Abstract
Biology of planktonic stomatopod larvae has long remained poorly understood and often considered “black boxes” in life history studies. From coralline crustose algal (CCA) reefs at Datan, Taoyuan, Taiwan, using light traps recently designed, we collected considerable number of stomatopod larvae and juveniles. Applying DNA barcoding techniques using mitochondrial cytochrome oxidase I (COI) and 16S ribosomal RNA (16S rRNA) gene sequences, 14 morphotypes were revealed to represent 12 distinct species, seven of which identified to species level by comparing against reference sequences available from online source (GenBank), whereas the other five do not cluster with any known sequences. All stomatopod larvae and juveniles were described and illustrated. We report Manningia pilaensis (De Man, 1888) and Levisquilla jurichi (Makarov, 1979) as new records of the stomatopod fauna of Taiwan and confirm the validity of Lysiosquillina maculata (Fabricius, 1793). Based on material we acquired from light traps, which include propelagic antizoea larvae (of L. maculata), and also postlarval and juvenile forms (of various squillid species), both positively phototactic, indicating the current understanding of negative-positive-negative phototactic tendency from early planktonic to postlarval stages through the development of stomatopod larvae, might not be as distinct as previously described.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stomatopod larvae and juveniles are morphologically very distinct among zooplankton. Compared to larvae of other crustacean groups, little is known about biology of those of stomatopods, and the subject remains poorly understood in life history studies. Stomatopods experience a bipartite lifecycle through development, passing through multiple planktonic larval stages before maturing into juveniles and adults, eventually settling on benthic habitats. Upon hatching from egg masses brooded by the female parent, juveniles undergo a brief propelagic phase, taking shelter in mother’s burrow, and then ascending into the water column to join with the zooplankton. The number of pelagic stages varies from one to nine depending on species (Ahyong et al. 2014; also see Greenwood and Williams 1984; Hamano and Matsuura 1987), after which individuals settle as postlarvae (form resembling adults). The morphology of planktonic larvae differs between superfamilies, with lysiosquillids (Lysiosquilloidea) first hatching into antizoea larvae, wanting of raptorial appendages, instead relying on yolk for nutrition. The gonodactyloids (Gonodactyloidea), parasquilloids (Parasquilloidea), and eurysquilloids (Eurysquilloidea) develop into erichthus larvae, with broad carapaces and abdomen (now pleon) (Provenzano and Manning 1978), whereas alima larvae are unique to squilloids (Squilloidea), having telson bearing more than four intermediate (IM) denticles (Morgan and Provenzano 1979; see synthesis by Ahyong et al. 2014). Some species have rather extreme or peculiar-looking larval forms (Haug et al. 2018). Larval behavior varies among stages: propelagic larvae are negatively phototactic, switching to positive phototaxis in the following planktonic stages, and again display positive thigmokinesis (preference towards the substrate) and negative phototaxis during later larval stages (Dingle 1969; Morgan and Goy 1987). Some stomatopod larvae possibly perform defensive behavior (Haug and Haug 2014). Anyhow, very little has been published on species diversity or taxonomy of stomatopod larvae in the western Pacific, particularly in East Asia.
Given nearly identical forms among closely related groups, and diagnostic features of adults not yet developed in earlier stages (not until later juvenile stages; see below), morphological identification of stomatopod larvae to species level has been notoriously difficult. Previous documentation of stomatopod larvae morphologies was performed by two major approaches, namely (1) sampling of planktonic forms, raising until reaching postlarval or juvenile stages, fostering reasonable identifications (listed by Provenzano and Manning 1978; see also e.g. Gurney 1946; Alikunhi 1952, 1967); or (2) recording stage-by-stage development of hatched individuals released from a positively identified ovigerous female (Provenzano and Manning 1978; Hamano and Matsuura 1987). Among the 70 stomatopod species (see below) so far recorded from Taiwan, only 23 had larval forms previously described in the literature (Table 1).
In recent years, DNA barcoding using mitochondrial cytochrome c oxidase subunit I (COI) (Hebert et al. 2003; also see Matzen da Silva et al. 2011) and 16S ribosomal RNA (16S rRNA) (Schubart et al. 1998, 2000), has been considered a faster and perhaps more accurate approach in assigning species in many animal groups, in comparison to traditional morphology-based identification techniques (e.g., Ward et al. 2005; Raupach and Radulovici 2015). This approach enables the assignment of unknown material (especially larvae or juveniles) by matching the sequences of identified adult voucher specimens (e.g., Webb et al. 2006; Bracken-Grissom et al. 2012; Chu et al. 2019; Li et al. 2019), and application of which in investigations on stomatopod fauna had been performed by Barber and Boyce (2006) from Red Sea, Tang et al. (2010) from shallow seas of Hong Kong, and Feller et al. (2013) and Palecanda et al. (2020) in Lizard Island, Australia. These studies revealed multiple unidentified larval forms from these regions, highlighting the still immense challenge that researchers face in recognizing and documenting biodiversity through alpha-taxonomy. Most of these molecular diversity approaches, however, do not contain morphological descriptions of identified larvae (except Feller et al. 2013).
Like various crustacean groups, stomatopods are common inhabitants of shallow reefs, dwelling within which, taking shelter, using the habitat as settlement sites and feeding and nursery grounds (Castro 1988; Steller et al. 2003; Glynn and Enochs 2011). Apart from extensive coral reefs common along tropical shores which structure formed by calcareous scleractinian corals, reefs of crustose coralline algal (CCA) are accumulations of non-geniculate coralline algae, which is a common phototrophic component of rocky shore and reef habitats. Performing similar ecological functions as coral reefs, CCA reefs are generally reported from temperate shores as extensive concretions, or reefs at shallow depths, limited by the availability of solar irradiance (see Ballesteros 2006). Along tropical and subtropical shores, on the other hand, CCA reefs are much more limited in distribution and are found only in sites of murky waters with lower light availability, while the structure is capable of resisting stronger wave actions (Bosence 1983; Adey 1998; Ballesteros 2006), growth of which reported to be very slow (Adey and Vassar 1975; also see Dethier and Steneck 2001). Between the tropics, these reefs are found at the Marshall Islands (Emery et al. 1954), Caribbean waters (Adey and Vassar 1975), and the Mediterranean (Ballesteros 2006). In this complex habitat, anyhow, in the course of assessing species diversity, detailed surveying of adult individuals of inhabiting organisms, often sampling done by scuba divers, can be excessively labor-intensive, and research efforts therefore are not as cost-effective (but see Bouchet et al. 2009), and our understanding on species diversity hence a gross underestimation (Plaistance et al. 2011). Alternatively as an innovative approach, by sampling zooplankton, planktonic young of these reef inhabitants, from the water column, likewise reflects species diversity. In the present study, a considerable number of stomatopods were collected by deploying light traps at a crustose coralline algal (CCA) reef in Taiwan. Adopting a synthetic approach, combining morphological examination (traditional taxonomy) and molecular barcoding techniques (COI and 16S rRNA sequences), we present species diversity of stomatopod larvae found in CCA reefs in northwestern Taiwan, attempting to contribute baseline information on stomatopod larval biology for the western Pacific region.
Materials and methods
Sites investigated
The only extensive CCA reef reported in the West Pacific situates in Taoyuan County, along the northwestern coast of the main island of Taiwan (Fig. 1). The CCA reefs in Datan, Taoyuan, are most extensive: measuring at least 27 km in length and a maximum width of 450 m, experiencing tidal inundation 4-m high (Dai et al. 2009; Liou et al. 2017; Fig. 1). The basal substratum of the Datan CCA reef is composed of sedimentary conglomerate with biogenic strata deposited during the Holocene (as recently as some 11,650 years ago), upon which the upper layers composed of scleractinian coral skeletons, overlain by masses of crustose coralline algae that are around 4500 years old (Wang et al. 2008; Dai et al. 2009). Dai et al. (2009) and Liou (2012) reported species diversity in the Datan CCA reefs being immensely rich. Species composition of non-geniculate coralline algae was recently reported by Liu et al. (2018), who described one new genus and three new species, whereas Kuo et al. (2019) reported the discovery of an endangered scleractinian coral Polycyanthus chiashanensis Lin, Kitahara, Tachikawa, Keshavmurthy & Chen, 2012, which previously only known from Kaohsiung, southern Taiwan, its type locality. Crustacean fauna, however, as reported by Lin et al. (2013), is represented by 25 species, most of which (21) being decapods, mostly common to intertidal rocky reef habitats. Only one stomatopod, Gonodactylus chiragra (Fabricius, 1781), was recorded.
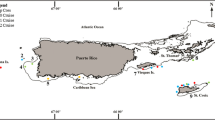
a Map of collection site at Datan, Taoyuan; b aerial view of the CCA reef (indicated by the white double arrows) (photograph taken by Chu yu-wei, Juvenile Art Center); c light trap designed in Chan et al. (2016) for collecting stomatopod larvae; d the CCA reef exposed during low tides; e, f light traps deployed subtidal region in CCA reef at night
Specimen collection and morphological examination
Zooplankton was collected along the shores of the Datan CCA reef (25° 02′ 18.8″ N, 121° 02′ 55.4″ E; see Fig. 1a, b) from May to October, 2018 (May 16, June 26, July 13, October 26), using light traps placed along the lower intertidal zone, design of the device following Chan et al. (2016) (Fig. 1c–f). The traps were deployed at the subtidal level during low tides in the evening, left overnight, and collected in the morning to noon the next day. The collected zooplankton was sieved using a 300-μm mesh net and preserved in 95% ethanol.
Sampled zooplankton was sorted and examined under stereomicroscopes (Olympus SZX7), and stomatopod larvae and juveniles were sorted based on morphology. The stomatopod material, consisting of antizoea (of lysiosquillids), erichthus and alima larvae, postlarvae and juveniles, was further identified as different morphotypes. Although postlarvae and juveniles share a body form similar to that of adults, postlarvae still retain their larval-type retina, although the structure is not as obvious in juveniles of squilloids (Ahyong et al. 2014). Representatives of each morphotype were examined and photographed, and key features (carapace, mouthparts, and telson) were illustrated with line drawings. The only antizoea morphotype collected was small in size, which was dehydrated using graded ethanol series, critical point-dried, coated with gold, and then observed and photographed under a scanning electron microscope (SEM; FEI Quanta 200, USA). Taxonomic schemes, measurements, and morphological terminology followed those presented by Ahyong (2001) and Ahyong et al. (2008). The abbreviations CL, TL, TS, AS, MD, SM, IM, and LT stand respectively for carapace length, total length, thoracic somites, abdominal somites, median, submedian, intermediate, and lateral. In planktonic larvae (antizoea, erichthus, and alima), given rostral and posterolateral spines often much elongated and easily broken off, CL is measured from the base of rostral spine, along midline to posterior margin, in addition of short postero-median spine, whereas TL is measured from the base of rostral spine, along midline to level of SM teeth of telson. Those of postlarva and juvenile individuals follow Manning (1998): CL from the base of rostral plate, along the midline to the posterior margin of carapace; TL from the anterior tip of rostral plate to the level of SM teeth of telson. The examined material was deposited into the collections of the Coastal Ecology Laboratory (CEL), Biodiversity Research Center, Academia Sinica, Taipei.
