Abstract
The inhibition behaviour on mild steel (MS) of Robinia pseudoacacia leaves extract (RPLE) in different concentrations in 0.5 M HCl as a green corrosion inhibitor was investigated by using electrochemical techniques, surface analysis. Electrochemical ımpedance spectroscopy (EIS) measurements demonstrated that RPLE augments the char transfer resistance and then diminishes the double layer capacitance due to the creation of a deterrent layer on the MS with increasing concentration. Polarization measurements have shown that RPLE reducing both anodic and cathodic current density values is a mixed-type inhibitor. Also in with and without RPLE environments, the inhibition efficiencies have decreased with increasing temperature while the activation energy value increases with the addition of RPLE to acidic solution. Thermodynamic parameters (such as \(\Delta G_{\text{ads}}^{o}\) and Kads) were obtained from electrochemical measurements. Adsorption of RPLE components on MS has been descriptived of FT-IR (Fourier Transform İnfrared Spectroscopy) and SEM (Scanning Electron Microscopy) data confirming the entity of a obstacle film on MS.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Metals and their alloys hava been utilized in many practices in industries [1, 2]. In the same way, acid solutions commonly have been used in industrial processes such as pickling, chemical cleaning, descaling, acid oil-well acidizing, too [3,4,5,6,7]. During this process, metals and their alloys used in many industrial equipments are dissolved by acid solutions and reasoned with economic losses [8]. Hence, the investigation of tehir corrosion problems in aggressive environments constitutes an important class of scientific research etc. To controlled of corrosion rate in these processes, a number of organic compounds containing N, S or O atoms, π electrons taking place in triple or/and conjugated double bonds, functional electronegative groups in their structures have been researched and mostly utilized as corrosion inhibitors [9,10,11]. However, due to their high cost, increasing health problems and ecological risks, researchers have sought to find high-yield, cheaper and non-toxic inhibitors. Researches has focused primarily on plant-derived corrosion inhibitors due to their low cost and eco-friendly properties [12, 13]. These inhibitors are the extracts of leaves, barks, seeds, fruits, gum and roots of plants obtained from readily available and renewable sources [14]. The plant extracts such as Hibiscus sabdariffa [15], Neem [16] Salvia hispanica [17], Ficus carica leaves [18], Aquilaria subintergra [19], Silybum marianum L. [20], Propolis [21], Asparagus racemosus [22], Piper guineense [23], Areca catechu [24], palm leaves and seeds [25], Citrus aurantifolia [26] have been reported as good inhibitors in aggressive environments. These plant extracts have included the water-soluble photochemicals (such as phenolic compounds, alkaloids, flavonoids, catechins, co-enzymes, terpenoids containing amino acids, proteins, vitamins, polysaccharides). Furthermore, it has been reported that the existence of these photochemicals reduces the corrosion rate by forming the obstacle film on the metal surface. These components have close likeness with the conventional organic corrosion inhibitors as chemical structure [27]. For these reasons, many studies have shown that extracts of plant could were used as influential and eco-friendly “green” corrosion inhibitors. N. O. Eddy et al. [15] studied the inhibitive and adsorption properties of ethanol extract of Hibiscus sabdariffa calyx were using gravimetric, gasometric, thermometric, and Fourier transform infrared methods of monitoring corrosion in 0.1 M HCI. They showed that the maximum corrosion inhibition of about 86% was obtained using 0.5 g/L extract of Hibiscus sabdariffa calyx using gasometric anda thermometric metods. To inhibit the corrosion of carbon steel API 5LX in the presence of different bacterial strains (Bacillus subtilis A1, Streptomyces parvus B7, Pseudomonas stutzeri NA3 and Acinetobacter baumannii MN3), neem extract was investigated using weight loss, electrochemical studies including potential measurements, potentiodynamic polarization and impedance and surface analysis includes Fourier transforminfrared spectroscopy (FT-IR) by P. Parthipan et al. [16]. Highest inhibition efficiency was observed in abiotic system with AILE (81%) using weight loss. R. Haldhar et al. [26] reported that the Citrus aurantifolia leaves extract was effective in inhibiting corrosion of MS in a 0.5 M sulfuric acid solution using weight loss, Tafel and EIS. Maximum inhibition efficiency was obtained as 96.46% at a concentration of 250 mg/L of Citrus aurantifolia leaves extract. Adsorption on the MS surface of the molecules followed the Langmuir adsorption isotherm.
