Abstract
The current nematode classification comprises three primarily marine basal Chromadorean orders: the Microlaimida Leduc et al., 2018; Desmodorida De Coninck, 1965; and Chromadorida Chitwood, 1933. The phylogenetic placement of several taxa within these orders, however, is unclear due to the paucity of taxonomically informative morphological characters for high-level classification and is yet to be tested by molecular phylogenetic analyses due to the absence of molecular sequences. Here, we describe Molgolaimus kaikouraensis sp. nov. and Aponema pseudotorosum sp. nov. from the continental slope of New Zealand and investigate phylogenetic relationships of these species and that of the rare desmodorid genera Onepunema and Pseudonchus, using SSU phylogenetic analyses for the first time. Whilst our analyses provided support for the current classification of Aponema within the family Microlaimidae and of Pseudonchus within the Desmodorida, we could not confirm relationships of Onepunema. We found no support for the placement of Molgolaimus with either the Desmodorida or Microlaimidae/Microlaimida as in the current and previous classifications. Instead, Molgolaimus was classified with the Chromadorida with moderate and strong support in maximum likelihood and Bayesian analyses, respectively. Congruence analysis suggests that in some cases at least, the structure of the female reproductive system is a more taxonomically informative trait for marine nematode classification than the male reproductive system or cuticle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Determining relationships among marine nematode higher taxa can be challenging due to the paucity of taxonomically informative traits and the common occurrence of convergent evolution within the phylum (Lorenzen 1981; van Megen et al. 2009; Bik et al. 2010). Although molecular phylogenetic analyses have helped improve our understanding of nematode classification and evolution over the last two decades (e.g. Meldal et al. 2007; Holterman et al. 2008), there remains considerable uncertainty about the relationships of some marine nematode groups for which limited or no molecular sequence data are yet available.
The current nematode classification, based on both morphology and SSU phylogenetic analyses, comprises three primarily marine basal Chromadorean orders: the Microlaimida Leduc et al., 2018; Desmodorida De Coninck, 1965; and Chromadorida Chitwood, 1933 (De Ley and Blaxter 2002; Leduc et al. 2018). This classification is similar to the higher classification proposed by Lorenzen (1981) where the suborder Chromadorina Filipjev, 1929 comprised the superfamilies Chromadoroidea Filipjev, 1917; Desmodoroidea Filipjev, 1922; and Microlaimoidea Micoletzky, 1922. These three orders are united by the presence of cheilorhabdia (12-fold-pleated vestibulum; Lorenzen 1981) and can be differentiated based mainly on the cuticle (punctated in Chromadorida versus smooth, striated or annulated in Microlaimida and Desmodorida) and female reproductive system (female reproductive system with outstretched ovaries in Microlaimida versus reflexed ovaries in Chromadorida and Desmodorida), although some exceptions do occur (Leduc et al. 2018).
The order Microlaimida is comprised of small- to medium-sized, mostly marine and free-living, nematode species common in both shallow water and deep-sea sediments. The order was recently erected based on results of SSU phylogenetic analyses, as well as lack of any morphological synapomorphy linking Desmodoroidea and Microlaimoidea which were previously both classified within the Desmodorida (Leduc et al. 2018). The Microlaimida comprises the Microlaimoidea, which includes the families Microlaimidae Micoletzky, 1922; Monoposthiidae Filipjev, 1934; and Aponchidae Gerlach, 1963. Molgolaimus Ditlevsen, 1921 was also included in the Microlaimida by Leduc et al. (2018) based on SSU phylogenetic analyses which placed Molgolaimus demani Jensen, 1978 in a monophyletic clade with Microlaimidae sequences. This analysis, however, overlooked the fact that Molgolaimus demani was synonymised with Microlaimus tenuispiculum De Man, 1922 by Lorenzen (1981) based on the structure of the reproductive system. Since no other molecular sequences of Molgolaimus were available, the classification of Molgolaimus remains to be tested in molecular phylogenetic analyses. In addition, several microlaimid genera such as Aponema Jensen, 1978 have not yet been sequenced. Aponema was originally placed in the family Molgolaimidae Jensen, 1978 (comprising Molgolaimus, Aponema, and Prodesmodora Micoletzky, 1923) by Jensen (1978) but was later moved to the Microlaimidae by Lorenzen (1981).