Molecular analysis
Total genomic DNA was extracted, either from entire antizoea individuals (given the small body size), or partially from larval and postlarval individuals of stomatopods (n = 38; from tissue of abdominal somites) using the DNeasy® Blood and Tissue Kit (Qiagen, California, USA) according to instructions provided by the manufacturer. Partial sequences of two mitochondrial DNA markers (COI and 16S rRNA) were amplified following the protocol from previous studies (Crandall and Fitzpatrick 1996; Folmer et al. 1994; Feller et al. 2013; Shih et al. 2019). Polymerase chain reactions (PCR) were conducted in DNA Engine Thermal Cycler (Bio-Rad, Richmond, California, USA), and the products were checked by electrophoresis on 1.5% agarose gel in 1 × TAE buffer. DNA purification and Sanger DNA sequencing were performed by Genomics BioSci & Tech Ltd. (New Taipei City, Taiwan). The sequences were assembled and edited in Geneious Prime 2020.1.1 (https://www.geneious.com).
The sequences were aligned with MUSCLE in Geneious Prime 2020.1.1 (https://www.geneious.com). Neighbor-joining (NJ) analysis with the Kimura 2-parameter (K2P) distance model and bootstrap values estimated from 1000 pseudoreplicates was conducted for COI and 16S rRNA, respectively, in MEGA X using referencing sequences downloaded from GenBank (Kumar et al. 2018; Table 2). K2P genetic distances were also calculated in MEGA X. Reference sequences downloaded from GenBank were involved in the previous molecular studies of stomatopods established in the past two decades (see Table 2).
Results
Species assignment by DNA barcoding
The neighbor-joining (NJ) phylogeny is shown in Fig. 2 (a, COI; b, 16S rRNA). Among the 38 larval and juvenile specimens examined, 25 clustered with available reference sequences with high bootstrap support (> 90%). These include Carinosquilla multicarinata (COI: MH168265; 16S rRNA: MH168221.1), Gonodactylus smithii (COI: DQ440603, MK397448, MT188302, AF205233, DQ440602, HM138788, MT188299 to MT188304; 16S rRNA: AF107615; but see below), Lysiosquillina maculata (COI: KM982431, KM982432, KM982436; 16S rRNA: AF107618, EU920935, HM138834), Oratosquilla oratoria (COI: MH168274; 16S rRNA: MH168214), and Oratosquillina inornata (COI: MH168266; 16S rRNA: MH168220.1) (Fig. 2, Table 2). Five specimens (CEL-stom-046, 047, 053, 055, and 059) were clustered with 16S rRNA reference sequence of Manningia pilaensis (KY236044), likewise one specimen (CEL-stom-062) clustered with 16S rRNA reference sequence of Levisquilla jurichi (MH168204), in both cases, but no affiliated COI reference sequence was available (Fig. 2). Seven representative specimens comprised of five distinct species (one in Gonodactylidae, three in Nannosquillidae, and one in Squillidae), but did not cluster with any reference sequences, and were thus considered unknown species. These taxa were nevertheless identifiable to the family level using morphological evidence (see discussion below).
Neighbor-joining (NJ) tree of a COI and b 16S rRNA sequences. Branch length represents Kimura 2-parameter (K2P) distances, and the bootstrap values are showed on the nodes only when > 90. “*” indicated those specimens that are not clustered to any reference sequence with COI but do cluster to reference sequence with 16S rRNA. Details of specimens from the present study (in bold, starting with CEL-stom) and those from reference sequences derived from GenBank are listed in Table 2
The frequency distributions of K2P genetic distances of COI and 16S rRNA sequences are shown in Fig. 3. For COI sequences, K2P genetic distances within species were below 2%, except for the specimens assigned as G. smithii, which showed distances above 4.5% (4.95 to 16.13%) to the reference sequences (Table S1; see below). Distances between species ranged from 6.97 to 30.74% (Table S1). As for 16S rRNA sequences, K2P genetic distances within species were below 0.91%, except for the specimens assigned as G. smithii, which showed distances ranged from 0 to 2.77% to the reference sequence, and between two reference sequences of Alima orientalis (3.47%). The distance between species ranges from 1.13 to 25.85% (Table S2). Specimens assigned as Carinosquilla multicarina and Oratosquillina inornata were represented by both alima larvae and juveniles in our material. Fourteen morphotypes, assigned as 12 distinct species, were verified by molecular approaches and are enumerated and illustrated below.
Systematic account
Superfamily Gonodactyloidea Giesbrecht, 1910.
Family Gonodactylidae Giesbrecht, 1910.
Gonodactylus smithii (Pocock, 1893)
Fig. 4
Gonodactylus smithii Pocock, 1893 (postlarva: CEL-stom-056): a carapace; b left mandible, posterior view; c left mandible, mesial view; d left maxillule; e left maxilla; f left maxilliped 1; g right maxilliped 2; h–j left maxillipeds 3 to 5; k telson and left uropod
Description (based on individual CEL-stom-056). Postlarva, CL 1.8 mm, TL 8.5 mm. Specimen overall subcylindrical in cross-section, devoid of conspicuous pigment. Eyestalk inflated, cornea spheroidal. Rostral plate as long as broad, triangular, anteriorly produced as an acute spine, lateral margins markedly concave. Carapace glabrous, gastric grooves extending 1/4 of length from bases of rostral plate (Fig. 4a). All maxillipeds epipod-bearing; raptorial appendage robust, propodus and dactylus unarmed along occlusal margin, dactylus mildly inflated proximally on flexor margin (Fig. 4g). TS5–8, to AS5 glabrous, devoid of longitudinal ridges. AS6 posteriorly armed with small but distinct SM, IM and LT spines (Fig. 4k). Telson longer than broad, markedly sculptured, MD carina stout; SM carina pronounced, each terminating with an elongated movable spine, between which armed with 11 to 12 SM denticles on each side; pronounced tooth situated between SM and IM tooth, each notch bearing a small denticle; LT tooth defined by a notch along the lateral margin. Uropod robust, penultimate segment of exopod bearing 7 stout LT spines and 1 distal spine (Fig. 4k).
Type locality. Arafura Sea.
Remarks. Molecular analyses showed that the COI sequences of several postlarval individuals from Taiwan (CEL-stom-056, 058, 061) are indeed conspecific (K2P distance 0.46 to 0.61%), while marginally matching with those identified as Gonodactylus smithii on GenBank (accession nos. DQ440603, MK397446, AF205223, DQ440602, HM138788, MT188299 to MT188304), with nucleotide (K2P) distances of 4.95 to 16.13%. Distances between sequences below 3% considered conspecific, those exceeding 5% being interspecific variation (Hebert et al. 2003). As addressed by Palecanda et al. (2020), in the case of G. smithii, based on reference published on GenBank and ones newly acquired, genetic divergence of COI sequences among their three putative species can vary by as much as 20%. Our material from Taiwan comprises of the fourth clade, sister to their “G. smithii species 1” (K2P distance 4.95 to 6.98%; Fig. 2a; Table S2), adding to the heterogeneity of the “G. smithii species complex.” This discrepancy might be result of genetic distance not as effective in species delineation and identification (see e.g., Meier et al. 2006), or alternatively, misidentification of relevant material.
Previous taxonomic studies based largely on morphological approaches gave the distribution range of G. smithii from the western Indian Ocean to the Red Sea, towards Vietnam, Australia, and New Caledonia (Serène 1947; Holthuis 1967; Manning 1968; Manning and Lewinsohn 1986; Manning 1995; Ahyong 2001). Hwang and Yu (1980) recorded the species based on material from Lanyu, Taiwan, under the name Pseudosquilla ornata (Ahyong et al. 2008). Taxonomic works already indicated marked variations particularly the form of the rostral plate, and the following extremely similar species are involved: G. acutirostris De Man, 1898 (type locality: Mergui) (considered synonymous with G. smithii by Serène (1954) and Holthuis (1967), but Manning and Lewinsohn (1986) and Manning (1995) kept both species distinct), G. chiragra var. anancyrus Borradaile, 1900 (type locality: New Britain, Papua New Guinea, and Loyalty Islands), G. minikoiensis Ghosh, 1990 (type locality: Minikoi, Lakshadweep), and G. arabica Ghosh, 1990 (type locality: Kavaratti, Lakshadweep). The above three species were placed under a synonymy of G. smithii by Manning (1995) and Ahyong (2001). Ahyong (2005) examined material from Sodwana Bay, South Africa, and remarked that the species G. smithii, as previously understood, was probably heterogeneous (also see Ahyong et al. 2008).
Gonodactylidae species
Fig. 5
Description (based on individual CEL-stom-027, and telson based on CEL-stom-026). Erichthus larva, CL 2.0 mm, TL 4.5 mm (CEL-stom-026). Carapace elongated, elevated along midline, extending towards posterior margin of AS2 (Fig. 5a), length excluding rostral and posterolateral spines approximate that of AS1 to telson; rostrum awl-shaped, ventrally armed with 4 oblique slender spines, anterolateral angle as a mere conical tubercle; posterior spine short, not extending beyond posterior margin, posterolateral spine prominent. Antennule triramous, flagella yet differentiated. Eyestalks inflated, cornea spheroidal. Only first two maxillipeds developed, both epipod-bearing, which small but discernable; raptorial claw propodus serrated along occlusal margin (Fig. 5g); dactylus not inflated, unarmed. AS6-telson yet differentiated despite faintly discernable margins, longer than broad, medially bearing 2 small posterior-oriented spines on proximal 1/5, laterally armed with 2 minute spines before posterolateral angle, posterior margin armed with approximately 24 minute spinules (Fig. 5h).
Remarks. Molecular evidence based on COI and 16S rRNA mitochondrial sequences place the present form, represented by two specimens (CEL-stom-026 and 027), among the Gonodactylidae, yet precise identities remain uncertain. Given the specimen CEL-stom-026 with telson and preceding somites damaged, telson of this form is illustrated based on another individual, CEL-stom-027.
Superfamily Eurysquilloidea Manning, 1977.
Family Eurysquillidae Manning, 1977.
