of the filtered extract. The chloroform phase was dried using a rotatory evaporator. The extract was suspended in 200 μL of methanol and derivatized with trifluoroacetic acid/acetic acid/water (20:10:70, v/v/v) (700 μL). Detection and quantification of the toxin were carried out following the methodology proposed by Trucksess et al. (1994). The HPLC system consisted of a Waters Alliance e2695 Separations Module, equipped with automatic injector, connected to a Waters 2475 Multi λ Fluorescence Detector. Chromatographic separations were performed on a stainless steel Supelcosil LC-ABZ C18 reversed-phase column (150 × 4.6 mm i.d., 5 μL particle size; Supelco, PA, USA) connected to a pre-column SecurityGuard KJO-4282 (20 × 4.6 mm i.d., particle size 5 μm, Phenomenex, Torrance, CA). AFB1 was quantified by correlating peak height of sample extracts and those of standard obtained from Sigma Chemical (St Louis, MO, USA) curves.
Analytical validation of AFB1 determination
For both assays carried out, a stock solution of AFB1 in methanol was prepared for recovery. Irradiated/AFB1-free maize grains (10 g) and MMEA (20 g) contained in a 250-mL Erlenmeyer flask were spiked with an equivalent of 0.5, 1.0, and 5 μg AFB1/g. Spiking was performed on triplicate and a single analysis of the blank sample was carried out. After evaporation of the solvent (18 h), the extraction solvent was added and the AFB1 concentration was detected, using the protocols detailed above for this mycotoxin. Mean AFB1 for culture medium and maize grains recoveries were 98.3% and 102.6%, respectively. Good linearity with a correlation coefficient higher than r2 > 0.992 was obtained for the calibration range. The limit of detection (LOD) for AFB1was calculated, based on signal-to-noise (S/N) ratios of 3:1 and were experimentally obtained injecting standard dilutions with the corresponding S/N ratio. The LOD for AFB1 on culture medium and maize grains were 0.7 ng/g and 2.2 ng/g, respectively. Precision was determined by intra-day and inter-day repeatability, making three injections of each spiked of culture medium and maize grains extracts per day during 3 days. The extracts used for inter-day injections were the same as those used in the first day and were properly kept at− 20 °C in darkness to avoid degradation of AFB1. The mean of toxin accumulation intra-day and inter-day relative standard deviation (RSD) values were calculated. Intra-day RSD was 5.23% and 6.21% and inter-day RSD was 14.87% and 15.73% for culture medium and maize grains, respectively.
Statistical analysis
All data were transformed to log10 (x + 1) to obtain the homogeneity of variance. Means were compared by the Fisher’s protected LSD test to determine the influence of the assayed abiotic factors (aW, temperature, and insecticide concentration) on each fungal strain between growth rate, lag phase prior to growth, and AFB1 concentration. The analyses were conducted using the software Infostat 2008p of the National University of Córdoba (Di Rienzo et al. 2008).
Results
Solid medium assay
Effect of CP on lag phase and growth rate
The effect of each single variable alone, aW, temperature, and pesticide concentration, two- three and four-way interactions were statistically significant (p < 0.01) in relation to lag phase and growth rate (Table 1).