Several rare taxa currently classified within the order Desmodorida, such as Onepunema Leduc & Verschelde, 2013 and Pseudonchinae Gerlach and Riemann, 1973, are yet to be sequenced and included in molecular phylogenetic analyses. The classification of Onepunema with the Desmodorida was mainly based on the presence of two reflexed ovaries in females, thick annulated cuticle, a cephalic capsule, and orange body colouration in glycerol preparations (Leduc and Verschelde 2013); this genus, however, is the only desmodorid taxon possessing two testes instead of just one, which has led to some debate regarding its phylogenetic placement (Armenteros et al. 2014; Leduc and Verschelde 2015). Pseudonchinae is an unusual group of predatory nematodes characterised by a bilaterally symmetrical buccal cavity and is the only subfamily of Desmodoridae for which no sequences are yet available.
In this study, we describe two new species, one belonging to the genus Molgolaimus and another to Aponema, based on specimens from the continental slope of New Zealand. We investigate the placement of these genera and of Onepunema and Pseudonchus (Pseudonchinae), using SSU phylogenetic analyses.
Material and methods
Sampling and morphological analyses
Sediment samples were obtained from the Conway Trough and Chatham Rise off the east coast of the South Island of New Zealand (Fig. 1). The Conway Trough is a north-south-oriented sedimentary basin approximately 40 km long and up to 10 km wide, reaching to within 3 km from the shore (Carter et al. 1982). It is separated from the highly productive Kaikoura canyon (De Leo et al. 2010; Leduc et al. 2014) to the north by a narrow sill. Chatham Rise is a submarine ridge extending eastwards from the South Island of New Zealand. It encompasses water depths from ca. 250 to 3000 m and lies beneath the Subtropical Front, a region associated with heightened primary productivity (Bradford-Grieve et al. 1997; Murphy et al. 2001).
Sampling was conducted using RV Tangaroa during the National Institute of Water and Atmospheric Research (NIWA) voyage TAN1701 (January 2017) to the Chatham Rise and TAN1708 (September 2017) to the Conway Trough and Kaikōura canyon. Sediment samples were collected using an Ocean Instrument MC-800A multicorer (internal diameter of core = 9.52 cm) at one site on Chatham Rise (station 133 at 860 m depth) and at two sites along the axis of Conway Trough (TAN1708 sites 28 and 30 at 491 and 570 m water depth, respectively). At each site, a subcore was obtained by pushing a cutoff syringe (29 mm internal diameter) into the sediment of one core. The sediment was then sliced into 0–1- and 1–5-cm layers and fixed in 10% buffered formalin. The remaining 0–5-cm layer of the sediment from the same core was transferred to a plastic bag and frozen at − 80 °C.
In the laboratory, frozen sediment samples were thawed overnight, then sieved trough a 45-μm mesh to retain nematodes. Nematodes were extracted using the ludox flotation method (Somerfield and Warwick 1996) and sorted under a dissecting microscope. A total of five species were isolated: Molgolaimus kaikouraensis sp. nov. and Aponema pseudotorosum sp. nov. from the Conway Trough samples and Onepunema enigmaticum Leduc & Vershelde, 2013; Pseudonchus virginiae Leduc & Verschelde, 2013; and Molgolaimus sp. from the Chatham Rise sample. A single specimen of each species (except for M. kaikouraensis sp. nov., for which two specimens were isolated) was mounted in a drop of seawater on a temporary slide to confirm its identity and images of key morphological features were taken to provide image vouchers. Nematodes from formalin-fixed sediment samples (M. kaikouraensis sp. nov. and Aponema pseudotorosum sp. nov. only) were extracted using the same method as for the frozen samples. Specimens for light microscopy were transferred to glycerol and mounted onto permanent slides (Somerfield and Warwick 1996).