Robinia pseudoacacia (Black Locust) growing in the Southeast U.S.A. at first is widely disbanded as savage and planted types growing in the world’s temperate zone. For a long time, the components of RPLE are usually used in phytotherapy and pharmacology [28, 29]. These effects of RPLE can be related with the existence of photochemicals (flavonoids, glycosids, steroids, terpenoids) [30]. In the same way, due to these effects, RPLE can be used as a corrosion inhibitor for the prevention of resource loss as economic because RPLE is cheap, easily accessible and eco-frendly.
The purpose of available work is to research the inhibition behaviour and mechanism of RPLE for MS using electrochemical techniques, surface analysis (SEM, FT-IR). The reason for the selection of RPLE as an inhibitor is the asset of components containing active centers for adsorption on MS. Also, RPLE is a source of photochemicals (components such as flavonoids, glycosids, steroids, terpenoids), extracted by simple methods, which are an environmentally acceptable, cost effective, easily available, renewable material source and not previously used in corrosion tests.
2 Experimental
2.1 Preparation of Plant Extract
The fresh leaves of R. Pseudoacacia in our city were collected. They were washed with distilled water, dried at 25 °C in vacuum oven, powdered into small pieces. 6.00 g of powdered leaves were waited in ultrasonic bath in 100 mL of aqueous ethylacetate (50:50) at 30 °C for 3 h. And then obtained solution was separated by separation funnel. The separated aqueous solution was evaporated in vacuum evaporator at 80 °C to obtain the extract. The extract was dried at room temperature and then stock solutions (0.12, 0.40, 1.20, 2.00 (g/L)) of RPLE was prepared in 0.5 M HCl and used as corrosion inhibitor in the tests. The preparation of extract is briefly described below (Fig. 1).
2.2 Preparation of Electrodes and Test Solutions
The working electrodes were cut in cylinder shape from MS bar with the chemical combination as follow (wt%: 0.263 C; 0.11 Si; 0.034 S; 0.023 P; 0.012 Cr; 0.01 Mn and balance Fe) and covered with polyester outside of surface area (0.5 cm2) for electrochemical tests. Prior to tests, the working electrodes were sanded with diverse emery papers ending with 1200 grade at last. And then, it was cleaned with double distilled water, ethanol, double distilled water back to back and lastly dried in open air. 0.5 M HCl agressive solution was readied from analytical grade HCl with distilled water. The different concentrations (0.12, 0.40, 1.20, 2.00 (g/L)) of the RPLE were prepared freshly for each experiment in 0.5 M HCl solution in open air. The test were applied by checking the cell temperature under the static conditions at 25 ± 1 °C.
2.3 Electrochemical Tests and Surface Analysis
All electrochemical tests were conducted in a traditional three electrode cell containing working electrode (MS), auxiliary electrode (Platinum sheet having 2 cm2 surface area) and reference electrode (Ag/AgCl (3 M KCl)). The potential data introduced in this work were pointed to reference electrode. To investigate the inhibiton mechanism and compute the activation energy belong to corrosion treatment, polarization plots were acquired without and with 2.00 g/L of RPLE in 0.5 M HCl solutions at diverse temperatures (298, 308, 318 and 328 K). The tests were carried out after 2 h immersion at open circuit potential (OCP). Potentiodynamic polarization plots were performed with the 0.001 V s-1 scan rate and in potential range (− 0.10; 0.00) V(Ag/AgCl). EIS experiments were determined at OCP by implementing 0.005 V peak-to-peak of the alternating current signal anda in range 105–10−2 Hz of the frequency. Also, linear polarization resistance (LPR) tests was screened in range ± 0.010 V(Ag/AgCl) of OCP with the 0.001 V s−1 scan rate. To defined the PZC of MS, the Cdl data corresponding to the impedance data of MS was examined in 0.5 M HCl solution including 2.00 g/L of RPLE at diverse potentials. Electrochemical data were ensured via computerized CHI 660b electrochemical analyzer with A1420 serial number.
The surface morphologies of the polished MS and MS after exposed for 2 h without and with 2.00 g/L of RPLE in 0.5 M HCl solutions at 298 K were designated by SEM analyzer (FESEM; Model: FEI Quanta 650). In addition, the adsorption on MS surface of RPLE components and RPLE were investigated by using Model: Perkin-Elmer RX1 (ATR) FT-IR spectrophotometer on the same conditions with SEM. The extract was scanned from 480 to 4000 cm−1 wave numbers.