Results presented derive from one representative Aspergillus section Flavi strain (AP55 and AF63) from each of the two fungal species studied. Table 2 shows the effect of different concentrations of CP on the lag phases of the Aspergillus section Flavi strains evaluated under several aW and temperature conditions. In general, in control treatments, the lag phases decreased, while the aW increased in all the strains tested. At 37 °C, 0.93 and 0.95 aW, the lag phases were the shortest, compared with those observed at 18 and 28 °C. Regarding pesticide treatment experiments, at 18 °C and 0.98 aW, the lag phase of strain AP55 remained constant with respect to control in all the doses of insecticide tested. At 0.95 aW with 0.6 and 1.4 mmol/L, significant increases of 54.1 and 57.4% on the lag phase, respectively, were registered (p < 0.01). At 28 °C and the lowest aW (0.93) with the highest dose of CP (1.4 mmol/L), a significant (p < 0.01) increase of 26.1% was observed in this parameter. At 37 °C, the lag phase increased when the aW decreased (p < 0.01). A significant effect of CP was observed at 0.93 and 0.98 aW where the lag phases increased in 400.5 and 483%, respectively, while the different doses of the insecticide also increased (p < 0.01). On the other hand, the lag phase of AF63 strain increased when aW decreased at 18 °C, and the same effect was observed when the different doses of CP increased. At 28 °C, 0.98 and 0.95 aW, the lag phases remained constant with respect to the control when increasing CP doses. In addition, at 0.93 aW and with the highest dose of CP (1.4 mmol/L), the lag phase showed an increase of 117.7% with respect to the control (p < 0.01). At 37 °C, the lag phase showed a significant increase with 0.93 aW and the highest doses of the insecticide (0.6 and 1.4 mmol/L), while at 0.95 and 0.98 aW with 0.06, 0.14, and 0.3 mmol/L, this parameter remained constant with respect to the control treatments (p < 0.01).
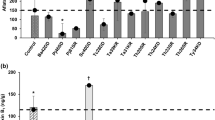
With regard to the growth rate of the two strains, aW was the most influential factor on growth rate in all conditions. In controls, the growth rate increased with the increase of aW (Fig. 1.1 and 1.2). In treatments with CP, at 18 and 28 °C, the growth rate of all strains assayed remained constant as the insecticide levels increased (Fig. 1, 1a and 1b and 2a and 2b). At 37 °C, the two strains had different behaviors in relation to CP; the growth rate of AP55 at 0.98 aW, with 0.06, 0.14, and 0.3 mmol/L of CP, did not show significant differences in growth rate with respect to controls. By comparison, with 0.6 and 1.4 mmol/L, this parameter decreased in 69.3 and 100%, respectively, compared with the control (p < 0.01) (Fig. 1, 1c). At the same temperature (37 °C), the growth rate of AF63, at 0.98 and 0.95 aW with 0.06 and 0.14 mmol/L of CP, remained constant as the insecticide increased, while from 0.3 mmol/L of CP this parameter decreased in 99% (p < 0.01) (Fig. 1, 2c). In summary, it can be observed that the highest growth rates were recorded in AP55 at 37 °C, 0.98 aW with 0.3 mmol/L of CP (Fig. 1, 1c), while the greatest inhibition of growth was observed in this strain at 37 °C with 1.4 mmol/L of the insecticide at 0.93 aW (Fig. 1, 1c). For AF63, the highest growth rate was observed at 37 °C with 0.98 aW and 1.4 mmol/L of CP.
Chlorpyrifos effects on growth rate of Aspergillus section Flavi strains under different aW (water activity) and temperature conditions on maize meal extract agar (MMEA). Mean values are based on triplicated data. Means in a row with a letter in common are not significantly different according to the LSD test (p < 0.01). 1 AP55, 2 AF63. a 18 °C, b 28 °C, c 37 °C
Chlorpyrifos effects on growth rate of Aspergillus section Flavi strains under different aW (water activity) conditions on maize grain at 25 °C. Mean values are based on triplicated data. Means in a row with a letter in common are not significantly different according to the LSD test (p < 0.01). 1 AP55, 2 AF63
Effect of CP on AFB1 production
Table 3 shows the effect of several amounts of CP on AFB1 production by two Aspergillus section Flavi strains growing under different aW (water activity) and temperatures conditions.