All measurements are in micrometres, and all curved structures are measured along the arc. The terminology used for describing the arrangement of morphological features such as setae follows Coomans (1979). Type specimens are held in the NIWA Invertebrate Collection (Wellington) and the National Nematode Collection of New Zealand (Auckland).
DNA extraction, PCR, and sequencing
Following observation and digital imaging under a compound microscope, a male specimen of each species was transferred to lysis buffer and kept frozen at − 80 °C prior to molecular analyses. DNA was extracted by the method of Zheng et al. (2002) with minor modifications. The DNA extract was stored at − 20 °C until used as PCR template.
Primers for LSU amplification were forward primer D2A (5′ ACAAGTACCGTGAGGGAAAGT 3′) and reverse primer D3B (5′ TGCGAAGGAACCAGCTACTA 3′) (Nunn 1992). Primers for the rDNA small subunit (SSU) were the first fragment forward primer 1096F, 5′-GGTAATTCTGGAGCTAATAC-3′ and reverse primer 1912R, 5′-TTTACGGTCAGAACTAGGG-3′, and the second fragment forward primer 1813F, 5′-CTGCGTGAGAGGTGAAAT-3′ and reverse 2646R, 5′-GCTACCTTGTTACGACTTTT-3′, respectively (Holterman et al. 2006). For both SSU and LSU, the 20-μl PCR contained 10-μl Go Tag® Green Master Mix (Promega Corporation, Madison, WI, USA), 1 μl (5 μM) each of forward and reverse primers and 2 μl of DNA template. The thermal cycling program was as follows: denaturation at 95 °C for 3 min, followed by 35 cycles of denaturation at 94 °C for 15 s, annealing at 53 °C for 30 s, and extension at 72 °C for 45 s. A final extension was performed at 72 °C for 7 min. The amplicons were electrophoresed on 1% TAE-agarose gel stained with SYBR® Safe, observed under UV illumination using the Gel-Doc system (BioRad, Hercules, CA, USA), and images processed using the Quantity One 1-D analysis software (BioRad). The PCR products were sequenced bi-directionally using the amplification primers by EcoGene (Auckland, New Zealand). Sequences were obtained with a 3130xl Genetic Analyzer (Applied Biosystems, USA) and assembled and edited with Sequencher 4.10.1 (Gene Codes Corp.).
Sequence alignment and phylogenetic inference
The ribosomal DNA SSU and LSU D2-D3 sequences were deposited in GenBank under accession numbers MK446235 and MK446240 (Molgolaimus kaikouraensis sp. nov.), MK446236 and MK446241 (Molgolaimus sp.), MK446237 and MK446242 (Aponema pseudotorosum sp. nov.), MK446238 and MK446243 (Onepunema enigmaticum), and MK446239 and MK446244 (Pseudonchus virginiae). The placement of the new sequences was first investigated through phylogenetic analysis of SSU sequences of representative genera of the orders Chromadorida, Microlaimida, and Desmodorida and rooted using Enoplea sequences. Initial D2-D3 of LSU analyses confirmed that the LSU rDNA gene is only informative at the species to family levels (e.g. Leduc et al. 2018); the analyses resulted in the polyphyly of several orders and deep phylogenetic relationships could not be resolved. D2-D3 of LSU sequences could therefore not be used to determine phylogenetic relationships at this broad taxonomic level. Because preliminary SSU analyses suggested a relationship between Molgolaimus and Chromadorida, detailed SSU and D2-D3 of LSU analyses were conducted using a more comprehensive set of sequences of the order Chromadorida (again rooted using Enoplea sequences). However, relationships between Molgolaimus and Chromadorida taxa could not be identified in D2-D3 of LSU analyses because support was either poor (ca. 50%; Bayesian analysis) or absent (< 50%; maximum likelihood analysis). DNA sequences were aligned using the MUSCLE (Edgar 2004a, b) with default parameters.