3 Results and Discussion
3.1 Electrochemical Impedance Spectroscopy
The MS corrosion in acidic solution with and without RPLE at distinct concentrations was researched by EIS measurement at 298 K after 1 h of immersed time. Nyquist plots obtained is existed in Fig. 2. All of the Nyquist plots at studied concentrations exhibit only one depressed capacitive loop displaying that MS corrosion is controlled with a charge transfer process [31]. Besides, the shape of Nyquist plots is usually the same, which display that the addition of RPLE in acidic media don’t affect the charge transfer mechanism [32]. In addition to, the diameters of Nyquist plots are generally noted as charge-transfer resistance but all resistances corresponding to the metal/solution interface must be computed. Hence, total resistance is the sum of the available resistances (charge-transfer resistance, film resistance, accumulation resistance, diffuse layer resistance) at the metal/solution interface and they are shown as Rct, Rf, Ra, Rd respectively [33]. Therefore, the diameters of Nyquist plots were considered Rp in place of Rct. As viewed in Fig. 2, the diameters of Nyquist plots increase with increased RPLE concentrations indicating that the RPLE components adsorb on MS surface and the corrosion resistance of MS increase. Also, this situation is associated with the creation of a inhibitive film on MS surface, and the film hinders to the charge and the mass transfers in the metal/solution interface.
The Bode and phase angel plots acquired for MS in acidic solution with and without RPLE at distinct concentrations at 298 K after 1 h of exposure time are given in Figs. 3 and 4. The one peaks in the phase-angle plots points to the presence of an equivalent circuit with one constant phase element in interface for the corrosion process (Fig. 4). The rise in the peaks expressions a more capacitive behaviour due to the adsorption of RPLE components at the interface with increasing RPLE concentration. In addition, it is observed that the maxima of the phase angle plots with RPLE are greater than that of the phase angle plot without RPLE. It has been monitored from Fig. 3 that as the RPLE concentration increases, the slope of the bode plots also increases. The adsorption of RPLE components on the MS surface may be liable for the corrosion inhibition effects of the extracts. Because, the adsorption of these components containing aromatic rings and heteroatoms (N, O) to the MS surface could obstruct the assault of corrosive ions in acidic solution by creating an obstacle for mass and charge transfer. The EIS diagrams are in harmony with each other and confirm the adsorption of RPLE components on the MS surface.
EIS data (straigth lines → fıt plots) were arranged with respect to the appropriate equivalent circuit model in Fig. 5 by using ZView2 software. The equivalent circuit model explicited as the Randles equivalent circuit contains the solution resistance (Rs) attached as series to both of the constant phase element of double layer (CPE) and Rct [34]. The Nyquist plots are not exactly semicircle as in EIS theory. The double layer in the interface of metal/solution in the Nyquist plots does not act like a real capacitor. Due to the volume difference of electrons and ions, the solution part of the double layer will be coated with a large volume by ions which is equivalent to the charge on the metal. In such cases, CPE used instead of Cdl (double layer capacitance), owing to the non-ideal capacitive state of CPE [35]. Rp, n, CPE values (acquired from EIS data and regulated by equivalent circuit) are given in Table 1. n is the surface inhomogeneitie degree (0 ≤ n ≥ ± 1). If the n value is 1, double layer capacitance act like a real capacitor [36]. The Rp values determined from LPR data were attached to Table 1, which corresponds to the percentage inhibition efficiency (η) values. η values were computed from Rp values in Eq. (1):
where Rp (with RPLE) and R′p (without RPLE) are the polarization resistance.
As viwed from Table 1, The values of Rp and n increases while CPE values diminish with the increasing concentration of RPLE in acidic media. The increment in Rct and the n values nearing to 1 is related with the creation of a barrier film on MS surface and increment of surface homogeneity on account of the adsorption of RPLE components [37, 38]. In acidic environments without RPLE, the n value is lower because the MS surface is damaged, with the addition of RPLE, the n value increases, which may be explained by the adsorption of RPLE components on the MS surface and less surface imperfections. The reducing in CPE values can be referred with a drop in local dielectric constant and/or by the thickening of the electrical double layer as a result of the adsorption of RPLE components [39]. Also, η values determined from the EIS and LPR data are compatible with each other.