In general, the insecticide did not have inhibitory effects on toxin production. AFB1 was stimulated as the incubation time increased, reaching the highest production at 14 days under all conditions tested. All the strains had the same behavior with respect to aW, specifically an increase in AFB1 levels when this factor was also increased. A. parasiticus AP55 produced similar amounts of toxin independently of the temperature assayed. However, A. flavus AF63 significantly increased toxin production when growing at 18 °C (p < 0.01). Regarding incubation time in strain AP55, from 0.06 to 0.6 mmol/L of CP at 18 °C and 7 and 14 days of incubation, the amounts of the toxin increased significantly with respect to the control treatments (p < 0.01). With A. flavus AF63, the increase in AFB1 levels was found from 0.3 to 1.4 mmol/L of CP, at 18 °C after 7, 14, and 21 days of incubation (p < 0.01). At 18 °C, the toxin concentration produced by the two strains showed an increase when the concentration of CP also increased, being significant at 0.6 mmol/L. The highest accumulation of AFB1 was observed in strain AP55, that is, 89 and 70% with respect to the control condition, at 7 and 14 days of incubation, respectively. In this CP concentration (0.6 mmol/L), an increase of 1056, 970, and 612% compared with the controls was found for AF63 at 7, 14, and 21 days, respectively.
Natural substrate assay
Effect of CP on lag phase and growth rate
Table 4 shows the effect of CP on lag phase under different levels of aW on maize grains at 25 °C. The ANOVA assays showed that the effects of each single variable (aW and pesticide concentration) and their interaction were statistically significant in relation to this parameter. AP55 in control treatments did not show significant differences on the lag phase when aW increased. By comparison, the lag phase of strain AF63 decreased significantly, while the levels of aW increased (p < 0.01). In CP treatments, an increase (110%) in the lag phase of strain AP55 with respect to control was registered when the doses of the insecticide also increased (p < 0.01). At 0.93 aW, a significant difference with respect to the other levels of aW was registered in all the concentrations of the insecticide. Regarding strain AF63, also with all the doses of the insecticide, the lag phase at 0.98 and 0.95 aW was lower than those observed at 0.93 aW. In addition, when the doses of CP increased, the lag phase was constant compared with the control treatments.
Regarding growth rate, the ANOVA assay only showed a significant effect on AP55 strain with insecticide concentration (Table 1). In the control treatments, both strains showed the same behavior; the growth rate was constant in the three levels of aW (Fig. 2). With respect to CP treatments, no significant differences were found among concentrations, with values between 5 and 6 mm/d, except with strain AP55, where the growth rate decreased significantly (30.3 %) with 0.14 mmol/L (p < 0.01).
Effect of CP on AFB1 production
Table 5 shows the effect of CP on AFB1 production under different conditions of aW on maize grains at 25 °C. The ANOVA assays showed that the effect of each single variable (aW and pesticide concentration) was statistically significant in relation to this parameter. In control treatments, strain AP55 showed a high production of AFB1 when aW increased, while for strain AF63, the AFB1 concentration remained constant on the three aW tested. In CP treatments, the response of the strains was variable according to the aW assayed. In strain AP55, an increase of more than 1000 times in the levels of AFB1 was observed with 1.4 mmol/L at 0.93 aW, compared with the control. In strain AF63, the highest CP concentration (1.4 mmol/L) stimulated the toxin accumulation on the three aW assayed (p < 0.01). Regarding the effect of the days of incubation, independent of aW and CP concentrations, a significant accumulation of AFB1 was observed from day 7 for both strains.
Discussion
The study provides information on the effect of different doses of the insecticide CP on growth parameters and AFB1 production by strains of Aspergillus section Flavi growing on maize-based medium and maize grains. The ecophysiology assays on maize grains were carried out to evaluate growth parameters and AFB1 production in the presence of CP at different levels of aW and optimal temperature with the purpose to simulate environmental conditions.