Manningia pilaensis (De Man, 1888)
Fig. 6
Manningia pilaensis (De Man, 1888) (erichthus larva: CEL-stom-046): a overall habitus, lateral view; b carapace and left maxilliped 2, dorsal view; c left mandible, posterior view; d mandible, mesial view; e left maxillule; f right maxilla; g left maxilliped 1; h left maxillipeds 3 to 5; i telson and left uropod
Description (based on individual CEL-stom-046). Erichthus larva, CL 2.5 mm, TL 7.2 mm. Carapace elongated, extending along midline to TS7, length excluding rostral and posterolateral spines less than that of AS1 to 4; rostrum compressed, blade-like, ventrally armed with at least 6 slender, oblique spines; anterolateral spine acute, not reaching bases of cornea; posterior spine strong but short, merely producing beyond outline of carapace, dorsal of which armed with another shorter accessory spine; posterolateral spine prominent, reaching posterior margin of AS2, ventrally armed with a short accessory spine (Fig. 5a). Antennules of three-segmented stalk, distally reaching to the tip of rostrum anteriorly. Eyestalks inflated, cornea spheroidal (Figs. 6a, b). All maxillipeds epipod-bearing, of which rounded; raptorial appendages propodus pectinated with minute spines along occlusal margin, proximally bearing 1 slender movable spine; dactylus slender, teeth along flexor margin barely discernable (Figs. 6a, b). AS6 posteriorly armed with small but distinct SM spines. Telson longer than broad, medially elevated, SM teeth pronounced, apexes fixed, breadth between exceeding half of telson width, medially divided by a shallow V-shaped notch, along the margin lined with 14 SM denticles on each side; IM and LT teeth prominent, apexes fixed, each with a small denticle. Uropod developed, distal segment of exopod armed with 2 LT spines.
Type locality. Elphinstone Island, Mergui Archipelago, Myanmar.
Remarks. Molecular analyses showed that the 16S rRNA mitochondrial sequences of several erichthus larvae (individuals CEL-stom-046, 047, 053, 055, 059) matched those of Manningia pilaensis (GenBank accession no. KY236044; K2P distance: < 0.64%). Distribution of this cryptic reef species ranges from the eastern Indian Ocean (Mergui Archipelago, Myanmar) to Vietnam and South China (Amoy = Xiamen) (see Manning 1967, 1995; also as M. serenei), and the herein report from Taiwan is not unexpected. The genus Manningia Serène, 1962, now includes 11 species (Ahyong 2001; see also Manning 1995), which largely found in the Indo-West Pacific, except for M. posteli Manning, 1977, which was recorded from Congo to Guinea, West Africa. The genus was described in a generic revision of Pseudosquilla Dana, 1852, by Serène (1962), raised to familial status by Manning (1977) under Gonodactyloidea (see Manning 1995), and later identified as a distinct superfamily by Ahyong (2001). Despite the lack of adult material from Taiwan, the current report nevertheless represents the first record of the Eurysquillidae and Eurysquilloidea from Taiwan (see Ahyong et al. 2008).
Superfamily Lysiosquilloidea Giesbrecht, 1910
Family Lysiosquillidae Giesbrecht, 1910
Lysiosquillina maculata (Fabricius, 1793)
Fig. 7
Lysiosquillina maculata (Fabricius, 1793) (antizoea larva): a overall habitus, lateral view; b carapace, dorsal view; c telson, dorsal view
Description (based on individual in SEM images). Antizoea larva, CL 1.2 mm, TL 1.9 mm. Carapace elongated, cylindrical, rostral spine slender, approximately 1/3 of CL, slightly curved upwards distally, entire dorsally and ventrally; posterior spine slender, short; posterolateral spine slender, approximately 1/5 of CL; corneal spheroidal, sessile, still undifferentiated from carapace (Fig. 7a, b). Antennule uniramous. Maxillipeds 1 to 5 biramous. Pleopods and uropods not yet developed (Fig. 7a). Telson depressed, unornamented, laterally armed with acute MG, LT, IM and SM teeth, posteriorly lined with 12 minute SM denticles (Fig. 7c).
Type locality. Manado, Indonesia (neotype; Ahyong 2001).
Remarks. Many small antizoea individuals were captured during our study, and COI and 16S rRNA mitochondrial gene sequences were amplified from eight representative individuals (CEL-stom-007, 064, 067, 069, 073, 074, 076, 077). Given unsatisfactory condition of the small and fragile antizoea specimens, sequencing from most individuals failed (over 70 individuals sequenced). Anyhow, COI sequences from all eight individuals cluster with Lysiosquillina maculata (GenBank accession nos. KM982431, KM982432, KM982436: from Lizard Island, Australia), with intraspecific genetic K2P distance ranging from 0 to 0.92% (Fig. 2a, Table S2). Phylogeny derived from 16S rRNA sequences also indicates our material being conspecific with Lysiosquillina maculata (GenBank accession nos. HM138834, AF107618, EU920935), with K2P distance less than 0.91% (Fig. 2a, Table S2). From morphological examinations, we interpret very subtle morphological variations among samples as intraspecific. We have no evidence suggesting multiple lysiosquilloid species being represented in this bulk antizoea lot. As such, we confirm the presence of L. maculata in Taiwan.
Records of Lysiosquillina maculata (previously Lysiosquilla) had been recorded in Taiwan starting from the early twentieth century (Fukuda 1913; Komai 1927; Lee and Wu 1966). However, Ahyong et al. (2008) considered the specimen represented by this name by Lee and Wu (1966) to be Lysiosquilla tredecimdentata instead. The same study only verified L. tredecimdentata and L. sulcirostris Kemp, 1913, as records of Lysiosquillidae from Taiwan. However, Lysiosquillina maculata sensu stricto was confirmed in Japan by Hamano (1989: material from Ryukyus), and its presence in Taiwan is not unexpected. While other large lysiosquillids often found in deeper waters of muddy substrates and captured by trawls, Lysiosquillina maculata prefers shallow subtidal sandflats in vicinity of coral reefs (S. T. Ahyong, pers. comm). This possibly accounts for the absence of L. maculata sensu stricto in past Taiwanese records (see “Discussion” section below).
In attempt to resolve records of Lysiosquillidae in Taiwan, we sequenced one large adult male individual from our laboratory collections identified as Lysiosquilla tredecimdentata Holthuis, 1941 (based on Ahyong et al. 2008), purchased from the Donggang fish market, Pingtung, Taiwan (CL: 27.3 mm, TL: 134.1 mm; as CEL-stom-093). Based on COI and 16S rRNA sequences, this specimen is shown to be distinct from antizoea individuals herein identified as L. maculata (genetic K2P distance: 14.94 to 15.14% for COI, 8.63% for 16S rRNA).
Family Nannosquillidae Manning, 1980
Nannosquillidae species 1
Fig. 8
Description (based on individual CEL-stom-045). Postlarva, CL 2.1 mm, TL 10.1 mm. Specimen overall dorsoventrally depressed, devoid of conspicuous pigment. Eyestalk stout, cornea slightly inflated. Rostral plate rounded triangular, slightly broader than long, distally rounded, laterally nearly straight; carapace depressed, glabrous, devoid of marked longitudinal grooves (Fig. 8a). All maxillipeds epipod-bearing, those of maxillipeds 1 and 2 more pronounced, transversely auricular, of maxillipeds 3 and 4 smaller, rounded; raptorial appendages robust; propodus broad, subovate, along occlusal margin pectinated, proximally bearing 2 elongated movable spines; dactylus armed with 6 teeth, along extensor margin bearing 2 low proximal lobes (Fig. 8a); propodus of maxilliped 4 distinctly subquadrate. Telson approximately 1.5 times broader than long, subquadrate; MD carina stout, posteriorly terminating as an acute spine; SM teeth fixed, prominent, breadth between occupying more than 1/3 of telson width, between which armed with 12 denticles on each side; IM and LT teeth prominent, lined with several small IM and 1 LT denticles (Fig. 8j). Uropod robust, protopod strongly bifurcate; endopod ovate, bearing marked proximal fold; penultimate segment of exopod laterally armed with 3 acute spines, distal segment subovate, mesial margin sinuous (Fig. 8j).
Remarks. Molecular evidence based on COI and 16S rRNA mitochondrial sequences showed that three forms—herein indicated as Nannosquillidae species 1 to 3—at species level not matching any of the available reference sequences. Based on COI sequences, the three nannosquillid species are shown to be distinct, while all showing some affinity to Alachosquilla vicina (Nobili, 1904) (GenBank accession no. KM982440 and AF107608) with high bootstrap values (Fig. 2), a nannosquillid having broad range of distribution across the Indo-West Pacific (Ahyong 2001). The three distinct species were each represented by limited number of postlarval individuals (sp. 1: 1 specimen; sp. 2: 1 specimen; sp. 3: 2 specimens), all bearing a marked proximal fold on the uropodal endopod (Figs. 8j, 9j, 10k), a diagnostic feature in adults of the family (see Manning 1995; Ahyong 2001; Ahyong et al. 2008), already well developed in earlier postlarval stages. Other diagnostic features allowing identification to generic level, such as form of rostral plate, are still wanting in our material, and not developed until early juvenile stages, whereas this Nannosquillidae species 1 probably belongs to the "Acanthosquilla" group (S. T. Ahyong, pers. comm.).
So far seven nannosquillid species have been recorded from Taiwan: Acanthosquilla derijardi Manning, 1970, A. manningi Makarov, 1978, A. multifasciata (Wood-Mason, 1895), Bigelowina phalangium (Fabricius, 1798), Pullosquilla litoralis (Michel & Manning, 1971), P. pardus (Moosa, 1991), and P. thomassini Manning, 1978 (Ahyong et al. 2008; Wang and Chiou 2017). Further molecular analyses may reveal the identities of the three postlarval forms reported herein.
Nannosquillidae species 2
Fig. 9
Description (based on individual CEL-stom-086). Postlarva, CL 2.1 mm, TL 10.8 mm. Specimen dorsoventrally depressed, devoid of conspicuous pigment. Eyestalks stout, cornea spheroidal. Rostral plate triangular, slightly broader than long, laterally mildly concave; carapace depressed, glabrous, longitudinal grooves nearly wanting (Fig. 9a). All maxillipeds epipod-bearing, those of maxillipeds 1 to 3 more pronounced, somewhat cordiform; raptorial appendage robust, propodus markedly broad, ovate, occlusal margin pectinated, proximally of 1 elongated movable spine, dactylus armed with 6 teeth, along extensor margin bearing 2 low proximal teeth (Fig. 9f); propodi of maxillipeds 3 and 4 distinctly subquadrate. Telson approximately 1.5 times broader than long, subquadrate, median carina stout, posteriorly of an acute spine; SM teeth fixed, prominent, breadth between occupying more than half of telson, each side armed with about 18 SM denticles, medial cleft wanting; IM and LT teeth prominent, each accompanied by small denticle (Fig. 9j). Uropod robust, protopod strongly bifurcated, endopod ovate, proximally folded; penultimate segment of exopod laterally armed with 3 small but distinct acute spines, distal segment ovate, lined with 1 longitudinal crest (Fig. 9j).