Phylogenies were built in Geneious 10.2.6 (http://www.geneious.com, Kearse et al. 2012). MrModelTest 2.3 (Nylander 2004) in conjunction with PAUP*4.0b10 (Swofford 2002) were used to select the best model using the Akaike Information Criterion. The substitution model (GTR (general time-reversible) + I (proportion of invariable sites) + G (gamma distribution)) was selected as the best-fit model for both SSU alignments (1657 and 1639 bp in the Chromadorida + Desmodorida + Microlaimida and Chromadorida only analyses, respectively). The trees were run with a chain length of 1100, 000 and burn-in length of 100, 000. The perimeter files from multiple runs were inspected for chain convergence in Tracer 1.5 (Rambaut and Drummond 2007), and the trees were edited in FigTree v1.4.2 (http://tree.bio.ed.ac.uk/software/figtree) and PowerPoint. These analyses were also conducted with PhyML 3.0 using the default settings in Geneious 10.2.6. The substitution model GTR, the NNI (default, fast) topology search, and the 1000 bootstrap replicates (Guindon et al. 2010) were selected for building the tree.
Results
Systematics
Family MolgolaimidaeJensen, 1978
Genus Molgolaimus Ditlevsen, 1921
Diagnosis (from Fonseca et al. (2006)). Cuticle finely striated or apparently smooth. Amphideal fovea round, posterior to the cephalic constriction. Inner and outer labial papillae small and in many species difficult to distinguish using light microscopy. Cephalic setae located slightly anterior or posterior to cephalic constriction. Buccal cavity small, weakly cuticularised, with small teeth. Pharynx narrow, cylindrical, with a pronounced posterior bulb, usually spherical. Pharyngeal lumen weakly cuticularised except in pharyngeal bulb where it can be heavily cuticularised. Secretory-excretory pore anterior to nerve ring, seldom posterior to it (may be obscured). Female reproductive system didelphic-amphidelphic, with reflexed ovaries. Position of genital branches variable. Male reproductive system monorchic with single anterior testis to the right or left of the intestine. Spicules of variable length and shape. Gubernaculum with or without apophysis. Precloacal supplements often present. Tail of varying shape and length, from short and conical to elongated and conico-cylindrical.
Type species.Molgolaimus tenuispiculum Ditlevsen, 1921
Remarks. This genus was originally described by Ditlevsen (1921) based on specimens from the Auckland Islands, southwest Pacific Ocean. Fonseca et al. (2006) classified the 33 valid species of the genus known at the time into four groups according to absolute spicule length. Two of these groups were further divided into subgroups based on relative spicule length as well as body length and ratios of body dimensions. Subsequently, Portnova (2009) described Molgolaimus haakonmosbiensis Portnova, 2009 and Shi and Xu (2017) transferred Microlaimus pecticauda Murphy, 1966 and Microlaimus spirifer Warwick, 1970 to Molgolaimus based on the structure of the female reproductive system.