In the present study, the different doses of insecticide did not affect the growth rate of the strains on MMEA at 18 and 28 °C. By comparison, at 37 °C, this parameter decreased only with the higher CP concentrations (0.6 and 1.4 mmol/L). On maize grains incubated at 25 °C, a decrease in growth rate was only observed in one strain (AP55) with 0.14 mmol/L. These data are showing that the CP in concentrations that can be found in the field (0.06 to 0.6 mmol/L) does not inhibit the growth of aflatoxigenic Aspergillus section Flavi strains. There is few information on the effects of insecticides, particularly CP, on the development of Aspergillus section Flavi on maize grains. Our results do not concur with those of Carranza et al. (2014), where the growth of Aspergillus section Nigri strains at 25 °C decreased with the increase in CP from 5 to 20 mg/mL (equivalent to 0.014 to 0.06 mmol/L). Mateo et al. (2017) studied the effect of azoles pesticides on A. flavus strains growth on maize. They observed a decrease of A. flavus growth with the increase of the concentrations of two fungicides (prochloraz, 0.01, 0.1, and 2 mg/L equivalent to 2.6 × 10-5, 2.6 × 10-4, and 5 × 10-3mmol/L, respectively, and tebuconazole, 0.5, 5, and 10 mg./L equivalent to 1.6 × 10-3, 1.6 × 10-2, and 3 × 10-2 mmol/L, respectively) in all conditions tested (0.99 and 0.95 of aW, at 25 and 37 °C). These authors observed marked differences in growth from 5 × 10-3of prochloraz and 3 × 10-2 mmol/L of tebuconazole. Our results from growth measurement partially agree with these authors, since this type of response was only observed at 37 °C from 0.6 mmol/L of CP. This fact may be attributed to the different patterns of susceptibility to pesticides in the different Aspergillus section Flavi strains and the nutritional characteristics of the culture media.
Regarding AFB1 production in control treatments, a significant stimulation of the toxin was observed when aW increased in MMEA. On the other hand, a significant stimulation of the toxin was observed at the lowest temperature, 18 °C. Contrarily, Gallo et al. (2016) showed that the optimal conditions for AFB1 production by A. flavus strains on almond medium were 28 °C and 0.96 aW. In the presence of CP, accumulation of AFB1 increased with the increase in CP concentration, especially in AF63 strain. A high concentration of the insecticide (0.6 mmol/L) in marginal environmental conditions (0.93 aW and 18 °C) would cause a stress effect on the strains and a stimulation of AFB1 production. It is important to highlight that CP in MMEA did not produce an inhibitory effect on the production of AFB1 and that significant increases in the production can be produced under certain conditions. Mateo et al. (2017) showed that with the highest levels of the fungicides (5 × 10-3 mmol/L of prochloraz and 3 × 10-2 mmol/L of tebuconazole), AFB1 production was inhibited and the AFB1 levels at 25 °C were higher than those observed at 37 °C. These results partially agree with the present work since the highest concentration of the toxin was also observed at the lowest temperatures assayed, but the different doses of CP did not produce an inhibitory effect on AFB1 accumulation despite the fact that the doses used were higher than those used by these authors.
Similarly, when the effect of CP on AFB1 production was studied on maize grains, a stimulation of toxin production was observed in concentrations usually used in the field (0.14 mmol/L) as well as with the highest concentration tested (1.4 mmol/L), which could be found in spill areas. In the presence of CP, the strains had a different behavior. In strain AP55, an increase of AFB1 was registered with increasing insecticide doses and aW levels. On the other hand, in strain AF63, AFB1 production remained constant with increasing CP doses in the three aW tested. Mateo et al. (2017) observed an accumulation of AFB1 in control treatments and registered an inhibition in AFB1 production with the highest doses of azole fungicides tested, at 25 °C and 0.95 aW. These results do not agree with those showed on the present study. It is important to highlight that the growth of strains and toxin production registered on maize grains was higher than on culture medium. Such a result could be explained by the better nutritional conditions on maize grains for growth and AFB1 production.
