Abstract
Symbiotic caridean shrimps were collected from diverse host invertebrates in a coral reef habitat of the southern East China Sea during the summer from April to August in 2014. Regular weekly sampling was conducted by scuba diving in the zone above 25 m depth at the Wang-Hai-Xiang Bay (also called as Fan-Zai-Aou Bay) in northeastern Taiwan. This region is influenced by the China Coast Current and the Kuroshio Current. In total, 27 species of shrimp in 20 genera and 3 families were identified, being taxonomically dominated by Palaemonidae (70.4% of species) and followed by Alpheidae (22.2%) and Hippolytidae (7.4%). The shrimp species were apportioned among hosts of various groups, as follows: scleractinians (45.2%), gorgonians (16.1%), echinoids (12.9%), crinoids (9.7%), actiniarians (6.5%), alcyonarians (3.2%), hydroids (3.2%), and sponges (3.2%). Six species are reported for the first time from the waters of Taiwan: Synalpheus neomeris (de Man, 1897); Cristimenes zanzibaricus (Bruce, 1967); Manipontonia paeneglabra Bruce, 2012; Miopontonia yongei Bruce, 1985; Palaemonella spinulata Yokoya, 1936; and Thaumastocaris streptopus Kemp, 1922. Of these, C. zanzibaricus also represents a northern range extension for the species. Five species, Arete indicus Coutière, 1903; Cuapetes amymone (de Man, 1902); Jocaste lucina (Nobili, 1901); Palaemonella pottsi (Borradaile, 1915); and Pontoniopsis comanthi Borradaile, 1915, showed higher than 60% prevalence on their hosts. These results provide useful baseline information on symbiotic shrimps prior to the establishment of a conservation area at this site in 2016, and also fill in faunal distribution gaps in the coral reef region between the southern Ryukyu arc and the Coral Triangle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical coral reefs of the Indo-West Pacific region are the most biologically diverse marine ecosystems in coastal areas (Paulay 1997; Stella et al. 2011; Veron 2000). Reefs are dominated by invertebrates (Stella et al. 2011), presumably because the complex morphology of corals provides an excellent refuge against predators (Austin et al. 1980; McCoy and Bell 1991; Patton 1994; Stella et al. 2010; Vytopil and Willis 2001). Shrimps of the infraorder Caridea, comprising approximately 3438 species (De Grave and Fransen 2011), are presently known as the second most species-rich group among the decapods. Many kinds of caridean shrimp can be found concealing themselves within the interstices of live macro-invertebrates.
In the Indo-West Pacific, caridean shrimps of the three families Hippolytidae, Alpheidae, and Palaemonidae are particularly common (Bruce 1972, 1976a; Glynn and Enochs 2011). Members of these families are known to have developed commensal habits in many cases (Bruce 1976a) and are associated with a variety of hosts, including species in the following phyla: Porifera, Cnidaria, Annelida, Mollusca, Arthropoda (crustaceans), Echinodermata, and Chordata (ascidians, fish) (Anker et al. 2005; Bruce 1972, 1976a, 1982; De Grave 1999; Fautin et al. 1995; Fransen 2006; Glynn and Enochs 2011; Guo et al. 1996; Marin 2007; Wei et al. 2005).
Obligate symbiotic species rely on the presence of their hosts for survival (Stella et al. 2011). Conversely, several caridean symbionts play a crucial role in increasing the survival rate of their hosts. They defend the hosts against predators (Glynn 1987; McKeon et al. 2012; Nakano and Fujii 2014), assist in clearing sediment away from their coral hosts (Stier et al. 2012), and enhance their host by providing nitrogenous nutrients (Spotte 1996). Some host species are reliant on these services and are unable to survive without their symbionts (Nakano and Fujii 2014; Stella et al. 2011; Stier et al. 2012).
Taiwan, formerly called Formosa, is an island located in the Western Pacific Ocean, with its northern part connected to the southern Ryukyu arc in the southernmost part of the East China Sea, and its southern side facing the South China Sea and the Coral Triangle through the Luzon Strait (Fig. 1). Taxonomy of caridean shrimps in Taiwan has been considerably studied since the early 1900s. First, two species of Palaemon were collected and described from Taiwan (Parisi 1919). Then, Maki and Tsuchiya (1923) published the first monograph on Taiwanese decapods, which provided excellent illustrations of caridean shrimps of genera such as Macrobrachium and Palaemon and still contributes to decapod studies in Taiwan. An early report on the symbiotic marine carideans (Holthuis 1952) of Taiwan recorded Anchistus custos (Forskål, 1775); Conchodytes biunguiculatus (Paul’son, 1875); and Conchodytes monodactylus Holthuis, 1952, all of which were associated with pen shells when collected from southern Taiwan in 1907. Later on, various symbiotic caridean shrimps in coral reef zones were studied by Taiwanese scientists, mainly by Chan and Yu (2002), Chang (2010), Chang et al. (2010), Jeng (1997, 1998, 2000), Jeng and Chang (1985), and Kou et al. (2013). Most symbiotic shrimps are small in size and cryptic in color (Bruce 1976a). Many of them might have been overlooked, still awaiting discovery to reveal their actual diversity.
In northeastern Taiwan, a number of recent studies on marine communities have focused on copepods (Chou et al. 2012), jellyfish (Tseng et al. 2015), symbiotic crabs (Limviriyakul et al. 2016a), and macro-algae (Lin et al. 2018). Baseline knowledge of marine carideans in Taiwan is quite sparse and relies on just a few publications. In contrast, knowledge about the diversity of anomuran and brachyuran crabs in Taiwan has become almost complete during recent decades, as shown in Baba et al. (2009), Chan et al. (2009), Chan et al. (2010), Chan and Yu (2002), McLaughlin et al. (2007), and Ng et al. (2001). Therefore, the unknown diversity of caridean shrimps still presents a challenge to biologists. In the present study, we collected specimens in the reef zone of northeastern Taiwan to evaluate the diversity of symbiotic caridean shrimp and thereby provide baseline taxonomic and distributional information for these animals in the coastal areas of the southern East China Sea.