3.2 Potentiodynamic Polarization Measurements
Potentiodynamic polarization plots were performed with and without RPLE at diverse concentration for MS at 298 K in 0.5 M HCl solution after 1 h of exposure time in order to comprehend the process of anodic and cathodic reactions (Fig. 6). In acidic solutions, the anodic direction of the corrosion process corresponds to the dissolution of the metal, while the cathodic direction corresponds to the generation of hydrogen gas from hydrogen ions (or to reduce oxygen gas). The inhibitor added to the acidic medium may affect the anodic or cathodic direction, or both. In polarization plots obtained are seen a decline in the cathodic and anodic current density values as associated with the rising RPLE concentration. This situation can be explained with the adsorption of RPLE components to active centers in both cathodic and anodic regions. Also, in cathodic direction, the paralell polarization plots display that the addition of RPLE to the aggressive environment diminisies the H+ ions on MS surface as well as that the hydrogen formation mechanism does not change. [40].
In the anodic polarization plots, although the current density values decrease up to the potential value~− 0.320 V/(Ag/AgCl) because of the adsorption of RPLE components to active centers in anodic regions, the current density values increase rapidly after this potential due to the desorption of the RPLE components. Therefore, the potential value ~− 0.320 V/(Ag/AgCl) can be expressed as the desorption potential. After from the desorption potential, the desorption rate of the RPLE components may be greater than their adsorption rate due to the prominent metal dissolution.
Potentiodynamic polarization parameters were identified from the polarization plots through extrapolation method (icorr (corrosion current density), Ecorr (corrosion potential), η (percentage inhibition efficiency) and cathodic Tafel slopes (βc)) [41] are represented in Table 2. η values was computed in accordance with following equation:
In Eq. (2), i′corr and icorr correspond to corrosion current densities without and with RPLE, in order of, identified by extrapolation to corrosion potential of cathodic current–potential plots.
It is obvious from Table 2 that icorr values decline significantly with rising RPLE concentration owing to the formated non- conductive layer by the adsorbed RPLE components in both regions (anodic and cathodic) on MS surface. Also, the Ecorr value tended towards the negative potantials (cathodic regions) in the existence of RPLE. This can be connected to the protective film formed on the MS surface, depending on the adsorption of the components of RPLE on the surface. Also, this tendency displays that RPLE act as the mixed type inhibitor having a greater effect on the cathodic regions. Furthermore, while βc values close together, η values increase with increasing RPLE concentration. The βc values close to each other indicate that the reaction mechanism does not changed by addition of RPLE to acid solution.
3.3 Adsorption Isotherm and Thermodynamic Parameters
In order to comprehend the adsorption shape of RPLE components on MS can be studied by researching the adsorption isotherms. However, the adsorption on metal surface of organic molecules contains a substitution process between the organic molecules and water molecules in the acidic solution, which is connected to the surface coverage degree of the molecule [42]. The surface coverage degree (θ) was computed from EIS results (Table 1) at different RPLE concentrations for the MS corrosion. The among diverse isotherms tested was defined to the Langmuir adsorption isotherm as the best fit isotherm (Fig. 7), which expressed as following:
In Eq. (3), Cinh, Kads and θ is expressed as the concentration of the RPLE, the adsorption equilibrium constant and the surface coverage degree, in order of. The plot of Cinh/θ against Cinh defined as the flat line for RPLE (Fig. 7). Kads value from the shift of the flat line for RPLE is defined as 14.97 M−1 pointing the monolayer adsorption of RPLE components on MS surface and the high adsorption ability of RPLE components [43]. Also, the powerful correlation value representing the availability of the Langmuir adsorption isotherm was found as R2 = 0.9993.
The connection between the standard free energy of adsorption \(\left( {\Delta G_{\text{ads}}^{o} } \right)\) and Langmuir adsorption isotherm is expressed by following equation:
where T and R are the absolute temperature, the universal gas Content, in order of. Water concentration was taken as \(C_{{H_{2} O}} = 55.5\). Mostly, if \(\Delta G_{\text{ads}}^{o}\) value is about 20 kJ mol−1, it is concerned with the physisorption but if \(\Delta G_{\text{ads}}^{o}\) value is − 40 kJ mol−1, it is concerned with chemisorption [44]. The \(\Delta G_{\text{ads}}^{o}\) value is computed about − 19.08 kJ mol−1 at 298 K according to Eq. (4), which points that the adsorption of RPLE components on MS is with electrostatic character. However, adsorption of molecules on metal surface can not be admited as completely physical or chemical adsorption. This situation can not be expressed clearly because the physical adsorption is accepted as the first digit of chemical adsorption. Therefore, in the adsorption of molecules on MS surface can also be probably the chemical adsorption as well as physical adsorption. In this study, \(\Delta G_{\text{ads}}^{o}\) value points that the in the adsorption of the RPLE components is also probable the chemical adsorption as well as the predominant physical adsorption [45].