List of valid Molgolaimus species based on classification of Fonseca et al. ( 2006 ) :
Group 1 (spicules < 35 μm long)
1a (spicules = 1 cloacal body diameters long)
M. citrus Gerlach, 1959
M. cuanensis (Platt, 1973) Jensen 1978
= Microlaimus cuanensis Platt, 1973
M. haakonmosbiensis Portnova, 2009
M. lazonus (Vitiello, 1970) Jensen 1978
= Microlaimus lazonus Vitiello, 1970
M. parallgeni (Vitiello, 1973) Jensen 1978
= Microlaimus parallgeni Vitiello, 1973
M. turgofrons (Lorenzen, 1971) Jensen 1978
= Microlaimus turgofrons Lorenzen, 1971
1b1 (spicules 1–3 cloacal body diameters long; see Fig. 13a in Fonseca et al. (2006))
M. drakus Fonseca, Vanreusel & Decraemer, 2006
M. exceptionregulum Fonseca, Vanreusel & Decraemer, 2006
M. gazii Muthumbi & Vincx, 1996
M. pecticauda (Murphy, 1966) Shi and Xu 2017
= Microlaimus pecticauda Murphy, 1966
M. mareprofundus Fonseca, Vanreusel & Decraemer, 2006
M. sapiens Fonseca, Vanreusel & Decraemer, 2006
M. spirifer (Warwick, 1970) Shi and Xu 2017
= Microlaimus spirifer Warwick, 1970
1b2 (spicules 1–3 cloacal body diameters long; see Fig. 13a in Fonseca et al. (2006))
M. abyssorum Muthumbi & Vincx, 1996
M. carpediem Fonseca, Vanreusel & Decraemer, 2006
M. falliturvisus Fonseca, Vanreusel & Decraemer, 2006
M. galluccii Fonseca, Vanreusel & Decraemer, 2006
M. kiwayui Muthumbi & Vincx, 1996
M. minutus Jensen, 1988
1c (spicules > 3 cloacal body diameters long)
M. typicus Furstenberg & Vincx, 1992
M. tyroi Muthumbi & Vincx, 1996
Group 2 (spicules 35–53 μm long)
M. allgeni (Gerlach, 1950) Jensen 1978
= Microlaimus allgeni Gerlach, 1950
M. australis Fonseca, Vanreusel & Decraemer, 2006
M. macilenti Fonseca, Vanreusel & Decraemer, 2006
M. nettoensis Fonseca, Vanreusel & Decraemer, 2006
M. sabakii Muthumbi & Vincx, 1996
M. xuxunaraensis Fonseca, Vanreusel & Decraemer, 2006
Group 3 (spicules 53–80 μm long)
M. liberalis Fonseca, Vanreusel & Decraemer, 2006
M. unicus Fonseca, Vanreusel & Decraemer, 2006
M. walbethi Fonseca, Vanreusel & Decraemer, 2006
Group 4 (spicules > 80 μm long)
4a (species not included in 4b)
M. gigasproximus Fonseca, Vanreusel & Decraemer, 2006
M. longispiculum Timm, 1961
M. tanai Muthumbi & Vincx, 1996
4b (b ratio = 8–11, spicules = 4–6 cloacal body diameters long)
M. gigaslongincus Fonseca, Vanreusel & Decraemer, 2006
M. pacificus Fonseca, Vanreusel & Decraemer, 2006
M. tenuispiculum Ditlevsen, 1921
Molgolaimus kaikouraensis sp. nov. Fu & Leduc
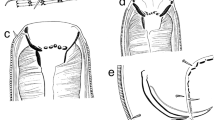
Molgolaimus kaikouraensis sp. nov. a Female anterior body portion; b female cephalic region; c male cephalic region; d entire female; e entire male; f male posterior body region; g female posterior body region. Arrows show position of precloacal supplements. Scale bar: a, b, and c = 30 μm; d and e = 105 μm; f and g = 44 μm
Material examined. Holotype male (NIWA 115477) and two paratype females (NIWA 115478) deposited in the NIWA Invertebrate Collection (Wellington). One paratype female (NNCNZ 3322) deposited in the National Nematode Collection of New Zealand (Auckland). All specimens collected on 11 September 2017.
Type habitat and locality. Subsurface (1–5 cm) muddy sediments, 570 m water depth, Conway Trough, off east coast of New Zealand’s South Island (42.6108° S, 173.5822° E).
Etymology. The species name is derived from the nearby town of Kaikoura.
Diagnosis
Molgolaimus kaikouraensis sp. nov. is characterised by body length 698–766 μm, striated cuticle, b ratio = 8–9, short cephalic setae 1–2 μm long, conico-cylindrical tail, female with vulva slightly pre-median, and male with sinusoidal spicules 4.4 cloacal body diameters long and two precloacal supplements.