Remarks. The three forms of Nannosquillidae species which have high bootstrap value in their COI and 16S rRNA phylogenies, share a proximally folded uropodal endopod, a diagnostic feature of the family. The three forms are nevertheless distinct and identifiable by (1) carapace of species 3 bearing marked longitudinal (gastric) grooves, which is vaguely defined or absent in species 1 and 2; (2) raptorial appendage dactylus of species 1 and 2 armed with 6 spines, whereas that of species 3 has 8; (3) form of telson, especially marginal armature, and relative width between both submedian spines; (4) uropodal endopod of species 2 and 3 sub- to rounded triangular, while that of species 1 ovate; and (5) penultimate segment of uropodal exopod laterally of 2 to 3 spines in species 1, 4 to 5 spines in species 2 and 6 acute spines in species 3. It remains nearly impossible to make solid identifications based solely on morphological characters of adults given in past taxonomic works. See also Remarks for Nannosquillidae species 1 above.
Nannosquillidae species 3
Fig. 10
Description (based on individual CEL-stom-063). Postlarva, CL 2.0 mm, TL 9.5 mm. Specimen dorsoventrally depressed, devoid of conspicuous pigment. Eyestalks stout, cornea spheroidal. Rostral plate rounded triangular, broader than long, distally rounded, lateral margins concave; carapace depressed, glabrous, longitudinal groove marked (Fig. 10a). All maxillipeds epipod-bearing, those of anterior 4 more pronounced; raptorial appendages robust; propodus occlusal margin pectinated, proximally bearing 2 elongated movable spines, dactylus armed with 8 teeth, along extensor margin bearing 2 low proximal lobes (Fig. 10g); propodi of maxillipeds 3 and 4 well developed, that of 3 rounded triangular, that of 4 distinctly subquadrate. Telson approximately 1.3 times broader than long, median carina stout, posteriorly terminating in an acute spine; SM teeth fixed, prominent, breadth between occupying more roughly half of telson width, medially separated by a shallow V-shaped cleft, on each side armed with 8 to 9 SM denticles; laterally armed with prominent IM and LT teeth, each armed with 1 small denticle (Fig. 10k). Uropod robust, protopod strongly bifurcate; endopod subtriangular, bearing marked proximal fold; exopod penultimate segment laterally armed with 6 acute spines, distal segment ovate (Fig. 10k).
Remarks. See Remarks for both Nannosquillidae species above.
Superfamily Squilloidea Latreille, 1802.
Family Squillidae Latreille, 1802.
Squillidae species
Fig. 11
Description (based on individual CEL-stom-082). Postlarva, CL 3.7 mm, TL 16.5 mm. Specimen squilloid-type, overall with dark spots arranged into short longitudinal markings symmetrical along midline. Eyestalks stout, cornea inflated, bilobed. Rostral plate slightly longer than broad, triangular, medial ridge conspicuous (Fig. 11a). Carapace overall glabrous, along midline furnished with dorsal pit, and a slightly elongated depression behind base of rostral plate; gastric groove apparent, extending 2/3 of carapace length; IM and LT carinae marked (Fig. 11a). Anterior 4 maxillipeds epipod-bearing, those of maxillipeds 3 and 4 small, rounded; raptorial appendage robust, propodus proximally armed with two slender movable spines, occlusal margin pectinated; dactylus armed with 5 spines (Fig. 11g). TS5 to 8 bearing marked submedian and lateral carinae, TS5 laterally bilobed, anterior one acute, curved, lower than the posterior lobe; TS6 single-lobed, anterior margin sinuous (Fig. 11k). AS armed with pronounced SM, IM and LT carinae; SM carinae of AS5 and 6 produced posteriorly (Fig. 11l). Telson longer than broad, median carina well-defined, prominent; submedian teeth fixed, separated medially by broad V-shaped notch, on each side armed with 5 to 7 SM denticles, IM (10 to 11 IM denticles) and LT carinae prominent (Fig. 11l). Uropod robust, protopod bifurcated, mesial tooth bearing a pronounced rounded lobe on outer margin; exopod penultimate segment laterally armed with eight stout spines laterally (Fig. 11l).
Remarks. Considering morphology of the single specimen examined (CEL-stom-082), the placement into Alima Leach, 1817, appears to be appropriate, given (1) lateral process of TS5 bilobed and TS6 and 7 single-lobed and (2) raptorial claw merus distally unarmed along outer inferodistal margin. Two species of Alima have been recorded from Taiwan, namely A. orientalis Manning, 1978, and A. hieroglyphica (Kemp, 1911) (Ahyong et al. 2008). Comparison of COI reference sequences of Alima species acquired from GenBank (voucher specimens from Lizard Island, Australia; Feller et al. 2013), our material match neither A. orientalis (KF205337, KF214292, MT179626; K2P distance 18.01 to 18.63%), A. pacifica (HM138774, KM982423, KM982434; 20.57 to 21.25%), nor a phylogenetically close squilloid Busquilla plantei Manning, 1978 (HM138775; 22.09%). Results derived from 16S rRNA sequences are likewise inconclusive: showing affinity with Alima orientalis Manning, 1978 (HM138814; 7.64%), but with low bootstrap support. As such, we remain to report the present form as Squillidae species.
Carinosquilla multicarinata (White, 1848)
Carinosquilla multicarina (White, 1848) (alima larva: CEL-stom-010): a carapace, dorsal view; b carapace, lateral view; c left mandible, posterior view; d left mandible, mesial view; e left maxillule; f left maxilla; g right maxilliped 1; h left maxilliped 2; i right maxillipeds 3 to 5; j telson and right uropod
Description: alima larva (based on individual CEL-stom-010). CL 6.0 mm, TL 14.4 mm. Carapace elevated along midline, elongated, posterior margin reaching TS7, posterolateral spine reaching AS1; rostral spine slender, elongated, about 1/3 length of carapace (spines exclusive); posterior spine short; lateral margin towards posterolateral spine armed with about 8 small denticles, separately dispersed along the ventral-internal margin (Fig. 12a, b). Antennules of three-segmented stalk, reaching to the tip of rostrum anteriorly, distally bearing triramous segmented flagella. Eyestalks inflated, cornea spheroidal (Fig. 12a). Maxillipeds well developed; anterior 2 maxillipeds epipod-bearing, of which rounded; raptorial claw propodus minutely pectinated along occlusal margin, proximally armed with 2 acute fixed spines, dactylus unarmed (Fig. 12c). Pereiopods well developed. AS6 and telson yet articulating despite faint suture discernable; AS6 armed posteriorly with SM spines (Fig. 12j). Telson markedly longer than broad, SM teeth pronounced (4 SM denticles on each side); IM (9 IM denticles) and LT teeth pronounced, the latter confluent with lateral margin (Fig. 12j). Uropod developed, penultimate segment of exopod laterally armed with 5 acute spines (Fig. 12j).
Description: juvenile (based on individual CEL-stom-085). CL 3.1 mm, TL 14.8 mm. Specimen squilloid-type, overall with dark streaks arranged into short longitudinal markings symmetrical along midline, including some along LT carinae on AS. Eyestalks stout, cornea inflated as adult individuals. Rostral plate triangular, median carina distinct (Fig. 13a). Carapace bearing faint but discernible median carina, anteriorly bifurcated, almost continuous; IM, LT, and reflected MG carinae marked (Fig. 13a). Mandibular palp absent. Anterior 4 maxillipeds epipod-bearing, those of maxillipeds 1 and 2 more pronounced, of 3 and 4 small, rounded; raptorial appendage robust, propodus proximally armed with 3 slender movable spines, occlusal margin pectinated; dactylus armed with 5 spines (Fig. 13a). TS6 to 8 with marked SM and LT carinae, TS6 markedly bilobed, TS7 concave on anterior margin (Fig. 13a). AS1 to 6 armed with conspicuous SM and IM, LT and MG carinae; SM carina of AS6 posteriorly armed. Telson longer than broad, MD carina well-defined, prominent, posteriorly terminating in distinct spine; SM teeth fixed, separated medially by narrow V-shaped notch (5 SM denticles on each side), IM (7 to 9 IM denticles) and LT carinae prominent (Fig. 13h). Uropod robust, protopod bifurcated, mesial margin serrated, mesial spine bearing a pronounced rounded lobe in outer margin; exopod penultimate segment armed with 8 stout LT spines (Fig. 13h).
Type locality. The Philippines, precise locality unspecified.
Remarks. COI and 16S rRNA mitochondrial sequences from alima larval and juvenile individuals match those of Carinosquilla multicarina (GenBank accession no. MH168265 and MH186221.1; genetic K2P distances: 0.47 to 0.62% for COI, 0.00% for 16S rRNA). It appears the diagnostic multiple longitudinal carinae on the adult carapace, a diagnostic feature of the genus, had not yet emerged in the earlier stages of the juvenile, as shown above.
Levisquilla jurichi (Makarov, 1979)
Fig. 14
Description (based on individual CEL-stom-062). Postlarva, CL 1.8 mm, TL 7.5 mm. Specimen dorsal ventrally depressed, devoid of conspicuous pigment. Eyestalks stout, cornea slightly inflated. Rostral plate triangular, slightly longer than broad, medial ridge faint, yet completely separated from carapace (Fig. 14a). Carapace depressed, glabrous, gastric groove marked, extending to posterior margin; posterior spine as faint, rounded tooth (Fig. 14a). At least anterior 2 maxillipeds epipod-bearing; raptorial appendage robust, propodus along occlusal margin pectinated, dactylus armed with 6 teeth (Fig. 14e). TS5 to 8 laterally single-lobed (Fig. 14i). AS1 to 5 glabrous; AS6 posteriorly armed with small but distinct SM and LT spines (Fig. 14i). Telson slightly broader than long, MD carina stout, SM tooth terminating as movable spine, distance between more than half of telson width (lined with approximately 14 SM denticles); IM (5 to 6 IM denticles) and LT teeth prominent (Fig. 14j). Uropod robust, protopod bifurcated, mesial margin serrated, mesial spine bearing large rounded lobe along outer margin; endopod elongated, margins serrated; exopod penultimate segment laterally armed with 4 spines (Fig. 14j).
Levisquilla jurichi (Makarov, 1979) (postlarva: CEL-stom-062): a carapace; b right maxillule; c left maxilla; d right maxilliped 1; e left maxilliped 2; f–h right maxillipeds 3 to 5; i thoracic somites 6 to 8; j telson and left uropod
Type locality. Kavieng, Papua New Guinea.