Materials and methods
Study area description
Wang-Hai-Xiang Bay, with an area of about 2.4 km2, is a semi-enclosed body of water in the vicinity of Keelung City in the northeastern coast of Taiwan in the southern East China Sea. The Wang-Hai-Xiang Chao-Jing Bay Resource Protected Area, covering an area of about 0.15 km2 in the eastern part of the Wang-Hai-Xiang Bay (25° 8′ 40.45″ N, 121° 48′ 17.16″ E; Fig. 1), was established on 12 May 2016. It serves as a multi-function fishery resource protection area and also supports travel, leisure, and diving activities. Animal collection, fishing, and all forms of habitat change and destruction are prohibited there except for proposes of academic research. Economic seaweeds, mainly Gelidiaceae spp. (Lin et al. 2018), however, could be harvested from November to June with a permit granted by the governmental management department.
Field sampling and sample treatment
Weekly collections were made under permit from April to August 2014, within the boundaries of what is now the Wang-Hai-Xiang Chao-Jing Bay Resource Protected Area. Symbiotic shrimp specimens were collected by scuba diving in the reef zone down to 24 meters (m) depth. Potential host animals (sponges, hydroids, actiniarians, scleractinians, gorgonians, alcyonarians, crinoids, and echinoids) were investigated. For each kind of potential host, the frequency of encounter, prevalence of symbiotic shrimps, and depth were recorded. Each host, along with its symbionts, was placed separately in a plastic zip-lock bag or box and taken to the Chao-Jing Ocean Center (National Museum of Marine Science and Technology) for identification. There, the specimens were photographed, preserved in 70% ethyl alcohol, and deposited in the center’s collection. The prevalence of each symbiont was calculated by dividing the number of host species individuals bearing the symbiont by the total number of host individuals checked.
Shrimp identification
In the laboratory, the caridean shrimps were identified under a dissecting microscope (Olympus SZX16) using the following keys: for Alpheidae, Anker and Jeng (2007), Chace Jr. (1988), Suzuki (1970), and Wang and Sha (2015); for Hippolytidae, Chace Jr. (1997) and Xu and Li (2015); for Palaemonidae, Bruce (1982), Chace Jr. and Bruce (1993), Berggren (1994), Bruce (2004), Mitsuhashi and Takeda (2008), Marin (2009, 2014), Bruce (2010), Marin and Anker (2011), and Fransen and Rauch (2013).
Results
Twenty-seven species of symbiotic shrimp, representing 20 genera and 3 families, were identified from the study area (Table 1). The following 5 species showed a particularly high prevalence of occurrence (more than 60%) on their hosts: Arete indicus Coutière, 1903; Cuapetes amymone de Man, 1902; Jocaste lucina Nobili, 1901; Palaemonella pottsi Borradaile, 1915; and Pontoniopsis comanthi Borradaile, 1915. Furthermore, 6 species, Synalpheus neomeris (de Man, 1897); Cristimenes zanzibaricus Bruce, 1967; Manipontonia paeneglabra Bruce, 2012; Miopontonia yongei Bruce, 1985; Palaemonella spinulata Yokoya, 1936; and Thaumastocaris streptopus Kemp, 1992, were recorded for the first time in the waters of Taiwan.
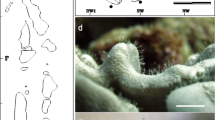
Symbiotic carideans were not distributed evenly among the host taxa. Among the shrimp species found by us, 3.2% were on sponges, 3.2% on hydroids, 6.5% on actiniarians, 45.2% on scleractinians, 16.1% on gorgonians, 3.2% on alcyonarians, 9.7% on crinoids, and 12.9% on echinoids (Fig. 2). Fourteen species, nearly half of the total (45.2%), were associated with scleractinian hosts, especially with branching corals. The proportions of symbiotic shrimps belonging to the three families were as follows: Alpheidae, 22.2% (6 species; Fig. 3); Hippolytidae, 7.4% (2 species; Fig. 4); and Palaemonidae, 70.4% (19 species; Fig. 5). Among these shrimps, the genus Synalpheus was dominant in species number, represented by 4 species in all. All of the species in the family Palaemonidae were so-called pontoniine shrimps, a name used for members of the former but no longer recognized as subfamily Pontoniinae (De Grave et al. 2015), which are almost exclusively tropical and subtropical marine symbionts. Such shrimps are rarely found in temperate or fresh waters (Bruce 1983).
Family Palaemonidae. aAncylocaris brevicarpalis; bCoralliocaris graminea; cCoralliocaris superba; dCoralliocaris viridis; eCristimenes zanzibaricus; fCuapetes amymone; gCuapetes elegans; hHamodactylus boschmai; iHamopontonia corallicola; jJocaste lucina; kLaomenes ceratophthalmus; lManipontonia paeneglabra; mMiopontonia yongei; nPalaemonella pottsi; oPalaemonella spinulata; pPontoniopsis comanthi; qStegopontonia commensalis; rThaumastocaris streptopus; sTuleariocaris zanzibarica. Scale bars each represent 5 mm
Discussion
The dominant hosts of symbiotic shrimps
The present results indicate that branching corals are not only important to the reef ecosystem on their own account but also contribute to the biodiversity of decapod crustaceans. Nearly half of the symbiotic shrimp species in the area were associated with scleractinian corals. Among these, 90% were found to live on branching species of Acropora and Pocilloporidae, while the remainder was associated with massive corals. Similarly, a study of symbiotic crabs in northeastern Taiwan found most of them to be associated with branching corals (Limviriyakul et al. 2016a). A substantial predilection for decapod symbionts to live on branching corals has also been reported elsewhere in the Indo-Pacific (Bruce 1972, 1976a, 1998; Fransen 2008, 2010, 2012; Limviriyakul et al. 2016b; Patton 1966; Stella et al. 2010; Stella et al. 2011; Vytopil and Willis 2001).