3.4 Effect of Temperature
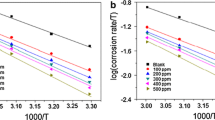
In some industries, inhibitors can be used at elevated temperatures. Often raising the test temperature can accelerate the corrosion process and significantly increase the corrosion rate. Therefore, it is useful to investigate the temperature variation of the corrosion performance of the inhibitors. The temperature influence on the inhibition performance of RPLE for MS was determined by potentiodynamic polarization measurements obtained with 2.00 g/L RPLE and without RPLE at various temperatures (298, 308, 318 and 328 K) in 0.5 M HCl (Figs. 8, 9). It is clear that the current density values rise with rising temperature at in 0.5 M HCl with 2.00 g/L RPLE and without RPLE in Figs. 8 and 9, respectively. The current density values obtained with 2.00 g/L RPLE in 0.5 M HCl were found as 0.016, 0.081, 0.217, 0.430 mA/cm2 according to the increase in temperature, respectively. The current density values without RPLE in 0.5 M HCl were found as 0.250, 0.225, 0.824, 2.36 mA/cm2, respectively. The current density values rise in both environments with rising temperature. For with RPLE medium can be clarified with increasing of the desorption of RPLE components on MS surface. In the acidic medium, this can be expressed by the acceleration in MS dissolution [46]. By comparing apparent activation energy (Ea) values at in 0.5 M HCl with and without RPLE can be acquired information about the effect of temperature on MS corrosion. By calculating the current density values from the current potential curves given in Fiğ. 8 and 9 the Ea values in acidic media with and without RPLE were computed from Arrhenius equation:
The plots of log icorr versus 1/T at in 0.5 M HCl without and with 2.00 g/L RPLE gave straight lines (Fig. 10). The Ea values computed from the slopes of the flat line for presence medias. They were defined as 65.68 kj mol−1 K-1, 88.13 kJ mol-1 K-1 at in 0.5 M HCl without and with 2.00 g/L RPLE, in order of. The high activation energy value in the existence of RPLE is concerned with the physical adsorption of RPLE components on MS surface compared to in 0.5 M HCl [47, 48]. In addition to, it may be associated with an increase in the thickness of the double layer due to the physical adsorption of RPLE components at the metal/solution interface.
3.5 Potential of Zero Charge (PZC) and the Inhibition Mechanism
Adsorption of molecules on metal surface depends mainly with the its charge, dipole moment, chemical structure and other ionic species in environment. The surface charge belong to metal is found by defining the location of Ecorr according to PZC. The double-layer capacitance (Cdl) acquired from EIS data against voltage (V) plots was used to detect the PZC. Mostly, a minimum value on the acquired plot is expressed as the surface charge of metal. According to the difference Er = Ecorr − PZC, the net metal surface charge can be identified in acidic media, Er refer to the Antropov’s ‘‘rational” corrosion potential [49, 50]. If the MS surface has a negative net charge and adsorption of cations is preferred. In contrast, adsorption of anions is preferred if Er is positive. The charges of metals during corrosion can be considered as one of the reasons determining the behavior of inhibitors. Therefore, the PZC of MS should be computed under worked conditions to express the inhibition behaviour of inhibitors.
In Fig. 11 is given the Cdl plot against the potential of MS in acidic medium with 2.00 g/L RPLE in after 1 h of waiting time. As observed in Fig. 11, the value of potential corresponding the minimum point of parabola is − 0.434 V (Ag/AgCl) called as PZC. According to Fig. 11, Er is negative value which is -0.454 V (Ag/AgCl). Hence, the surface charge of MS at Ecorr is negative. Then, on the negatively charged MS surface may adsorb the protonated RPLE components in acidic solutions [51, 52] to slow hydrogen gas formation., neutral and cationic RPLE components may be adsorbed in anodic regions by chemical absorption mechanism involving the sharing of electrons between vacant d orbits of the MS surface and tehir heteroatoms containing free electron pairs.