Remarks. Molecular analyses based on 16S rRNA sequences show the present specimen to be phylogenetically closest to Levisquilla jurichi (Makarov, 1979) (GenBank accession no. MH168204; K2P distance 1.60%), a species found from the Indo-West Pacific to the Andaman Sea, Vietnam, New Caledonia, and Australia (Manning 1995; Ahyong 2001). The only postlarval individual at hand (CEL-stom-062) was soft, probably freshly molted, was in poor condition; we hence failed to retrieve either mandible. Other morphological features, nevertheless, share in common with adults of Levisquilla: (1) single-lobed lateral extensions of thoracic somites 5 to 8; (2) SM carina of telson bearing articulated apexes; and (3) mesial margin of uropodal protopod serrated. Given minimal K2P distance in comparison with L. jurichi, and morphological features agreeing with the genus, we identify the present specimen as L. jurichi, representing a new record for the stomatopod fauna of Taiwan. Previously, the only species in this genus recorded from Taiwan is L. inermis (Manning, 1965) (Ahyong et al. 2008).
Oratosquillina inornata (Tate, 1883)
Oratosquillina inornata (Tate, 1883) (alima larva: CEL-stom-050): a carapace and left maxilliped 2, dorsal view; b carapace, lateral view; c left mandible, posterior view; d left mandible, mesial view; e left maxillule; f left maxilla; g left maxilliped 1; h left maxillipeds 3 to 5; i telson and left uropod
Oratosquillina inornata (Tate, 1883) (juvenile: CEL-stom-048): a carapace; b left mandible, posterior view; c left mandible, mesial view; d left maxillule; e left maxilla; f–h left maxillipeds 1 to 5; i thoracic somites 5 to 8; j telson and left uropod
Description: alima larva (based on individual CEL-stom-050). CL 6.4 mm, TL 16.5 mm. Carapace elevated along midline, elongated, posteriorly reaching TS6; rostral spine slender, elongated, slightly less than half of carapace length; anterolateral spine prominent, reaching base of eyestalk; posterolateral spine reaching AS1; posterior spine prominent, short; lateral margin towards posterolateral spine ventrally armed with about 9 small denticles, the 4 near the posterior margin more pronounced, separately dispersed along the ventral-internal margin (Fig. 15a, b). Antennules of three-segmented stalk, reaching to the tip of rostrum anteriorly, distally triflagellate. Eyestalks inflated, cornea spheroidal (Fig. 15a). Maxillipeds well developed, anterior 2 epipod-bearing, of which rounded; raptorial claw propodus minutely pectinated along occlusal margin, proximally armed with 2 acute fixed spines, dactylus slender, unarmed (Fig. 15a). Pereiopods well developed. AS6 and telson yet articulating despite faint suture discernable; AS6 armed posteriorly with SM spines (Fig. 15i). Telson markedly longer than broad, SM teeth separated by broad V-shaped notch (13 to 15 SM denticles on each side); IM (9 IM denticles) and LT teeth pronounced, confluent with lateral margin (Fig. 15i). Uropod developed, penultimate segment of exopod laterally armed with about 3 small but acute spines (Fig. 15i).
Description: juvenile (based on individual CEL-stom-048). CL 3.1 mm, TL 15.2 mm. Specimen squilloid-type, overall with dark spots arranged into longitudinal lines symmetrical along midline, more pronounced along posterior margins of exposed TS and AS. Eyestalks stout, cornea inflated as adult individuals. Rostral plate rounded triangular, median carina distinct, yet completely separated from carapace (Fig. 16a). Carapace bearing faint but discernable median carina, dorsal pit marked; IM and LT carinae conspicuous (Fig. 16a). Mandibular palp absent. Anterior 4 maxillipeds epipod-bearing, those of anterior 2 more pronounced, the other 2 smaller, rounded; raptorial appendage robust, propodus proximally armed with 1 slender movable spine, occlusal margin pectinated; dactylus armed with 6 robust spines (Fig. 16g). TS6 to 8 with marked SM and LT carinae, TS5 to 7 laterally bilobed (Fig. 16i). AS1 to 6 armed with conspicuous SM and IM carinae. Telson longer than broad, MD carina well-defined, prominent, interrupted on proximal 1/5, posteriorly terminating with a distinct spine; SM teeth fixed, separated medially by narrow V-shaped notch (4 SM denticles on each side), IM (8 IM denticles) and LT carinae prominent (Fig. 16j). Uropod robust, protopod bifurcated, mesial margin serrated, mesial tooth bearing a pronounced rounded lobe in outer margin; exopod penultimate segment laterally armed with 9 stout lateral spines (Fig. 16j).
Type locality. Gulf St. Vincent, South Australia.
Remarks. COI and 16S rRNA sequences from individuals of this species suggest that they are Oratosquillina inornata, a common shallow-sea species in Taiwan (GenBank accession no. MH168266 and MH168220; genetic K2P distances: COI: < 0.47%; 16S rRNA: < 0.23%). Like C. multicarinata above, there are several crucial morphological features in adults but not juveniles: the anterior part of the carapace and a bracket-shaped pair of ridges separated from the median ridge behind in adults (Fig. 16a).
Oratosquilla oratoria (De Haan, 1844)
Fig. 17
Description (based on individual CEL-stom-080). Juvenile, CL 4.2 mm, TL 20.0 mm. Specimen squilloid-type, overall with dark spots arranged into short longitudinal lines symmetrical along midline. Eyestalks stout, cornea inflated as adult individuals. Rostral plate triangular, median carina distinct (Fig. 17a). Carapace overall glabrous, along midline bearing faint but discernable carina, anteriorly bifurcated, almost continuous, followed by dorsal pit; gastric groove marked, extending from anterior margin to 9/10 carapace length; IM and LT carinae marked (Fig. 17a). Mandibular palp absent. Anterior 4 maxillipeds epipod-bearing, which of anterior 2 more pronounced, those of 3 and 4 smaller, rounded; raptorial appendage robust, propodus proximally armed with 2 slender movable spines, occlusal margin pectinated; dactylus armed with 6 spines (Fig. 17g). TS5 to 8 bearing distinct SM and LT carinae, TS5 to 7 laterally bilobed (Fig. 17k). AS1 to 6 each armed with pronounced SM, IM and LT carinae, SM carinae of AS6 produced posteriorly. Telson longer than broad, MD carina well-defined, prominent, posteriorly terminating with a distinct spine; SM teeth fixed, separated medially by narrow V-shaped notch, on each side armed with 5 SM denticles; IM (IMD 8) and LT carinae prominent (Fig. 17l). Uropod robust, protopod bifurcated, mesial tooth bearing a pronounced rounded lobe in outer margin; exopod penultimate segment laterally armed with 7 stout LT spines (Fig. 17l).
Oratosquilla oratoria (De Haan, 1844) (juvenile: CEL-stom-080): a carapace; b left mandible, posterior view; c left mandible, mesial view; d left maxillule; e left maxilla; f–j left maxillipeds 1 to 5; k thoracic somites 5 to 8; l telson and uropod
Type locality. Japan, precise locality unspecified.
Remarks. Morphological features of juveniles of this form match well with those of O. oratoria, including bilobed lateral processes of TS5 to 7 (Fig. 17k) and anteriorly bifurcated median carina on carapace (Fig. 17a). Juveniles examined, however, appear to differ from typical adult forms by a weakly indicated anterolateral spine, and telson with prelateral lobe nearly undefined (Fig. 17l). Molecular analyses show that the COI and 16S rRNA sequences of the material examined match well with those of O. oratoria (GenBank accession no. MH168274 and MH 168214; genetic K2P distances: 0.31 to 0.78% for COI and < 0.45% for 16S rRNA).
Discussion
Although taxonomic accuracy of metazoan mitochondrial gene sequences in GenBank is generally considered reliable (Leray et al. 2019; Meiklejohn et al. 2019), caution should be maintained on credibility of individual sequences. In the present study, we have used two marker genes and the results were largely consistent. Furthermore, some of the sequences cluster with reference sequences with high support value (such as Lysiosquillina maculata clade in 16S rRNA NJ tree: Fig. 2b). As such, we are confident that the taxonomic assignments made in the present study being reliable. In this study, based on COI and 16S rRNA gene sequences, 14 morphotypes of stomatopods larvae/juveniles are revealed, representing 12 distinct species, five of which could not be assigned with certainty to species level based on comparisons to sequences currently in GenBank. We herein report erichthus larva assigned to Manningia pilaensis (De Man, 1888) and a postlarva of Levisquilla jurichi (Makarov, 1979), as new records of Taiwan.
The planktonic stomatopod larvae collected using light traps comprised of several postlarval and juvenile forms, among which squilloid species (Squillidae) were especially prominent. This shows that the transition of phototactic behavior from planktonic to postlarval (settlement) stages may not be as distinct as previously assumed (see Dingle 1969; Morgan and Goy 1987). Additionally, large quantities of antizoea larvae of Lysiosquillina maculata (more than 70 individuals, represented by eight sequenced samples above) were captured, and these “premature” juveniles, not yet equipped with thoracic appendages, a feature that is unique to the lysiosquilloids, are at least positively phototactic at some point during this phase.
Two recorded species belong to the Gonodactylidae, a group of stomatopods which found to be species-rich in reef-associated habitats were recorded. One species was assigned as Gonodactylus smithii (which is involved in a yet-deciphered species complex, see above), reported by Ahyong et al. (2008) as a coral reef species from various sites in Taiwan. Among those sites was Danshuei, Taipei, which a pronouncedly estuarine locality, indicating substantial plasticity in habitat preference, and the present record from CCA reefs in Taoyuan is not surprising. The remaining eight species, three of the Nannosquillidae and five of Squillidae, generally prefer habitats with soft substrates (see also discussions on the ecology of Pullosquilla by Wang and Chiou (2017)). It appears the planktonic larvae and juveniles of these species also utilize and take advantage of the more complex structure of algae reefs in these shallow, murky waters, emphasizing the ecological value of this precious habitat.
The stomatopod fauna of Taiwan has been relatively well documented in the past decades, and was synthesized and illustrated in considerable detail by Ahyong et al. (2008). Post-2008 additions to the Taiwanese fauna include papers by Yeh and Hsueh (2010) and Wang and Chiou (2017), adding Taku spinosocarinatus (Fukuda, 1909), and three species of Pullosquilla (P. litoralis (Michel & Manning, 1971), P. thomassini (Manning, 1978), and P. pardus (Moosa, 1991)), respectively, making the total number of species 67. The present work, adopting molecular barcoding approaches on taxonomic assignment, added new records of Manningia pilaensis and Levisquilla jurichi, also confirming the validity of Lysiosquillina maculata, bringing the total number of stomatopod species in Taiwan to 70, which is comparable to the number of stomatopod species elsewhere in East Asia: 104 species in Chinese and adjacent seas (15 Taiwan-exclusive records included; Wang and Liu 2008), 68 from Japanese seas (Ahyong 2012), and 120 from the vicinity of the South China Sea (Moosa 2000). However, as Ahyong et al. (2008) argued, a considerable portion of the Taiwanese records was obtained from commercial trawlers, therefore heavily biased towards estuarine seas, and shallow to deeper depths on sandy-muddy substrates. The bias is reflected by the dominating richness of the Squilloidea (two-thirds of the Taiwanese fauna), a group common as burrowers in these habitats. Structures such as rocky and coral reefs, based on reported species counts of associated gonodactylids, were apparently poorly surveyed. The above findings show, despite decades of research, sampled by traditional surveying methods, fauna inhabiting structurally complex habitats such as reefs, are not as adequate in documenting stomatopod fauna of Taiwan. There is, nevertheless, high potential for an innovative approach that synergizes morphology-based taxonomy, molecular barcoding techniques, and online database resources.