This study suggests that, in general, the insecticide CP, when applied in pest control, could have an indirect effect stimulating growth and AFB1 production on non-target organisms present in the same ecosystem, such as Aspergillus section Flavi strains in low (0.06 mmol/L) and high concentrations (1.4 mmol/L). CP was able to inhibit the mycelial growth in marginal conditions of aW (0.95 and 0.93), at the highest concentration and at 37 °C. This fact establishes the importance of the use of adequate doses of the insecticide and avoiding the application of doses higher than 0.14 mmol/L to prevent the growth and AFB1 production on natural substrates such as maize grains. Doses higher than those recommended do not ensure the inhibition of mycelial growth and AFB1 production and could lead to undesired effects on the organoleptic characteristics of maize grains. In addition, this study suggests that lower doses, compared with those usually recommended for this insecticide, and with an unsuitable distribution on the substrate, may cause stimulation of growth rates and AFB1 production.
These ecophysiology assays provide important information with regard to the environmental conditions and CP concentrations that favor fungal growth and AFB1 production. The results indicate that CP levels in maize grains should be considered as a factor of good agricultural practice, in order to avoid growth of aflatoxigenic fungi and AFB1 production. Health risks for humans and livestock animals as well as economic losses could thereby be minimized.
References
Alvarenga AAA, Méndez JM, Ríos DF (2017) Aflatoxinas, un riesgo real. Reportes Cient LA FACEN 4:68-71.
AOAC - Official methods of analysis 16th Ed (1995) Association of official analytical chemists. Washington DC
Barberis C, Astoreca A, Asili R, Fernandez-Juri G, Chulze S, Magnoli C, Dalcero A (2009) In vitro control of growth and ochratoxin A production by butylated hydroxyanisole in Aspergillus section Nigri species. Food Control 20:709–715. https://doi.org/10.1016/J.FOODCONT.2008.09.003
Barberis C, Astoreca A, Fernandez-Juri MG, Dalcero AM, Magnoli C (2010) Effect of antioxidant mixtures on growth and ochratoxin a production of Aspergillus section Nigri species under different water activity conditions on peanut meal extract agar. Toxins (Basel) 2:1399–1413. https://doi.org/10.3390/toxins2061399
Benito N, Carranza CS, Magnoli CE, Barberis CL (2018) Effect of atrazine on growth and production of AFB1 in Aspergillus section Flavi strains isolated from maize soils. Mycotoxin Res:1–10. https://doi.org/10.1007/s12550-018-0330-5
Bhatnagar-Mathur P, Sunkara S, Bhatnagar-Panwar M, Waliyar F, Sharma KK (2015) Biotechnological advances for combating Aspergillus flavus and aflatoxin contamination in crops. Plant Sci 234:119–132. https://doi.org/10.1016/J.PLANTSCI.2015.02.009
Cabrini SM, Calcaterra CP (2016) Modeling economic-environmental decision making for agricultural land use in Argentinean Pampas. Agric Syst 143:183–194. https://doi.org/10.1016/j.agsy.2015.12.016
Carranza CS, Bergesio MV, Barberis CL, Chiacchiera SM, Magnoli CE (2014) Survey of Aspergillus section Flavi presence in agricultural soils and effect of glyphosate on nontoxigenic A. flavus growth on soil-based medium. J Appl Microbiol 116:1229–1240. https://doi.org/10.1111/jam.12437
Carranza CS, Aluffi ME, Barberis CL, Magnoli CE (2016a) Evaluation of chlorpyrifos tolerance and degradation by non-toxigenic Aspergillus section Flavi strains isolated from agricultural soils. Int J Curr Microbiol AppSci 5:1–18. https://doi.org/10.20546/ijcmas.2016.507.001