3.6 SEM and FT-IR Analysis
The morfologies of MS surface was perused by SEM data. In Fig. 12a–c presents SEM data obtained on polished MS and MS after 2 h of immersion time in acidic solution without and with 2.00 g/L RPLE. As can be seen, all sides of polished MS surface are not smooth and there are scratches on the surface that can be used as active centers for the initiation of corrosion reactions (Fig. 12a). As observed in Fig. 12b, on the without RPLE surface is seen damages such as some cracks, small pits and the accumulations of corrosion products stemming from the assault of agressive solution. Whereas, in the existence of RPLE apparent the creation of a inhıbitive layer on MS (Fig. 12c). It is obvious that the RPLE components protects it against to corrosion as generating a barrier layer on MS surface.
FT-IR spectrum of RPLE and its corrosion products on MS surface was applied to identify the functional groups existing in it and the interaction between MS and RPLE components. The FT-IR spectrum belong to the extract and the corrosion products on MS surface are showed in Fig. 13. In Fig. 13 is shown the diverse bonds existing in the RPLE and its corrosion products. The RPLE absorbs mainly at 3259.51, 1607.25, 1406.47, 1244.83, 1068.30, 925.98 cm−1, which are credited to –O–H stretching [16], –C = O stretching vibration [53], –C–H bending vibration [54], –C–O stretching, –C–N stretching [55], –C–H bendine [56], respectively. There is a shift in the spectrum of RPLE corrosion products according to the spectrum of RPLE components. This case exhibites that the inhibition may occur as a result of the interaction between the RPLE components and MS [21, 57, 58]. In Fig. 13 is clear that there are the existence of O, N atoms and different functional groups in RPLE components. These functional groups act as active centers that interact with the metal surface to prohibit corrosion reactions. Conventional organic inhibitors contain these atoms and functional groups, too.
3.7 The Inhibition Mechanism
The FT-IR belong to RPLE displayed the existence of heteroatom (such N, S and O), which may be derived from aromatic rings and hydrocarbons from other phytochemicals of the extracts. On the MS surface, there are active anodic and cathodic sites in contact with corrosive species, which results in a vigorous dissolution of MS. MS surface can be protected against the charge and mass transfer which causes corrosion by adsorption of the natural organic components organic components presented in the extracts, which prevents corrosion. Acorrding to the PZC, the inhibition mechanism and adsorption of the RPLE can be expressed by the adsorption of protonated RPLE components on MS surface in agressive solution. The protonated RPLE components adsorb by competing with water molecules. RPLE components control the cathodic and anodic regions. While the adsorption on the cathodic sites is formed by the protonated RPLE components in the acidic medium, the adsorption on the anodic sites takes place through the donor–acceptor interaction with Fe ions on MS surface of the lone pair electrons on the heteroatoms belong to RPLE components retarding the evolution of hydrogen (Fig. 14). For this reason, RPLE can be considered as an effective inhibitor because of forming a protective layer that limits acid attack on the MS surface due to its existing components.
4 Conculusion
Naturally obtained RPLE acted as an influential corrosion inhibitor for MS at low concentrations acidic media; While the inhibition efficiency of RPLE increments with the rising concentration, it diminishes with rising temperature. This demonstrates a state of its physical adsorption. In EIS measurement, the inhibition efficiency achieved 92% in 0.5 M HCl with 2.00 g/L RPLE at 25 °C. Polarization measurements display that RPLE components behave as an effective mixed-type inhibitor on both the cathodic and anodic regions of MS surface, but they are more effective in cathodic regions. With FTIR results, it can be eventuated that RPLE could adsorb to the MS surface to compose a denser and firmer preventive film closing both cathodic and anodic active centers. The presence of the obstacle film occured with RPLE components on the metal surface, is evidenced by EIS measurements. The corrosion performance of the RPLE was also tested at diverse temperatures and at diverse acid concentrations, indicating that RPLE can effectively delay MS corrosion in 0.5 M HCl. However, the inhibitory activity of RPLE diminished by increasing of acid concentration and temperature. The adsorption of RPLE components on MS surface confirms to Langmuir adsorption isotherm with mono layer. The possible formation of RPLE/metal complex on MS surface supported by SEM/FT-IR analyzes and PZC measurements. SEM analysis certified that the RPLE remarkably delayed surface destruction. ΔGads and Eads values have shown that in the adsorption of the RPLE components to MS surface takes place in the chemical adsorption together with the predominant physical adsorption. ΔGads notified that the adsorption of RPLE on MS at diverse temperatures was checked initially by the physical adsorption process. Also, RPLE is easily extracted, extremely inexpensive, environmentally safe and effective in slowing MS corrosion in acidic media, not formerly tried in corrosion studies. Whence, RPLE can be used as a potential corrosion inhibitor in the acidic environment for MS.