The growth form of scleractinian corals is a factor affecting the diversity of coral-associated organisms (Pratchett et al. 2009; Stella et al. 2010; Vytopil and Willis 2001). A branching morphology benefits symbionts by providing more surface area on which to live, greater protection against predators, and more food in the form of coral tissue and mucus (Castro 1988; Vytopil and Willis 2001). Furthermore, more complex branching is correlated with a higher diversity of associated organisms (Stella et al. 2010).
Non-branching corals, including massive, encrusting and mushroom corals, harbor fewer species of symbiotic carideans (Bruce 1976a, 1998). Many species of these corals that do harbor associated shrimp, such as Euphyllia glabrescens (Chamisso & Eysenhardt, 1821), Fungia spp., Galaxea fascicularis (Linnaeus, 1767), Goniopora spp., Heliofungia actiniformis (Quoy & Gaimard, 1833), and Physogyra lichtensteini (Milne Edwards & Haime, 1851), have long-extended or swollen polyps (Bruce 1976a; De Grave 1998; Hoeksema et al. 2012; Marin 2014). In the present study, we found Hamopontonia corallicola Bruce, 1970, is associated with massive corals of the genus Goniopora.
The dominant species of symbiotic shrimps
The five species of symbiotic shrimp with particularly high prevalence in the investigated area have varied distribution records worldwide. The alpheid shrimp Arete indicus (Fig. 3b) is common throughout the Indian Ocean and Western Pacific Ocean. It inhabits intertidal rocky shores (Suzuki 1970), which is consistent with our record of A. indicus in the 0–3-m-depth zone. This cryptically colored shrimp is most commonly associated with Echinometra mathaei (Blainville, 1825) (Banner and Banner 1973), but it also occurs on various other genera of echinoids, including Centrostephanus, Diadema, and Heliocidaris (Banner and Banner 1973; Bruce 1982). All of the specimens in the present study were found beneath the rock-boring sea urchin, E. mathaei, and most of the sea urchins were giving shelter to a couple of shrimps. The prevalence of A. indicus in the area was extraordinary; approximately 90% of E. mathaei specimens harbored the shrimp species, and the echinoid was also abundant in the intertidal zone of the study area, attaining densities of 60 individuals per square meter or more. In addition, A. indicus was usually observed together with an anomuran crab, Petrolisthes virgatus Paulson, 1875, which lives in the cavities excavated in the substrate by the urchin (Limviriyakul et al. 2016a).
The pontoniine shrimps Cuapetes amymone (Fig. 5f) and Jocaste lucina (Fig. 5j) are obligatory symbionts of scleractinian branching corals and both species have been widely recorded sympatrically across the Indo-West Pacific (Electronic Supplementary Material, Table S1). C. amymone has been found from the shallows to a depth of 23 m (Bruce 2004) whereas the maximum recovered depth for J. lucina is 54 m (Li 2008). C. amymone is usually associated with the genera Acropora, Pocillopora, Seriatopora, and Stylophora (Bruce 1972, 2004; Stella et al. 2011) whereas J. lucina is mainly associated with Acropora, and only rarely with Pocillopora (Bruce 1972), Stylopora (Bruce 1998), Porites (Bruce 1981a), hydroids of the genus Millepora (Bruce 1981a), and dead coral heads (Head et al. 2015). In the present study, we collected C. amymone from various genera of branching corals, and J. lucina from Acropora spp. As has been noted before (Limviriyakul et al. 2016a), we found these two species occurring together on the same host colony, and also with Tetralia crabs, Cymo crabs, Coralliocaris shrimps, and coral gobies.
The symbiotic shrimps Palaemonella pottsi (Fig. 5n) and Pontoniopsis comanthi (Fig. 5p) occur on crinoids and are broadly distributed throughout the Indo-West Pacific (ESM, Table S1), most commonly associated with genera such as Anneissia, Comanthus, Comaster, Comatella, Comatula, Heterometra, Lamprometra, and Phanogenia (Bruce 1970, 1982, 1994; Li and Bruce 2006). Both species have been reported from shallow water to 25–30 m depth (De Grave 2000). All of our specimens of Pa. pottsi and Po. comanthi were collected from crinoids, with the prevalence of more than 60% and 85%, respectively. Studies from Vietnam also report that the number of Pa. pottsi specimens collected per host is much lower than those of Po. comanthi (Britayev and Mekhova 2011; Dgebuadze et al. 2012). Both shrimps have their own particular niche on the host. In this study, Pa. pottsi were approximately 2.0–4.2 mm in carapace length and lived in the center near the tegmen and mouth of the host. In contrast, Po. comanthi were relatively small, with a carapace length of approximately 0.9–1.6 mm, and inhabited the arms of crinoids. The aggregation habits of both shrimps were also dissimilar: Pa. pottsi usually lived in a heterosexual pair on a crinoid while Po. comanthi lived in large groups of adults and juveniles of both sexes. Most of the crinoids in the study area harbored at least 1 individual of Po. comanthi, but none was found on small crinoids with an arm length of less than 7 cm. These two species were commonly found to live together and with another symbiotic shrimp, Synalpheus stimpsonii (de Man, 1888), and also with the symbiotic anomuran and brachyuran crabs Allogalathea elegans (Adams and White, 1848) and Permanotus purpureus (Gordon, 1934).