Carranza CS, Barberis CL, Chiacchiera SM, Dalcero AM, Magnoli CE (2016b) Isolation of culturable mycobiota from agricultural soils and determination of tolerance to glyphosate of nontoxigenic Aspergillus section Flavi strains. J Environ Sci Heal B 51:35–43. https://doi.org/10.1080/03601234.2015.1080491
Chavas JP, Shi G, Lauer J (2014) The effects of GM technology on maize field. Crop Sci 54:1331–1335. https://doi.org/10.2135/cropsci2013.10.0709
Cooper J, Dobson H (2007) The benefits of pesticides to mankind and the environment. Crop Prot 26:1337–1348. https://doi.org/10.1016/J.CROPRO.2007.03.022
Dayo E, Oluwaniyi TT (2015) Mycoflora, proximate composition and nutritional changes during the storage of Oryza sativa. J Seek 40:108–116
Di Rienzo J, Casanoves F, Balzarini M, Gonzalez L, M T, Robledo C (2008) InfoStat Versión 2018
EC - European Commission Implementing Regulation (EU) 2020/17 of 10 January 2020 concerning the non-renewal of the approval of the active substance chlorpyrifos-methyl, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concer. https://eur-lex.europa.eu/legal-content/GA/TXT/?uri=CELEX:32020R0017. Accessed 28 Jul 2020
ESFA - European Food Safety Authority (2019) Statement on the available outcomes of the human health assessment in the context of the pesticides peer review of the active substance chlorpyrifos. EFSA J 17:e05809. https://doi.org/10.2903/j.efsa.2019.5809
Food Safety (2020) Chlorpyrifos & Chlorpyrifos-methyl | Food Safety. https://ec.europa.eu/food/plant/pesticides/approval_active_substances/chlorpyrifos_chlorpyrifos-methyl. Accessed 2 Sept 2020
Gallo A, Solfrizzo M, Epifani F, Panzarini G, Perrone G (2016) Effect of temperature and water activity on gene expression and aflatoxin biosynthesis in Aspergillus flavus on almond medium. Int J Food Microbiol 217:162–169. https://doi.org/10.1016/j.ijfoodmicro.2015.10.026
Gareis M, Ceynowa J (1994) Einfluß des Fungicids Matador (Tebuconazole/Triadimenol) auf die Mykotoxinbildung durch Fusarium culmorum. Z Lebensm Unters Forsch 198:244–248. https://doi.org/10.1007/BF01192603
Geisen R (1996) Multiplex polymerase chain reaction for the detection of potential aflatoxin and sterigmatocystin producing fungi. Syst Appl Microbiol 19:388–392. https://doi.org/10.1016/S0723-2020(96)80067-1
Hua F, Yunlong Y, Xiaoqiang C, Xiuguo W, Xiaoe Y, Jingquan Y (2009) Degradation of chlorpyrifos in laboratory soil and its impact on soil microbial functional diversity. J Environ Sci 21:380–386. https://doi.org/10.1016/S1001-0742(08)62280-9
IARC – International Agency for Research on Cancer (2002) Monograph on the evaluation of carcinogenic risk to humans. Vol 82: some traditional herbal medicines, some mycotoxins, naphthalene and styrene. pp. 84-86. Lyon, France
John EM, Shaike JM (2015) Chlorpyrifos: pollution and remediation. Environ Chem Lett 13:269–291. https://doi.org/10.1007/s10311-015-0513-7
Klich MA (2002) Identification of common Aspergillus species. Utrecht, The Netherlands; Centraalbureau voor Schimmelcultures. ISBN 90-70351-46-3
Magan N, Aldred D (2007) Post-harvest control strategies: minimizing mycotoxins in the food chain. Int J Food Microbiol 119:131–139. https://doi.org/10.1016/j.ijfoodmicro.2007.07.034
Magan N, Hope R, Cairns V, Aldred D (2003) Post-harvest fungal ecology: impact of fungal growth and mycotoxin accumulation in stored grain. Eur J Plant Pathol 109:723–730. https://doi.org/10.1023/A:1026082425177
Mandeel QA (2005) Fungal contamination of some imported spices. Mycopathologia 159:291–298. https://doi.org/10.1007/s11046-004-5496-z