References
M.K. Bagga, R. Gadi, O.S. Yadav, R. Kumar, R. Chopra, G. Singh, J. Environ. Chem. Eng. 4, 4699 (2016)
M.E. Mashuga, L.O. Olasunkanmi, E.E. Ebenso, J. Mol. Struct. 1136, 127 (2017)
H. Bentrah, Y. Rahali, A. Chala, Corros. Sci. 82, 426 (2014)
J. Haque, C. Verma, V. Srivastava, M.A. Quraishi, E.E. Ebenso, Results Phys. 9, 1481 (2018)
P. Singh, E.E. Ebenso, L.O. Olasunkanmi, I. Obot, M. Quraishi, J. Phys. Chem. C 120, 3408 (2016)
M. Faustin, A. Maciuk, P. Salvin, C. Roos, M. Lebrini, Corros. Sci. 92, 287 (2015)
H.M.A. El-Lateef, Corros. Sci. 92, 104 (2015)
L.L. Liao, S. Moa, H.Q. Luo, N.B. Li, J. Colloid Interface Sci. 520, 41 (2018)
S. Masroor, M. Mobin, M.J. Alam, S. Ahmad, RSC Adv. 7, 23182 (2017)
R. Haldhar, D. Prasad, A. Saxena, R. Kumar, Sustaın. Chem. Pharm. 9, 95 (2018)
A. Saxena, D. Prasad, R. Haldhar, G. Singh, A. Kumar, J. Environ. Chem. Eng. 6, 694 (2018)
P. Tiwari, M. Srivastava, R. Mishra, G. Ji, R. Plakası, J. Environ. Chem. Eng. 6, 4773 (2018)
M. Mobin, R. Aslam, J. Aslam, Mater. Chem. Phys. 191, 151 (2017)
M. Gopiraman, P. Sakunthala, R. Kanmani, A.R. Vincent, N. Sulochana, Ionics 17, 843 (2011)
L.B. Molina-Ocampo, M.G. Valladares-Cisneros, J.G. Gonzalez-Rodriguez, Int. J. Electrochem. Sci. 10, 388 (2015)
P. Parthipan, J. Narenkumar, P. Elumalai, P.S. Preethi, A.U.R. Nanthini, A. Agrawal, A. Rajasekar, J. Mol. Liq. 240, 121 (2017)
I. Hermoso-Diaz, J. Gonzalez-Rodriguez, J. Uruchurtu-Chavarin, Int. J. Electrochem. Sci. 11, 4253 (2016)
T.H. Ibrahim, E.E. Gomes, I.B. Obot, M. Khamis, J. Adhes. Sci. Technol. 31, 2697 (2017)
M. Farsak, H. Keles, M. Keles, Measurement 109, 334 (2017)
S.M. Sajadi, M. Nasrollahzadeh, M. Maham, J. Colloid Interface Sci. 469, 93 (2016)
S. Varvara, R. Bostan, O. Bobis, L. Găină, F. Popa, V. Mena, R.M. Souto, Appl. Surf. Sci. 426, 1100 (2017)
A. Saxena, D. Prasad, R. Haldhar, J. Mater. Sci. 53, 8523 (2018)
E.E. Oguzie, C.E. Ogukwe, J.N. Ogbulie, F.C. Nwanebu, C.B. Adindu, I.O. Udeze, K.L. Oguzie, F.C. Eze, J. Mater. Sci. 47, 3592 (2012)
K.P. Vinod Kumar, M. Sankara Narayanan Pillai, G. Rexin Thusnavis, J. Mater. Sci. 46, 5208 (2011)
S.A. Umoren, M.M. Solomon, I.B. Obot, R.K. Suleiman, J. Adhes. Sci. Technol. 32, 1934 (2018)
R. Haldhar, D. Prasad, N. Bhardwaj, J. Adhes. Sci. Technol. 33, 1169 (2019)
E.E. Oguzie, C.K. Enenebeaku, C.O. Akalezi, S.C. Okoro, A.A. Ayuk, E.N. Ejike, J. Colloid Interface Sci. 349, 283 (2010)
N.C. Veitch, P.C. Elliott, G.C. Kite, G.P. Lewis, Phytochemistry 71, 479 (2010)
I.C. Marinas, E. Oprea, E.-I. Geana, C. Chifiriuc, V. Lazar, J. Serb. Chem. Soc. 79, 1363 (2014)
M.A. Kaloo, B.A. Bhat, G. Rafiqi, Int. J. Pharm. Pharmacol. 2, 2581 (2018)
N.R. Honesty, G. Kardaş, A.A. Gewirth, Corros. Sci. 83, 59 (2014)