Newly recorded species in Taiwan
One species of Alpheidae and 5 species of Palaemonidae represent new records for Taiwan. The alpheid shrimp Synalpheus neomeris (Fig. 3d) has been found on soft corals from East Africa to Australia. Typically found in pairs, it is usually associated with Dendronephthya spp. and Xenia spp. (Chace Jr. 1988) but has also been recorded in association with sponges, bryozoans, and dead coral heads (Banner and Banner 1975; Chace Jr. 1988). It ranges from the shallow subtidal to 250 m depth (Banner and Banner 1983; Chace Jr. 1988). In the present study, specimens were found on alcyonarians of the genus Dendronephthya.
Cristimenes zanzibaricus (Fig. 5e) was collected from the echinoids Diadema setosum and Echinothrix sp. at 5–10 m depth. This report represents the first record of this shrimp in the northern hemisphere and the second locality for it in the Pacific Ocean. In East Africa (Tanzania, Kenya, and Seychelles) and Australia (Bruce 1967, 1976b, 1983; Fransen 1994), C. zanzibaricus has been found to live with the echinoid hosts Astropyga radiata (Leske, 1778); Centrostephanus tenuispinus H.L. Clark, 1914; Diadema savignyi (Audouin, 1829); Diadema setosum (Leske, 1778); and Echinothrix calamaris (Pallas, 1774) (Bruce 1967, 1976b, 1982) from the subtidal to 12 m depth (Fransen 1994).
Manipontonia paeneglabra (Fig. 5l) was found on the gorgonian Mopsella sp. and on a hydroid from relatively shallow depths of 18–22 m. The present record is just the fourth report and the northernmost record of this species, and the first for which the hosts are known. It was first described by Bruce (2012) in Indo-West Pacific waters, and it is distributed in the South China Sea (Paracel Islands), Singapore, Australia, and Papua New Guinea (Anker and De Grave 2016; Bruce 2012; Muséum national d’Histoire naturelle 2013). The previously known depth range was 82.3–105 m, and nothing was known about the shrimp’s habitat or host (Bruce 2012).
Miopontonia yongei (Fig. 5m) was sampled from a gorgonian host, Ellisella sp., at 20 m depth. This rare species of Palaemonidae has previously been found associated with Ellisella plexauroides (Toeplitz, 1919) and with an antipatharian, Cirrhipathes anguina (Dana, 1846) (Okuno 1998; Wagner et al. 2012). It has been reported at 40–80 m depth from just a few localities: Indonesia (Bali and Raja Ampat), Philippines, Japan, and Australia (Bruce 1985; Fransen 2008; Gan et al. 2015; Okuno 1998; Williams and Boyko 2012).
The pontoniine shrimp Palaemonella spinulata (Fig. 5o) is distributed in East Africa and the West Pacific Ocean (ESM, Table S1) from the intertidal to 60 m depth (Bruce 1994; Poupin 2003), often living among coral heads, algae, dead coral, and coral rubble (Li and Bruce 2006; Stella et al. 2011). It is probably a free-living micro-predator in coral reefs (Bruce 1981b). In the present study, specimens were collected from gorgonians and from branching scleractinian corals of the genera Acropora and Seriatopora.
Thaumastocaris streptopus (Fig. 5r), a widespread shrimp species, has been reported from most areas of the Indo-West Pacific as well as the Gulf of Mexico (ESM, Table S1). It is usually found living in pairs within the spongocoel of several genera of Porifera, such as Acarnus, Callyspongia, Haliclona, Leucetta, Mycale, Oceanapia, Petrosia, Siphonochalina, and Xestospongia (Bruce 1981b, 2005; De Grave 2000), but it is occasionally associated with scleractinian corals of the genera Pocillopora and Stylophora (Preston and Doherty 1990). In our study, all specimens of this shrimp were found inside Callyspongia sponges except for one specimen from a small barrel sponge, Xestospongia testudinaria Lamarck, 1815. Barrel sponges are well-known for their large size and have a relatively wide spongocoel radius compared with the other known hosts of this shrimp. It may be that T. streptopus can live in any sponge with a narrow exhaust tube, including small barrel sponges.
Diversity of pontoniine shrimps in Taiwan
The world species richness of marine caridean shrimps is dominated by the family Palaemonidae (981 species), followed by Alpheidae (663) and Hippolytidae (338) (De Grave and Fransen 2011). So-called pontoniine shrimps, members of the family Palaemonidae, are among the most diverse invertebrate-associated taxa on tropical reefs in the Indo-Pacific (Bruce 1972, 1976a; De Grave 2001), with more than 602 species known (De Grave and Fransen 2011). Approximately 60–70% of them are involved in some form of symbiotic association (De Grave 2001). The present study identified 19 pontoniine shrimps, with 5 species recorded from Taiwan for the first time, thereby raising the total number of pontoniine shrimp species from Taiwan to 83 (ESM, Table S1). These represent approximately 14% of the known species of pontoniine shrimps, more than a previous estimate by Shao (1998) that the marine “pontoniine” species around Taiwan represent one tenth of those worldwide.
Conclusion
The present study revealed 27 species of symbiotic caridean shrimps at the study site in northeastern Taiwan; among them, 6 species were reported from Taiwan for the first time. The high species richness of these shrimps in this part of the southern East China Sea shows that this region holds great promise for advanced studies on caridean shrimps. It also provides a hint of the state of health of the diverse benthic fauna in the investigated areas, because some of these symbiotic shrimps, which have the ability to mitigate environmental stresses and enhance nutrient flow to their hosts, are considered to increase the persistence and resilience of their hosts. Currently, coral reef fauna are threatened by various anthropogenic impacts and climate change. Subtropical reefs in Taiwan have the valuable potential to serve as climate change refuges for vulnerable tropical coral reef species. To effectively maintain and manage these coral reefs, more complete knowledge of the reef fauna biodiversity is essential. Our present data have established which species of symbiotic caridean shrimp were dominant before the establishment of the Wang-Hai-Xiang Chao-Jing Bay Resource Protected Area in northeastern Taiwan, thereby, providing baseline information to enable future monitoring of the effectiveness of the Protected Area.