References
Adey WH (1998) Coral reefs: algal structured and mediated ecosystems in shallow, turbulent, alkaline waters. J Phycol 34:393–406. https://doi.org/10.1046/j.1529-8817.1998.340393.x
Adey WH, Vassar JM (1975) Colonization, succession and growth rates of tropical crustose coralline algae (Rhodophyta, Cryptonemiales). Phycologia 14:55–69
Ahyong ST (2001) Revision of the Australian Stomatopod Crustacea. Rec Aust Mus Supp 26:1–326
Ahyong ST (2005) Coral reef mantis shrimps from the vicinity of Sodwana Bay, South Africa (Crustacea: Stomatopoda). Proc Biol Soc Washington 118:158–164. https://doi.org/10.2988/0006-324X(2005)118[158:CRMSFT]2.0.CO;2
Ahyong ST (2012) Stomatopod crustacea of the KUMEJIMA 2009 expedition, Japan. Zootaxa 3367:232–251. https://doi.org/10.11646/zootaxa.3367.1.22
Ahyong ST, Jarman SN (2009) Stomatopod interrelationships: preliminary results based on analysis of three molecular loci. Arthr Syst Phyl 67:91–98
Ahyong ST, Chan T-Y, Liao Y-C (2008) A catalogue of the mantis shrimps (Stomatopoda) of Taiwan. National Taiwan Ocean University, Keelung, 190pp
Ahyong ST, Haug JT, Haug C (2014) Stomatopoda. In: Martin JW, Olesen J, Høeg JT (eds) Atlas of crustacean larvae. John Hopkins University Press, Baltimore, pp185–189
Ahyong ST, Hwang H-S, Kim W (2018) Review of the mantis shrimp genus Taku Manning, 1995 (Stomatopoda: Gonodactyloidea: Takuidae). J Crust Biol 38:451–459. https://doi.org/10.1093/jcbiol/ruy033
Alikunhi KH (1944) Final pelagic larva of Squilla hieroglyphica Kemp. Curr Sci 13(9):237–238
Alikunhi KH (1950) Observations on some larval and post-larval stomatopods. J Bombay Nat Hist Soc 49:101–107
Alikunhi KH (1952) An account of the stomatopod larvae of the Madras plankton. Rec Indian Mus 49:239–319
Alikunhi KH (1958) Notes on a collection of stomatopod larvae from the bay of Bengal, off the Mahanadi estuary. J Zool Soc India 10:120–147
Alikunhi KH (1967) An account of the post-larval development, moulting and growth of the common stomatopods of the Madras coast. In Proceedings of the Symposium on Crustacea, held at Ernakulam from January 12–15, 1965, pt. 2. Mandapam Camp: Marine Biological Association of India. pp 824–939
Alikunhi KH, Aiyar RG (1942) On some Squilla larvae from the Madras plankton. Curr Sci 11(2):56–58
Alikunhi KH, Aiyar RG (1943) Growth in some stomatopods. Curr Sci 12(3):80–82
Ballesteros E (2006) Mediterranean coralligenous assemblages: a synthesis of present knowledge. Ocean Mar Biol Annu Rev 44:123–195
Barber P, Boyce SL (2006) Estimating diversity of Indo-Pacific coral reef stomatopods through DNA barcoding of stomatopod larvae. Proc R Soc B 273:2053–2061. https://doi.org/10.1098/rspb.2006.3540
Barber PH, Erdmann MV (2000) Molecular systematics of the Gonodactylidae (Stomatopoda) using mitochondrial cytochrome oxidase C (subunit 1) DNA sequence data. J Crust Biol 20:20–36. https://doi.org/10.1163/1937240X-90000004
Bok MJ, Porter ML, Cronin TW (2015) Ultraviolet filters in stomatopod crustaceans: diversity, ecology and evolution. J Exp Biol 218:2055–2066. https://doi.org/10.1242/jeb.122036
Borradaile LA (1900) On the Stomatopoda and Macrura brought by Dr Willey from the South Seas. Zoological results based on material collected in New Nritain, New Guinea, Loyalty Islands and elsewhere. collected during the years 1895, 1896 and 1897. Part IV. Cambridge University Press, Cambridge, pp 395–428
Bosence DWJ (1983) Coralline algal reef frameworks. J Geol Soc Lond 140:365–376. https://doi.org/10.1144/gsjgs.140.3.0365
Bouchet P, Ng PKL, Largo D, Tan SH (2009) PANGLAO 2004—investigations of the marine species richness in the Philippines. Raff Bull Zool Supp 20:1–19
Bracken-Grissom HD, Felder DL, Vollmer NL, Martin JW, Crandall KA (2012) Phylogenetics links monster larva to deep-sea shrimp. Ecol Evol 2:2367–2373. https://doi.org/10.1002/ece3.347
Brooks WK (1886) Report on the Stomatopoda collected by H. M. S. Challenger during the years 1873–76. The Vovage of the H. M. S. Challenger, Zoology 16:1–116
Castro P (1988) Animal symbioses in coral reef communities: a review. Symbiosis 5:161–184
Chan T-Y, Ho KC, Li CP, Chu KH (2009) Origin and diversification of the clawed lobster genus Metanephrops (Crustacea: Decapoda: Nephropidae). Mol Phyl Evol 50:411–422. https://doi.org/10.1016/j.ympev.2008.11.020
Chan BKK, Shao K-T, Shao Y-T, Chang Y-W (2016) A simplified, economical, and robust light trap for capturing benthic and pelagic zooplankton. J Exp Mar Biol Ecol 482:25–32. https://doi.org/10.1016/j.jembe.2016.04.003
Chu C, Loh KH, Ng CC, Ooi AL, Konishi Y, Huang SP, Chong VC (2019) Using DNA barcodes to aid the identification of larval fishes in tropical estuarine waters (Malacca Straits, Malaysia). Zool Stud 58:30. https://doi.org/10.6620/ZS.2019.58-30
Cook CE, Yue Q, Akam M (2005) Mitochondrial genomes suggest that hexapods and crustaceans are mutually paraphyletic. Proc R Soc B 272:1295–1304. https://doi.org/10.1098/rspb.2004.3042
Costa FO, deWaard JR, Boutillier J, Ratnasingham S, Dooh RT, Hajibabaei M, Hebert PDN (2007) Biological identifications through DNA barcodes: the case of the Crustacea. Can J Fish Aquat Sci 64:272–295. https://doi.org/10.1139/f07-008
Crandall KA, Fitzpatrick JF Jr (1996) Crayfish molecular systematics: using a combination of procedures to estimate phylogeny. Syst Biol 45:1–26. https://doi.org/10.1093/sysbio/45.1.1
Dai C-F, Wang S-W, Chang J-S, Jeng A-I (2009) Handbook for ecological tours of Guanyin algae reef. Liquefied natural gas engineering office. CPC Corporation, Taiwan
Dana JD (1852–1855) Crustacea, part I. United States exploring expedition during the years 1838, 1839, 1840, 1841, 1842, under the command of Charles Wilkes, U.S.N. 13. C. Sherman, Philidelphia, 685 pp (1852), atlas (1855)
de Haan W (1833–1850) Crustacea. In: von Siebold PF (ed) Fauna japonica sive Descriptio Animalium, quae in Itinere per Japoniam, Jussu et Auspiciis Superiorum, qui Summum in India Batava imperium Tenent, Suspecto, Annis 1823–1830 Collegit, Notis, Observationibus et Adumbrationibus Illustravit. Lugduni-Batavorum, 243pp
de Man JG (1887–1888) Report on the podophthalmous Crustacea of the Mergui archipelago, collected for the trustees of the Indian museum, Calcutta, by Dr. John Anderson F.R.S., superintendent of the museum. J Linn Soc Zool 22:1–305
de Man JG (1898) Bericht über die von Herrn Schiffscapitän storm zu Atjeh, an den westlichen Küsten von Malakka, Borneo und Celebes sowie in der Java-see-gesammelten Decapoden und Stomatopoden. Sechster (Schluss-) Theil. Zool Jb Abt Syst 10:677–708
Dethier MN, Steneck RS (2001) Growth and persistence of diverse intertidal crusts: survival of the slow in a fast-paced world. Mar Ecol Prog Ser 223:89–100
Dingle H (1969) Ontogenetic changes in phototaxis and thigmokinesis in stomatopod larvae. Crustaceana 16:108–110
ĎuriŠ Z (2018) Madeirasquilla tuerkayi, a new genus and species of mantis shrimps from Madeira Island, eastern Atlantic (Crustacea: Stomatopoda: Nannosquillidae). Zootaxa 4399:553–562. https://doi.org/10.11646/zootaxa.4399.4.5
Elliott J (2002) DNA evidence for morphological and cryptic Cenozoic speciations in the Anaspididae, ‘living fossils’ from the Triassic. J Evol Biol 13:624–633. https://doi.org/10.1046/j.1420-9101.2000.00207.x
Emery KO, Tracey JI Jr, Ladd HS (1954) Geology of bikini and nearby atolls. Geol Surv Prof Pap 260A:265pp
Fabricius JC (1781) Species Insectorum Exhidentes Eorum Differentias Specificas, Synonyma Auctorum, Loca Natalia, Mmetamorphosin Adiectis, Observationibus, Descriptionibus. Hamburgii et Kilon i, 552pp
Fabricius JC (1787) Mantissa insectorum sistens eorum species nuper detectas: adjectis characteribus genericis, differentiis specificis, emendationibus, observationibus. Proft Hafniae I, 348pp
Fabricius JC (1793) Entomologia Systematica Emendata et Aucta. Secundum classes, Ordines, genera, species. Adjectis Synonimis, Locis, Observationibus, Descriptionibus 2. Hafniae, 519pp
Fabricius JC (1798) Entomologia Systematica emendata et aucta, secundum classes, ordines, genera, species adjectis synonimis locis observationibus descriptionibus. Hafniae. I-IV. Supplementum Entomologiae Systematicae Copenhagen, 572pp
Feller KD, Cronin TW (2014) Hiding opaque eyes in transparent organisms: a potential role for larval eyeshine in stomatopod crustaceans. J. Exp Biol 217:3263–3273. https://doi.org/10.1242/jeb.108076
Feller KD, Cronin TW (2016) Spectral absorption of visual pigments in stomatopod larval photoreceptors. J Comp Phys A 202:215–223. https://doi.org/10.1007/s00359-015-1063-y
Feller KD, Cronin TW, Ahyong ST, Porter ML (2013) Morphological and molecular description of the late-stage larvae of Alima leach, 1817 (Crustacea: Stomatopoda) from Lizard Island, Australia. Zootaxa 3722:22–32
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Foxon GEH (1939) Stomatopod larvae. Sci Rep 6:251–266
Fukuda T (1909) The Stomatopoda of Japan. Dobutsugaku Zasshi 21:54–62