Mateo EM, Gómez JV, Gimeno-Adelantado JV, Romera D, Mateo-Castro R, Jiménez M (2017) Assessment of azole fungicides as a tool to control growth of Aspergillus flavus and aflatoxin B1 and B2 production in maize. Food Addit Contam A 34:1039–1051. https://doi.org/10.1080/19440049.2017.1310400
Ministerio de Agricultura Presidencia de la Nación (2018) Estimaciones agrícolas. Distribución de la superficie agropecuaria según el uso actual de los suelos. Available on: https://datos.agroindustria.gob.ar/dataset/estimaciones-agricolas. Accessed 14 Dec 2019
Nesci A, Etcheverry M (2002) Aspergillus section Flavi populations from field maize in Argentina. Lett Appl Microbiol 34:343–348. https://doi.org/10.1046/j.1472-765X.2002.01094.x
Pechanova O, Pechan T (2015) Maize-pathogen interactions: an ongoing combat from a proteomics perspective. Int J Mol Sci 16:28429–28448. https://doi.org/10.3390/ijms161226106
Pildain MB, Cabral D, Vaamonde G (2005) Poblaciones de Aspergillus flavus en maní cultivado en diferentes zonas agroecológicas de la Argentina, caracterización morfológica y toxigénica. RIA 34:3–19
Pina JI (2012) Clasificación toxicológica y etiquetado de productos fitosanitarios. Criterios regulatorios locales e Internacionales. ILSI Argentina 3:10–39
Salem A Ben, Rouard N, Devers M, Béguet J, Martin-Laurent F, Caboni P, Chaabane H, Fattouch S (2018) Environmental fate of the insecticide chlorpyrifos in soil microcosms and its impact on soil microbial communities. In: Euro-Mediterranean Conference for Environmental Integration. Springer, Cham, pp 387–389
Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B (2010) Food and indoor fungi: Second Edition. 2nd. Utrecht, The Netherlands: CBS-KNAW Fungal Biodiversity Centre, pp. 390
Samson RA, Visagie CM, Houbraken J, Hong S-B, Hubka V, Klaassen CHW, Perrone G, Seifert KA, Susca A, Tanney JB, Varga J, Kocsubé S, Szigeti G, Yaguchi T, Frisvad JC (2014) Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol 78:141–173. https://doi.org/10.1016/J.SIMYCO.2014.07.004
Trucksess MW, Stack ME, Nesheim S, Albert RH, Romer TR (1994) Multifunctional column coupled with liquid chromatography for determination of aflatoxins B1, B2, G1, and G2 in corn, almonds, brazil nuts, peanuts, and pistachio nuts: collaborative study. J AOAC Int 77:1512–1521
WHO – World Health Organization (2016) The international code of conduct on pesticide management – guidelines on highly hazardous pesticides. Rome, Food and Agriculture Organization of the United Nations; Geneva, World Health Organization. Available on: https://apps.who.int/iris/bitstream/handle/10665/205561/9789241510417_eng.pdf;jsessionid=054FFB235F014FDC710705192FBAF8ED. Accessed 16 Dec 2019
Williams WP, Ozkan S, Ankala A, Windham GL (2011) Ear rot, aflatoxin accumulation, and fungal biomass in maize after inoculation with Aspergillus flavus. F Crop Res 120:196–200. https://doi.org/10.1016/J.FCR.2010.10.002
Wu F, Guclu H (2013) Global maize trade and food security: Implications from a social network model. Risk Anal 33:2168–2178. https://doi.org/10.1111/risa.12064
Funding
This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT-PICT- 0943/14) and Secretaría de Ciencia y Técnica, Universidad Nacional de Río Cuarto (SECYT-UNRC- 18/453).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Magnoli, K., Benito, N., Carranza, C. et al. Effects of chlorpyrifos on growth and aflatoxin B1 production by Aspergillus section Flavi strains on maize-based medium and maize grains. Mycotoxin Res 37, 51–61 (2021). https://doi.org/10.1007/s12550-020-00412-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-020-00412-w