References
Anker A, De Grave S (2016) An updated and annotated checklist of marine and brackish caridean shrimps of Singapore (Crustacea, Decapoda). Raffles Bull Zool Supplement No 34:343–454
Anker A, Jeng MS (2007) Establishment of a new genus for Arete borradailei Coutière, 1903 and Athanas verrucosus Banner and Banner, 1960, with redefinitions of Arete Stimpson, 1860 and Athanas Leach, 1814 (Crustacea: Decapoda: Alpheidae). Zool Stud 46:454
Anker A, Murina GV, Lira C, Caripe JAV, Palmer AR, Jeng MS (2005) Macrofauna associated with echiuran burrows: a review with new observations of the innkeeper worm, Ochetostoma erythrogrammon Leuckart and Rüppel, in Venezuela. Zool Stud 44:157–190
Austin AD, Austin SA, Sale PF (1980) Community structure of the fauna associated with the coral Pocillopora damicornis (L.) on the Great Barrier Reef. Aust J Mar Freshw Res 31:163–174
Baba K, Macpherson E, Lin CW, Chan TY (2009) Crustacean fauna of Taiwan: squat lobsters (Chirostylidae and Galatheidae). National Taiwan Ocean University, Keelung
Banner DM, Banner AH (1973) The alpheid shrimp of Australia. Part I: the lower genera. Rec Aust Mus 28:291–382
Banner DM, Banner AH (1975) The alpheid shrimp of Australia. Part 2: the genus Synalpheus. Rec Aust Mus 29:267–389
Banner AH, Banner DM (1983) An annotated checklist of the alpheid shrimp from the western Indian Ocean. Travaux et Documents de l’ORSTOM 158:1–164
Berggren M (1994) Periclimenes nomadophila and Tuleariocaris surec, two new species of pontoniine shrimps (Decapoda: Pontominae), from Inhaca Island, Mocambique. J Crust Biol 14:782–782
Britayev TA, Mekhova ES (2011) Assessment of hidden diversity of crinoids and their symbionts in the bay of Nhatrang, Vietnam. Org Divers Evol 11:275–285
Bruce AJ (1967) Notes on some Indo-Pacific Pontoniinae. III-IX. Description of some new genera and species from the western Indian Ocean and the South China Sea. Zool Verhandel 87:1–73
Bruce AJ (1970) Observations on the Indo-West-Pacific species of the genus Palaemonella Dana, 1852 (Decapoda, Pontoniinae). Crustaceana 19:273–287
Bruce AJ (1972) A review of information upon the coral hosts of commensal shrimps of the subfamily Pontoniinae, Kingsley, 1878 (Crustacea, Decapoda, Palaemonidae). In: Mukundan G, Pillai CSG (eds) Proceedings of the symposium on corals and coral reefs, India, 1972. The Marine Biological Association of India, pp 399–418
Bruce AJ (1976a) Coral reef caridea and “commensalism”. Micronesica 12:83–98
Bruce AJ (1976b) A report on a small collection of shrimps from the Kenya National Marine Parks at Malindi, with notes on selected species. Zool Verhandel 145:1–72
Bruce AJ (1981a) Pontoniine shrimps from Viti Levu, Fijian Islands. Micronesica 17:77–95
Bruce AJ (1981b) Pontoniine shrimps of Heron Island. Atoll Res Bull 245:1–33
Bruce AJ (1982) The shrimps associated with Indo-West Pacific echinoderms, with the description of a new species in the genus Periclimenes Costa, 1844 (Crustacea: Pontoniinae). Austral Mus Mem 16:191–216
Bruce AJ (1983) The pontoniine shrimp fauna of Australia. Austral Mus Mem 18:195–218
Bruce AJ (1985) Notes on some Indo-Pacific Pontoniinae, XLII. Miopontonia yongei gen. nov., sp. nov., from the Australian north west shelf (Decapoda, Caridea). Crustaceana 48:167–178
Bruce AJ (1994) A synopsis of the Indo-West Pacific genera of the Pontoniinae (Crustacea: Decapoda: Palaemonidae). Koeltz Scientific Books, Königstein
Bruce AJ (1998) New keys for the identification of Indo-West Pacific coral associated pontoniine shrimps, with observations on their ecology (Crustacea: Decapoda: Palaemonidae). Ophelia 49:29–46
Bruce AJ (2004) A partial revision of the genus Periclimenes Costa, 1884 (Crustacea: Decapoda: Palaemonidae). Zootaxa 582:1–26
Bruce AJ (2005) Pontoniine shrimps from Papua New Guinea, with designation of two new genera, Cainonia and Colemonia (Crustacea: Decapoda: Palaemonidae). Mem Queensl Mus 51:333–383
Bruce AJ (2010) Palaemonella dijonesae sp. nov.(Crustacea: Decapoda: Pontoniinae) from Western Australia. Zootaxa 2372:151–156
Bruce AJ (2012) Notes on Indo-Pacific Pontoniinae, LII. A third species of the genus Manipontonia Bruce, Okuno & Li, 2005. Crustaceana 85:1377–1383
Castro P (1988) Animal symbioses in coral reef communities: a review. Symbiosis 5:161–184
Chace FA Jr (1988) The caridean shrimps (Crustacea: Decapoda) of the Albatross Philippine Expedition, 1907-1910, part 5: family Alpheidae. Smithson Contrib Zool 466:1–99
Chace FA Jr (1997) The caridean shrimps (Crustacea: Decapoda) of the albatross Philippine expedition, 1907-1910, part 7: families Atyidae, Eugonatonotidae, Rhynchocinetidae, Bathypalaemonidae, Processidae, and Hippolytidae. Smithson Contrib Zool 587:1–106
Chace FA Jr, Bruce AJ (1993) The caridean shrimps (Crustacea: Decapoda) of the Albatross Philippine Expedition, 1907-1910, part 6: superfamily Palaemonoidea. Smithson Contrib Zool 543:1–152