L.L. Liao, S. Mo, J.L. Lei, H.Q. Luo, N.B. Li, J. Colloid Interface Sci. 474, 68 (2016)
A. Ongun Yüce, R. Solmaz, G. Kardaş, Mater. Chem. Phys. 131, 615 (2012)
E.H. Naoual, F. Rabiaa, T. Mustapha, J. Charafeddine, B. Fouad, Appl. Surf. Sci. 357, 1294 (2015)
K. Ramya, R. Mohan, A. Joseph, J. Taiwan Inst. Chem. Eng. 45, 3021 (2014)
A.K. Singh, M.A. Quraishi, Corros. Sci. 52, 152 (2010)
M.M. Solomon, S.A. Umoren, I.I. Udosoro, A.P. Udoh, Corros. Sci. 52, 1317 (2010)
I.B. Obot, N.O. Obi-Egbedi, A.O. Eseola, Ind. Eng. Chem. Res. 50, 2098 (2011)
Z. Salarvand, M. Amirnasr, M. Talebian, K. Raeissi, S. Meghdadi, Corros. Sci. 114, 133 (2017)
A. Ongun Yüce, G. Kardaş, Corros. Sci. 58, 86 (2012)
C. Verma, M.A. Quraishi, A. Singh, J. Mol. Liq. 212, 804 (2015)
V. Srivastava, J. Haque, C. Verma, P. Singh, H. Lgaz, R. Salghi, M.A. Quraishi, J. Mol. Liq. 244, 340 (2017)
I. Ahamad, R. Prasad, M. Quraishi, Mater. Chem. Phys. 124, 1155 (2010)
M. Srivastava, P. Tiwari, S.K. Srivastava, A. Kumar, G. Ji, R. Prakash, J. Mol. Liq. 254, 357 (2018)
X. Zhang, Y. Zheng, X. Wang, Y. Yan, W. Wu, Ind. Eng. Chem. Res. 53, 14199 (2014)
H. Keleş, Mater. Chem. Phys. 130, 1317 (2011)
X. Li, S. Deng, H. Fu, Corros. Sci. 55, 280 (2012)
G. Sığırcık, T. Tüken, M. Erbil, Appl. Surf. Sci. 324, 232 (2015)
H.M. Hazwan, K.M. Jain, Mater. Chem. Phys. 125, 461 (2011)
M. Lebrini, M. Lagrenee, H. Vezin, L. Gengembre, F. Bentiss, Corros. Sci. 47, 485 (2005)
A. Ostovari, S.M. Hoseinieh, M. Peikari, S.R. Shadizadeh, S.J. Hashemi, Corros. Sci. 51, 1935 (2009)
A.K. Singh, Ind. Eng. Chem. Res. 51, 3215 (2012)
A.A. Khadom, A.N. Abd, N.A. Ahmet, S. Afr, J. Chem. 25, 13 (2018)
L.L. Liao, S. Mo, H.Q. Luo, N.B. Li, J. Colloid Interface Sci. 499, 110 (2017)
N.O. Eddy, B.I. Ita, S.N. Dodo, E.D. Paul, Green Chem. Lett. Rev. 5, 43 (2012)
H. Hassannejad, A. Nuri, J. Mol. Liq. 254, 377 (2018)
M. Farsak, A. Ongun Yüce, G. Kardaş, ChemistrySelect 2, 3676 (2017)
S. Garai, P. Jaisankar, J.K. Singhc, A. Elango, Corros. Sci. 60, 193 (2012)
Acknowledgements
Author is highly thankful to Prof. Dr. Gülfeza KARDAŞ for valuable contributions and Çukurova University Faculty of Arts and Sciences Department of Chemistry Physical Chemistry Research Laboratory for supporting this work, ÇÜMERLAB for characterization of SEM images. The author also thanks to Assist. Prof. Murat FARSAK who contributed in the experimental work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yüce, A.O. Corrosion Inhibition Behavior of Robinia pseudoacacia Leaves Extract as a Eco-Friendly Inhibitor on Mild Steel in Acidic Media. Met. Mater. Int. 26, 456–466 (2020). https://doi.org/10.1007/s12540-019-00509-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12540-019-00509-7