Fukuda T (1911) Supplement to Japanese Stomatopoda. Dobutsugaku Zasshi 23:173–175
Fukuda T (1913) On two species of Japanese Stomatopods, with list of Stomatopoda found in Japanese seas. Dobutsugaku Zasshi 25:69–72
Ghosh HC (1990) Stomatopoda: Crustacea. Fauna of Lakshadweep. State Fauna Series 2:199–212
Giesbrecht W (1910) Stomatopoden, Erster Theil. Fauna Flora Golfes Neapel Monogr 33:1–239
Glynn PW, Enochs IC (2011) Invertebrates and their roles in coral reef ecosystems. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer Science+Business Media B.V, Netherlands, pp 273–325. https://doi.org/10.1007/978-94-007-0114-4_18
Greenwood JD, Williams BG (1984) Larval and early post-larval stages in the abbreviated development of Heterosquilla tricarinata (Claus, 1871) (Crustacea, Stomatopoda). J Plankton Res 6:615–635
Gurney R (1946) Notes on Stomatopod larvae. Proc Zool Soc London 116(1):133–175. https://doi.org/10.1111/j.1096-3642.1946.tb00112.x
Hamano T (1989) Biology of Stomatopods. 18. Taxonomy and key of Japanese Stomatopoda 16. Family Lysiosquillidae. Aquabiol 11:468–471
Hamano T, Matsuura S (1987) Egg size, duration of incubation, and larval development of the Japanese mantis shrimp in the laboratory. Nippon Suisan Gakkaishi 53:23–39
Haug C, Haug JT (2014) Defensive enrolment in mantis shrimp larvae (Malacostraca: Stomatopoda). Cont Zool 83:185–194
Haug C, Wagner P, Bjarsch JM, Braig F, Haug JT (2018) A new “extreme” type of mantis shrimp larva. Nauplius 26:e2018019. https://doi.org/10.1590/2358-2936e2018019
Hebert PDN, Cywinska A, Ball SL, deWaard JR (2003) Biological identifications through DNA barcodes. Proc R Soc B 270:313–321. https://doi.org/10.1098/rspb.2002.2218
Holthuis LB (1941) Biological results of the Snellius expedition XII. The Stomatopoda of the Snellius Expedition. Temminckia 6:241–294
Holthuis LB (1967) The second Israel South Red Sea expedition, 1965, report no. 1. The stomatopod Crustacea collected by the 1962 and 1965 Israel South Red Sea expeditions. Israel J Zool 16:1–45
How MJ, Porter ML, Radford AN, Feller KD, Temple SE, Caldwell RL, Marshall NJ, Cronin TW, Roberts NW (2014) Out of the blue: the evolution of horizontally polarized signals in Haptosquilla (Crustacea, Stomatopoda, Protosquillidae). J Exp Biol 217:3425–3431
Hwang J-J, Yu H-P (1980) A fauna-list of the Crustacea from Lan-Yu Island. Ann Taiwan Mus 23:151–179
Kemp S (1911) Preliminary descriptions of new species and varieties of Crustacea Stomatopoda in the Indian museum. Rec Indian Mus 6:93–100
Kemp S (1913) An account of the Crustacea Stomatopoda of the Indo-Pacific region based on the collection in the Indian museum. Mem Indian Mus 4:1–217
Komai T (1927) Stomatopoda of Japan and adjacent localities. Mem Coll Sci Kyoto Imp Univ Ser B 3:307–354
Komai T (1932) An enormous swarm of stomatopod larvae. Annot Zool Japon 13:351–354
Komai T, Tung YM (1929) Notes on the larval stages of Squilla oratoria with remarks on some other stomatopod larvae found in the Japanese seas. Annot Zool Japan 12:187–237
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Kuo C-Y, Chung A, Keshavmurthy S, Huang Y-Y, Yang S-Y, Chen CA (2019) Lonely giant on the sand: unexpected massive Taiwanese coral, Polycyathus chaishanensis in the Datan algal reef demands a conservation focus. Galaxea J Coral Reef Stud 21:11–21. https://doi.org/10.3755/galaxea.21.1_11
Latreille PA (1802) Histoire naturelle, générale et particulière, des Crustacés et des Insectes, 3. F. Dufart, Paris, 467 pp
Latreille PA (1828) Squille, Squilla. Encyclopédie Méthodique. Entomologie ou Historire naturelle des Crustacés, des Arachnides et des Insectes 10. Paris: Asasse, Paris, pp467–475
Leach WE (1817–1818) A general notice of the animals taken by Mr. John Cranch, during the expedition to explore the source of the River Zaire. In: Tuckey JK, Narrative of an Expedition to Explore the River Zaire, Usually Called the Congo, in South Africa, in 1816, under the Direction of Captain J. K. Tuckey, R.N., to Which is Added, the Journal of Professor Smith, Some General Observations on the Country and its Inhabitants, and an Appendix: Containing the Natural History of that Part of the Kingdom of Congo Through Which the Zaire Flows. John Murray, London, pp407–409
Lee S-C, Wu S-K (1966) The stomatopod crustacea of Taiwan. Bull Inst Zool Acad Sin 5:41–58
Leray M, Knowlton N (2015) DNA barcoding and metabarcoding of standardized samples reveal patterns of marine benthic diversity. Proc Natl Acad Sci U S A 112:2076–2081. https://doi.org/10.1073/pnas.1424997112
Leray M, Knowlton N, Ho S-L, Nguyen BN, Machida RJ (2019) GenBank is a reliable resource for 21st century biodiversity research. Proc Natl Acad Sci U S A 116:22651–22656. https://doi.org/10.1073/pnas.1911714116
Li JJ, Shih YJ, Ho PH, Jiang GC (2019) Description of the first zoea of the cavernicolous crab Karstama boholano (Ng, 2002) (Crustacea: Decapoda: Sesarmidae) from Taiwan, with notes on ecology. Zool Stud 58:36. https://doi.org/10.6620/ZS.2019.58-36
Lin M-F, Kitahara MV, Tachikawa H, Keshavmurthy S, Chen CA (2012) A new shallow-water species, Polycyathus chaishanensis sp. nov. (Scleractinia: Caryophylliidae), from Chaishan, Kaohsiung, Taiwan. Zool Stud 51(2):213–221
Lin H-J, Hsu H-F, Liao W-S, Lee C-L, Liu P-J, Lin S-M (2013) Biodiversity of the algal reefs in Taoyuan. J Wetlands 2:1–24
Liou C-Y (2012) Rescue algal reefs in Taiwan: vanishing treasure chest of biodiversity. Endemic Species Research Institute, Council of Agriculture, Executive Yuan, Taiwan Available at: https://www.tesri.gov.tw/A15_7/content/10026
Liou C-Y, Yang S-Y, Chen CA (2017) Unprecedented calcareous algal reefs in northern Taiwan merit high conservation priority. Coral Reefs 36:1253
Liu L-C, Lin S-M, Caragnano A, Payri C (2018) Species diversity and molecular phylogeny of non-geniculate coralline algae (Corallinophycidae, Rhodophyta) from Taoyuan algal reefs in northern Taiwan, including Crustaphytum gen. Nov. and three new species. J Appl Phycol 30:3455–3469. https://doi.org/10.1007/s10811-018-1620-1
Makarov RR (1978) New data on crustaceans of the families Lysiosquillidae and Gonodactylidae (Crustacea, Stomatopoda) from Tonkin Bay (Vietnam). Zool Zhur Mos 57:176–189
Makarov RR (1979) Mantis shrimps (Crustacea: Hoplocarida: Stomatopoda) in collections of expeditions of the research vessel “Akademic Kripovich”. Biol Morya Vladivostok 3:14–23
Manning RB (1965) Stomatopoda from the collection of his majesty the emperor of Japan. Crustaceana 9(3):249–262
Manning RB (1967) Notes on the genus Manningia with description of a new species (Crustacea: Stomatopoda). Proc US Nat Mus 122:1–13. https://doi.org/10.5479/si.00963801.122-3589.1
Manning RB (1968) Stomatopod Crustacea from Madagascar. Proc US Nat Mus 124:1–61. https://doi.org/10.5479/si.00963801.124-3641.1
Manning RB (1970) Some Stomatopod crustaceans from Tuléar, Madagascar. Bull Mus Natl Hist Nat 2 Sér 41(6):1429–1441
Manning RB (1977) A monograph of the west African stomatopod Crustacea. Atlantide Rep 12:25–181
Manning RB (1978) New and rare Stomatopod Crustacea from the indo-West Pacific region. Smiths Contr Zool 264:1–36
Manning RB (1980) The superfamilies, families, and genera of recent stomatopod crustacea, with diagnoses of six new families. Proc Biol Soc Washington 93(2):362–372
Manning RB (1995) Stomatopod Crustacea of Vietnam: the legacy of Raoul Serène. Crust Res, Sp no 4:1–339
Manning RB (1998) Stomatopods. In: Carpenter KE, Niem VH (eds) FAO species identification guide for fishery purposes. The living marine resources of the Western Central Pacific. Vol. 2. Cephalopods, crustaceans, holothurians and sharks. FAO, Rome, pp827–849
Manning RB, Lewinsohn C (1986) Notes on some stomatopod Crustacea from the Sinai peninsula, Red Sea. Smith Cont Zool 433:1–19. https://doi.org/10.5479/si.00810282.433
Matzen da Silva J, Creer S, dos Santos A, Coata AC, Cunha MR, Costa FO, Carvalho GR (2011) Systematic and evolutionary insights derived from mtDNA COI barcode diversity in the Decapoda (Crustacea: Malacostraca). PLoS One 6:e19449. https://doi.org/10.1371/journal.pone.0019449
Meier R, Kwong S, Vaidya G, Ng PKL (2006) DNA barcoding and taxonomy in Diptera: a tale of high intraspecific variability and low identification success. Syst Biol 55:715–728
Meiklejohn KA, Damaso N, Robertson JM (2019) Assessment of BOLD and GenBank -- their accuracy and reliability for the identification of biological materials. PLoS One 14(6):e0217084. https://doi.org/10.1371/journal.pone.0217084
Michel A (1968) Dérive des larves de stomatopodes de l'Est de l'Océan Indien. Cah ORSTOM Sér Océan 6(1):13–41
Michel A, Manning RB (1971) A new Austrosquilla (Stomatopoda) from the Marquesas Islands. Crustaceana 20(3):237–240
Moosa MK (1991) The Stomatopoda of New Caledonia and Chesterfield Islands. In: Richer de Forges B (ed) Le benthos des fonds meubles des lagons de Nouvelle-Calédonie vol 1, pp 147–219