Chan TY, Yu HP (2002) Decapod crustacean fauna study in Taiwan. J Fish Soc Taiwan 29:163–171
Chan TY, Ng PKL, Ahyong ST, Tan SH (2009) Crustacean fauna of Taiwan: brachyuran crabs, volume I–Carcinology in Taiwan and Dromiacea, Raninoida, Cyclodorippoida. National Taiwan Ocean University, Keelung
Chan TY, Osawa M, Boyko CB, Ahyong ST, Macpherson E (2010) Crustacean fauna of Taiwan: crab-like anomurans (Hippoidea, Lithodoidea and Porcellanidae). National Taiwan Ocean University, Keelung
Chang SC (2010) Taxonomic studies on families Hippolytidae and Processidae (Crustacea, Decapoda, Caridea) of Taiwan. National Taiwan Ocean University, Master thesis
Chang SC, Komai T, Chan TY (2010) First record of the hippolytid shrimp genus Lebbeus White, 1847 (Decapoda: Caridea) from Taiwan, with the description of three new species. J Crust Biol 30:727–744
Chou C, Tseng LC, Ou CH, Chen QC, Hwang JS (2012) Seasonal succession of planktonic copepods in bight environments of northeastern Taiwan. Zool Stud 51:1380–1396
De Grave S (1998) Pontoniinae (Decapoda, Caridea) associated with Heliofungia actiniformis (Scleractinia) from Hansa Bay, Papua New Guinea. Belg J Zool 128:13–22
De Grave S (1999) Pontoniinae (Crustacea: Decapoda: Palaemonidae) associated with bivalve molluscs from Hansa Bay, Papua New Guinea. Bull Inst R Sci Nat Belgique, Biol 69:125–141
De Grave S (2000) Caridean shrimps (Crustacea, Decapoda) from Hansa Bay, Papua New Guinea: Palaemonidae and Gnathophyllidae. Bull Inst R Sci Nat Belgique, Biol 70:119–148
De Grave S (2001) Biogeography of Indo-Pacific Pontoniinae (Crustacea, Decapoda): a PAE analysis. J Biogeogr 28:1239–1253
De Grave S, Fransen CHJM (2011) Carideorum catalogus: the recent species of the dendrobranchiate, stenopodidean, procarididean and caridean shrimps (Crustacea: Decapoda). Zool Meded 85:195–588
De Grave S, Fransen CHJM, Page TJ (2015) Let’s be pals again: major systematic changes in Palaemonidae (Crustacea: Decapoda). PeerJ 3:e1167
Dgebuadze PY, Mehova ES, Britayev TA (2012) Recolonization of the Himerometra robustipinna (Himerometridae, Crinoidea) by macrosymbionts: an in situ experiment. Symbiosis 58:253–258
Fautin DG, Guo CC, Hwang JS (1995) Costs and benefits of the symbiosis between the anemoneshrimp Periclimenes brevicarpalis and its host Entacmaea quadricolor. Mar Ecol Prog Ser 129:77–84
Fransen CHJM (1994) Marine palaemonoid shrimps of the Netherlands Seychelles Expedition 1992-1993. Zool Verhandel 297:85–152
Fransen CHJM (2006) On Pontoniinae (Crustacea, Decapoda, Palaemonidae) collected from ascidians. Zoosystema 28:713–746
Fransen CHJM (2008) Pontoniine shrimps. In: Hoeksema BW, van der Meij SET (eds) Cryptic marine biota of the Raja Ampat island group. Preliminary results of the Raja Ampat Expedition (2007), Ekspedisi Widza Nusantra (E-Win) of the Indonesian Institute of Science (LIPI). Progress Report. National Museum of Natural History Naturalis, Leiden, pp 16–18
Fransen CHJM (2010) Palaemonoid shrimps. In: Hoeksema BW, van der Meij SET (eds) Crossing marine lines at Ternate: capacity building of junior scientists in Indonesia for marine biodiversity assessments. Preliminary results of the Ternate Expedition (2009). 2nd edn. National Museum of Natural History Naturalis, Leiden, pp 26–30
Fransen CHJM (2012) Palaemonoid shrimps. In: Kassem K, Hoeksema BW, Affendi YA (eds) Semporna Marine Ecological Expedition. Kota Kinabalu, Malaysia. WWF-Malaysia, NCB Naturalis, Universiti Malaysia Sabah, pp 101-108
Fransen CHJM, Rauch C (2013) Hamodactylus macrophthalmus spec. nov., a new coral-associated pontoniine shrimp (Decapoda, Caridea, Palaemonidae) from Indonesia. Zootaxa 3635:286–296
Gan ZB, Li X, Chan TY, Chu KH, Kou Q (2015) Phylogeny of Indo-West Pacific pontoniine shrimps (Crustacea: Decapoda: Caridea) based on multilocus analysis. J Zool Syst Evol Res 53:282–290
Glynn PW (1987) Some ecological consequences of coral-crustacean guard mutualisms in the Indian and Pacific Oceans. Symbiosis 4:301–323
Glynn PW, Enochs IC (2011) Invertebrates and their roles in coral reef ecosystems. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, New York, pp 273–325
Guo CC, Hwang JS, Fautin DG (1996) Host selection by shrimps symbiotic with sea anemones: a field survey and experimental laboratory analysis. J Exp Mar Biol Ecol 202:165–176
Head CI, Bonsall MB, Koldewey H, Pratchett MS, Speight M, Rogers AD (2015) High prevalence of obligate coral-dwelling decapods on dead corals in the Chagos Archipelago, Central Indian Ocean. Coral Reefs 34:905–915
Hoeksema BW, Van der Meij SET, Fransen CHJM (2012) The mushroom coral as a habitat. J Mar Biol Assoc UK 92:647–663
Holthuis LB (1952) The Decapoda of the Siboga Expedition. Part XI. The Palaemonidae collected by the Siboga and Snellius Expeditions with remarks on other species II. Subfamily Pontoniinae. Siboga Expeditie 39a:1–253
Jeng MS (1997) Studies on the land and aquatic decapod crustacean fauna of the Kenting National Park (II)–communities of decapod crustaceans around the sea. Kenting National Park, Ministry of the Interior, Pingtung
Jeng MS (1998) Shrimps and crabs of Kenting National Park. Kenting National Park, Ministry of the Interior, Pingtung
Jeng MS (2000) Decapod crustaceans associated with unbleached and bleached colonies of the coral, Seriatopora hystrix from near the thermal discharge of a nuclear power plant in Taiwan. Crustac Issues 12:77–84
Jeng MS, Chang KH (1985) Snapping shrimps (Crustacea: Decapoda: Alpheidae) of Taiwan. Bull Inst Zool, Acad Sin 24:241–256
Kou Q, Li X, Chan TY, Chu KH, Huang H, Gan Z (2013) Phylogenetic relationships among genera of the Periclimenes complex (Crustacea: Decapoda: Pontoniinae) based on mitochondrial and nuclear DNA. Mol Phylogen Evol 68:14–22
Li X (2008) Report on some species of Palaemonidae (Crustacea, Decapoda) from French Polynesia. Zoosystema 30:203–252
Li X, Bruce AJ (2006) Further Indo-West Pacific palaemonoid shrimps (Crustacea: Decapoda: Palaemonoidea), principally from the New Caledonian region. J Nat Hist 40:611–738
Limviriyakul P, Tseng LC, Hwang JS, Shih TW (2016a) Anomuran and brachyuran symbiotic crabs in coastal areas between the southern Ryukyu arc and the Coral Triangle. Zool Stud 55:1–14
Limviriyakul P, Tseng LC, Shih TW, Hwang JS (2016b) Symbiotic decapods in reef area of northeastern Taiwan. Journal of Ocean and Underwater Technology 26:13–24
Lin SM, Tseng LC, Ang PO Jr, Bolton J, Liu LC (2018) Long term study on seasonal changes in floristic composition and structure of marine macroalgal communities along the coast of northern Taiwan, southern East China Sea. Mar Biol 165:83
Maki M, Tsuchiya H (1923) A monograph of the Decapoda Crustacea of Formosa. Report of the Department of Agriculture, Government of Research Institute of Taihoku, vol 3
Marin I (2007) Pontoniine shrimps (Decapoda: Caridea: Palaemonidae) inhabiting boring sponges (Porifera: Demospongia) from Nhatrang Bay, Vietnam, with description of three new species. Zool Meded 81:217–240
Marin I (2009) Crinoid-associated shrimps of the genus Laomenes AH Clark, 1919 (Caridea: Palaemonidae: Pontoniinae): new species and probable diversity. Zootaxa 1971:1–49
Marin I (2014) A new species of the pontoniine shrimp genus Hamopontonia Bruce, 1970 associated with caryophyllid coral Euphyllia glabrescens (Chamisso & Eysenhardt, 1821) in Nhatrang Bay, Vietnam. Zootaxa 3815:131–140
Marin I, Anker A (2011) A partial revision of the Philarius gerlachei (Nobili, 1905) species complex (Crustacea, Decapoda, Palaemonidae), with description of four new species. Zootaxa 2781:1–28
McCoy ED, Bell SS (1991) Habitat structure: the evolution and diversification of a complex topic. In: Belll SS, McCoy ED, Mushinsky HR (eds) Habitat structure: the physical arrangement of objects in space, vol 8. Population and community biology series. Chapman and Hall, New York, pp 3–27
McKeon CS, Stier AC, McIlroy SE, Bolker BM (2012) Multiple defender effects: synergistic coral defense by mutualist crustaceans. Oecologia 169:1095–1103
McLaughlin PA, Rahayu DL, Komai T, Chan TY (2007) A catalog of the hermit crabs (Paguroidea) of Taiwan. National Taiwan Ocean University, Keelung
Mitsuhashi M, Takeda M (2008) Identity of the coral-associated pontoniine shrimp species, Coralliocaris nudirostris (Heller, 1861) and C. venusta Kemp, 1922 (Crustacea: Decapoda: Palaemonidae), with descriptions of two new species. Zootaxa 1703:1–24
Muséum national d’Histoire naturelle (2013) Collection: Crustaceans (IU), Specimen MNHN-IU-2013-10603. http://coldb.mnhn.fr/catalognumber/mnhn/iu/2013-10603. Accessed 8 Mar 2016
Nakano R, Fujii T (2014) The soft-coral associated pistol shrimp Synalpheus neomeris (De Man)(Decapoda: Alpheidae) defends its host against nudibranchs in Okinawa, Japan. Raffles Bull Zool 62:759–763
Ng PKL, Wang CH, Ho PH, Shih HT (2001) An annotated checklist of brachyuran crabs from Taiwan (Crustacea: Decapoda). National Taiwan Museum Special Publication Series, vol 11. National Taiwan Museum, Taipei
Okuno J (1998) Miopontonia yongei Bruce, 1985 (Decapoda, Caridea, Palaemonidae): new host record and colour pattern. Crustaceana 71:349–353
Parisi B (1919) I decapodi Giapponesi del Museo di Milano. VII. Natantia. Atti Soc Ital Sci Nat Mus Civico Storia Nat Milano 58:59–99
Patton WK (1966) Decapod Crustacea commensal with Queensland branching corals. Crustaceana 10:271–295
Patton WK (1994) Distribution and ecology of animals associated with branching corals (Acropora spp.) from the Great Barrier Reef, Australia. Bull Mar Sci 55:193–211
Paulay G (1997) Diversity and distribution of reef organisms. In: Birkeland C (ed) Life and death of coral reefs. Chapman and Hall, New York, pp 298–353