Moosa MK (2000) Marine biodiversity of the South China Sea: a checklist of stomatopod crustacea. Raff Bull Zool Supp 8:405–457
Morgan SG, Goy JW (1987) Reproduction and larval development of the mantis shrimp Gonodactylus bredini (Crustacea: Stomatopoda) maintained in the laboratory. J Crust Biol 7:595–618. https://doi.org/10.1163/193724087X00379
Morgan SG, Provenzano AJ Jr (1979) Development of pelagic larvae and postlarva of Squilla empusa (Crustacea, Stomapoda), with an assessment of larval characters within the Squillidae. Fish Bull 77(1):61–90
Nobili G (1904) Diagnoses préliminaires de vingt-huit espèces nouvelles de stomatopodes et décapodes macroures de la Mer Rouge. Bull Mus Hist Nat 10:228–238
Palecanda S, Feller KD, Porter ML (2020) Using larval barcoding to estimate stomatopod species richness at Lizard Island, Australia for conservation monitoring. Sci Rep 2020(1):10990. https://doi.org/10.1038/s41598-020-67696-x
Plaisance L, Knowlton N, Paulay G, Meyer C (2009) Reef-associated crustacean fauna: biodiversity estimates using semi-quantitative sampling and DNA barcoding. Coral Reefs 28:977–986. https://doi.org/10.1007/s00338-009-0543-3
Plaistance L, Caley MJ, Brainard RE, Knowlton N (2011) The diversity of coral reefs: What are we missing? PloS ONE 6(10):e25026. https://doi.org/10.1371/journal.pone.0025026
Pocock RI (1893) Report upon the stomatopod crustaceans obtained by P. W. Bassett-Smith, Esq., Surgeon R. N., during the cruise, in the Australian and China seas, of H.M.S. “Penguin,” Commander W. U. Moore. Ann Mag Nat Hist Ser 6 11:473–479
Porter ML, Zhang Y, Desai S, Caldwell RL, Cronin TW (2010) Evolution of anatomical and physiological specialization in the compound eyes of stomatopod crustaceans. J Exp Biol 213:3473–3486. https://doi.org/10.1111/j.1755-0998.2009.02643.x
Provenzano AJ Jr, Manning RB (1978) Studies on development of Stomatopod Crustacea II. The later larval stages of Gonodactylus oerstedii Hansen reared in the laboratory. Bull Mar Sci 28(2):297–315
Radulovici AE, Sainte-Marie B, Dufresne F (2009) DNA barcoding of marine crustaceans from the estuary and gulf of St Lawrence: a regional-scale approach. Mol Ecol Res 9:181–187. https://doi.org/10.1111/j.1755-0998.2009.02643.x
Raupach MJ, Radulovici AE (2015) Looking back on a decade of barcoding crustaceans. Zookeys 539:53–81. https://doi.org/10.3897/zookeys.539.6530
Raupach MJ, Barco A, Steinke D, Beermann J, Laakmann S, Mohrbeck I, Neumann H, Kihara TC, Pointner K, Radulovici A, Segelken-Voigt A, Wesse C, Knebelsberger T (2015) The application of DNA barcodes for the identification of marine crustaceans from the North Sea and adjacent regions. PLoS One 10:e0139421. https://doi.org/10.1371/journal.pone.0139421
Schubart CD, Reimer J, Diesel R (1998) Morphological and molecular evidence for a new endemic freshwater crab, Sesarma ayatum sp. n., (Grapsidae, Sesarminae) from eastern Jamaica. Zool Scr 27:373–380. https://doi.org/10.1111/j.1463-6409.1998.tb00468.x
Schubart CD, Niegel JE, Felder DL (2000) Use of the mitochondrial 16S rRNA gene for phylogenetic and population studies of Crustacea. Crust Issues 12:817–830
Serène R (1947) Sur des Stomatopodes rares trouves en Indochine et n'existant pas dans les collections du Museum. Bull Mus Nat Hist Natl Paris, 2 Sér 19:381–389
Serène R (1954) Observations biologiques Sur les stomatopodes. Mém Inst Océan Nhatrang 8:1–93
Serène R (1962) Révision du genre Pseudosquilla (Stomatopoda) et définition de genres nouveaux. Bull Inst Océan Monaco 59:1–27
Shanbhogue SL (1975) Descriptions of stomatopod larvae from the Arabian Sea with a list of stomatopod larvae and adults from the Indian Ocean and a key for their identification part I. J Mar Biol Ass India 17(2):196–238
Shen H, Braband A, Scholtz G (2013) Mitogenomic analysis of decapod crustacean phylogeny corroborates traditional views on their relationships. Mol Phylogenet Evol 66:776–789. https://doi.org/10.1016/j.ympev.2012.11.002
Shih H-T, Hsu P-Y, Shahdadi A, Schubart CD, Li J-J (2019) The synonymy of the supratidal crab species Parasesarma cognatum Rahayu & Li, 2013 with P. liho Koller, Liu & Schubart, 2010 (Decapoda: Brachyura: Sesarmidae) based on morphological and molecular evidence, with a note on P. paucitorum Rahayu & Ng, 2009. Zool Stud 58:21. https://doi.org/10.6620/ZS.2019.58-21
Steller DL, Riosmena-Rodríguez R, Foster MS, Roberts CA (2003) Rhodolith bed diversity in the Gulf of California: the importance of rhodolith structure and consequences of disturbance. Aq Cons Mar Fr Ecosyst 13:S5–S20
Tang RWK, Yau C, Ng W-C (2010) Identification of stomatopod larvae (Crustacea: Stomatopoda) from Hong Kong waters using DNA barcodes. Mol Ecol Resour 10:439–448. https://doi.org/10.1111/j.1755-0998.2009.02794.x
Tate R (1883) Descriptions of some new species of Squilla from South Australia. Trans Proc R Soc S Aust 6:48–53
Toon A, Finley M, Staples J, Crandall KA (2009) Decapod phylogenetics and molecular evolution. In: Martin JW, Crandall KA, Felder DL (eds) Decapod crustacean Phylogenetics, crust Iss 18, CRC Press, Boca Raton, pp15–29
Townsley SJ (1953) Adult and larval stomatopod crustaceans occurring in Hawaiian waters. Pac Sci 7:399–437
Van Der Wal C, Ahyong ST, Ho SYW, Lo N (2017) The evolutionary history of Stomatopoda (Crustacea: Malacostraca) inferred from molecular data. PeerJ 5:e3844. https://doi.org/10.7717/peerj.3844
Van Der Wal C, Ahyong ST, Ho SYW, Lins LSF, Lo N (2019) Combining morphological and molecular data resolves the phylogeny of Squilloidea (Crustacea : Malacostraca). Invert Syst 33:89–100. https://doi.org/10.1071/IS18035
Vereshchaka AL, Kulagin DN, Lunina AA (2018) A phylogenetic study of krill (Crustacea: Euphausiacea) reveals new taxa and co-evolution of morphological characters. Cladist 35:150–172. https://doi.org/10.1111/cla.12239
Wang J-W, Chiou T-H (2017) Three new records of Nannosquillidae from Taiwan with notes on their ecology (Crustacea, Stomatopoda, Lysiosquilloidea). Zookeys 721:33–43. https://doi.org/10.3897/zookeys.721.20588
Wang Y-L, Liu R (2008) Hoplocarida Calmann, 1904. In: Liu RY (ed) Checklist of marine biota of China seas. Science Press, Beijing, pp654–660
Wang S-W, Dai C-F, Shea K-S (2008) Holocene reefal limestones of coastal Taoyuan. In: Proceedings of the Vth Symposium on Stratigraphy of Taiwan. Central Geological Survey, Ministry of Economic Affairs, R.O.C, pp150
Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PDN (2005) DNA barcoding Australia’s fish species. Phil Trans R Soc Lond B Biol Sci 360:1847–1857. https://doi.org/10.1098/rstb.2005.1716
Webb KE, Barnes DKA, Clark MS, Bowden DA (2006) DNA barcoding: a molecular tool to identify Antarctic marine larvae. Deep-Sea Res II 53:1053–1060. https://doi.org/10.1016/j.dsr2.2006.02.013
White A (1848) Descriptions of two new species of Crustacea. Proc Zool Soc Lond 144:pl.VII(1, 1a)
Wood-Mason J (1895) Figures and descriptions of nine species of Squillidae from the collection in the Indian museum. Calcutta, pp 1–11
Yeh T-C, Hsueh P-W (2010) Taku spinosocarinatus (Fukuda, 1909): first record of a takuid stomatopod from Taiwan. Crustaceana 83:369–373. https://doi.org/10.1163/001121610X12627655658285
Acknowledgments
The authors would like to acknowledge a grant for CCA reef research from Biodiversity Research Center, Academia Sinica, Taiwan. Thanks also to Hee-Seung Hwang (Seoul National University, Korea) for the initial discussion on stomatopod taxonomy, Noah Last (Third Draft Editing) for his English language editing, and Dr. Shane Ahyong (Australian Museum, Sydney) and one anonymous reviewer for providing much helpful comments to improve the quality of the article. We also thank the Taiwan Power Company for offering temporary entrance permits in accessing the Datan algal reefs. Thanks to Chu yu-wei, Juvenile Art Center, for providing the aerial photograph of Datan algal reef.
Funding
This study was funded by research grant from Academia Sinica to BKKC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the “Acknowledgments” section, if applicable.
Data availability statement
Authors deposited sequences of the specimens in NCBI GenBank (https://www.ncbi.nlm.nih.gov/genbank/).
Author contribution statement
BKKC and RJM designed the study. PCT, BKKC, RJM, WPH, and HRL conducted field samplings and laboratory sorting. WPH conducted the SEM observations. YFT and HRL conducted molecular analysis. KW prepared line drawings. KW, BKKC, and YFT wrote the MS.
Additional information
Communicated by E. Macpherson
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(XLSX 297 kb)
Rights and permissions
About this article
Cite this article
Wong, K.J.H., Tsao, YF., Tsai, PC. et al. To the light side: molecular diversity and morphology of stomatopod larvae and juveniles (Crustacea: Malacostraca: Stomatopoda) from crustose coralline algal reefs in Taiwan. Mar. Biodivers. 51, 20 (2021). https://doi.org/10.1007/s12526-020-01150-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-020-01150-z