Poupin J (2003) Crustacea Decapoda and Stomatopoda of Easter Island and surrounding areas: a documented checklist with historical overview and biogeographic comments. Atoll Res Bull 500:1–50
Pratchett MS, Wilson SK, Graham NAJ, Munday PL, Jones GP, Polunin NVC (2009) Coral bleaching and consequences for motile reef organisms: past, present and uncertain future effects. In: Oppen MJH, Lough JM (eds) Coral bleaching. Ecological Studies, vol 205. Springer, Berlin, pp 139–158
Preston NP, Doherty PJ (1990) Cross-shelf patterns in the community structure of coral-dwelling Crustacea in the central region of the Great Barrier Reef. I. Agile shrimps. Mar Ecol Prog Ser 66:47–61
Shao KT (1998) Marine ecology. National Press Company, Ming Wen Book Co., Ltd., Taipei, Taiwan
Spotte S (1996) Supply of regenerated nitrogen to sea anemones by their symbiotic shrimp. J Exp Mar Biol Ecol 198:27–36
Stella JS, Jones GP, Pratchett MS (2010) Variation in the structure of epifaunal invertebrate assemblages among coral hosts. Coral Reefs 29:957–973
Stella JS, Pratchett MS, Hutchings PA, Jones GP (2011) Coral-associated invertebrates: density, ecological importance and vulnerability to disturbance. Oceanogr Mar Biol Annu Rev 49:43–104
Stier AC, Gil MA, McKeon CS, Lemer S, Leray M, Mills SC, Osenberg CW (2012) Housekeeping mutualisms: do more symbionts facilitate host performance? PLoS One 7:e32079
Suzuki H (1970) Taxonomic review of four alpheid shrimps belonging to the genus Athanas with reference to their sexual phenomena. Sci Rep Yokohama Natl Univ, Sect 2 Biol Geol Sci 17:1–37
Tseng LC, Chou C, Chen QC, Hwang JS (2015) Jellyfish assemblages are related to interplay waters in the southern East China Sea. Cont Shelf Res 103:33–44
Veron JEN (2000) Corals of the world. Australian Institute of Marine Science, Townsville
Vytopil E, Willis B (2001) Epifaunal community structure in Acropora spp. (Scleractinia) on the Great Barrier Reef: implications of coral morphology and habitat complexity. Coral Reefs 20:281–288
Wagner D, Luck DG, Toonen RJ (2012) The biology and ecology of black corals (Cnidaria: Anthozoa: Hexacorallia: Antipatharia). Adv Mar Biol 63:67–132
Wang Y, Sha Z (2015) A review of the genus Synalpheus (Crustacea: Decapoda: Caridea: Alpheidae) from China seas. Zoological Systematics 40:357–435
Wei TP, Hwang JS, Tsai ML, Fang LS (2005) New records of gall crabs (Decapoda, Cryptochiridae) from Orchid Island, Taiwan, northwestern Pacific. Crustaceana 78:1063–1077
Williams JD, Boyko CB (2012) The global diversity of parasitic isopods associated with crustacean hosts (Isopoda: Bopyroidea and Cryptoniscoidea). PLoS One 7:e35350
Xu P, Li X (2015) Report on the Hippolytidae Bate (sensu lato) from China seas. Zoological Systematics 40:107–165
Acknowledgments
We are grateful to T.-W. Shih’s colleagues in the National Museum of Marine Science and Technology for their assistance with field sampling. Thanks are also due to Professor Tin-Yam Chan (Institute of Marine Biology, National Taiwan Ocean University), for his help and advice concerning shrimp taxonomy work. Dr. Mark J. Grygier (Center of Excellence for the Ocean, National Taiwan Ocean University) provided advice on English composition. Thanks to the reviewers and editor team of Marine Biodiversity for their thoughtful comments and corrections.
Funding
This study was funded by the Ministry of Science and Technology (MOST) of Taiwan through grant nos. NSC-1021324062 and 103AS-14.3.2-FA-F1(5-1) to T.-W. Shih; MOST 105–2621-M-019-001 and MOST 105–2918-I-019-001 to J.-S. Hwang; and MOST 105–2811-M-019-008, MOST 106-2811-M-019-004, and MOST 107-2811-M-019-004 to L.-C. Tseng.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities. The study is compliant with CBD and Nagoya protocols.
Data availability statement
All relevant data pertaining to this study are included in this published article and its supplementary information files.
Author contribution statement
TWS and JSH wrote the proposal and designed the study. Specimens collected by PL, LCT, and YHT. PL completed the taxonomic work of shrimp species identification and took photographs of shrimps. PL and LCT analyzed the data and drafted the manuscript. TWS and JSH finalized the manuscript. All authors read and approved the final manuscript.
Additional information
Communicated by E. Macpherson
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 38 kb)
Rights and permissions
About this article
Cite this article
Limviriyakul, P., Tseng, LC., Tsai, YH. et al. Baseline diversity and host relationships of symbiotic caridean shrimps on the coast of northern Taiwan, southern East China Sea, prior to the establishment of a conservation area. Mar. Biodivers. 50, 35 (2020). https://doi.org/10.1007/s12526-020-01052-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-020-01052-0