Description
Males
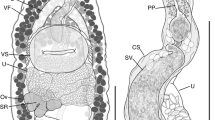
Body short, cylindrical, tapering gradually towards both ends. Cuticle striated. Cephalic region offset from rest of the body by a slight constriction. Six minute inner labial papillae and 6 minute outer labial sensilla present; four cephalic setae, 2 μm long, located at the level of buccal cavity and anterior to cephalic constriction. Amphideal fovea crypto-circular with a broken and lightly cuticularised outline, ca. 1.0 cbd from anterior end. Subcephalic and somatic setae not observed. Buccal cavity small, narrow, with lightly cuticularised walls; one small dorsal tooth and two smaller ventrosublateral teeth present. Pharynx muscular, surrounding buccal cavity, consisting of narrow, cylindrical anterior portion and with conspicuous spherical posterior bulb; pharyngeal lumen lightly cuticularised except in posterior bulb where it is heavily cuticularised. Nerve ring located near the middle of pharynx length. Secretory-excretory system present; ventral gland small, at the level of cardia, pore inconspicuous. Cardia well-defined, not surrounded by the intestine.
Reproductive system monorchic with outstretched anterior testis to the left of intestine. Testis large, with relatively short germinal zone. Mature sperm cells large, spherical or globular. Spicules 4.4 cloacal body diameters long, thin, sinusoidal, with slight capitulum. Gubernaculum parallel to the spicules, without apophyses, narrow, strongly cuticularised. Two precloacal supplements present. Tail conico-cylindrical, caudal glands not observed; tail tip not swollen and without terminal setae.
Females
Similar to males, but with slightly smaller amphids, larger maximum body diameter, longer pharynx, and shorter tail with swollen tip. Reproductive system didelphic-amphidelphic, with reflexed ovaries. Ovaries located to the left or right of the intestine, on the same or opposite sides of the intestine. Spermathecae not observed. Vulva slightly pre-median. Pars proximalis vaginae surrounded by constrictor muscle.
Remarks
Molgolaimus kaikouraensis sp. nov. belongs to Fonseca et al.’s (2006) group 4b based on spicule length and body dimensions. This group also includes M. gigaslongincus, M. pacificus, and M. tenuispiculum. Molgolaimus kaikouraensis sp. nov. is most similar to M. pacificus and M. gigaslongincus in values of b, spicule dimensions and tail length. Molgolaimus kaikouraensis sp. nov. differs from both latter species in the shorter body length (698–767 μm versus 865–1060 μm in M. pacificus and 850–1240 μm in M. gigaslongincus), stouter body shape (a = 19–25 versus 36–44 in M. pacificus and 25–42 in M. gigaslongincus), and shape of the tail tip (rounded or swollen versus pointed in M. pacificus and M. gigaslongincus).
Family MicrolaimidaeMicoletzky, 1922
Genus Aponema Jensen, 1978
Diagnosis (modified after Jensen (1978)). Cuticle with transverse striations. Pharynx with thick cuticular internal lining of posterior rounded bulb. One or two testes present. Copulatory apparatus strongly cuticularised, gubernaculum with dorso-caudal apophyses.
Type species.Aponema torosum (Lorenzen, 1973) Jensen 1978
Remarks. The genus was most recently revised by Tchesunov (2014), who listed six valid species described by Lorenzen (1973), Pastor de Ward (1980), Portnova (2009), Muthumbi and Vincx (1999), and Miljutin and Miljutina (2009). One species, A. subtile Leduc & Wharton, 2008, however, was omitted from the list (Leduc and Wharton 2008). Aponema pontica Revkova, 2017 was subsequently described by Revkova (2017).
List of valid Aponema species:
A. abyssalis (Miljutin & Miljutina, 2009) Tchesunov 2014
= Microlaimus abyssalis Miljutin & Miljutina, 2009
A. decraemerae Muthumbi & Vincx, 1999
A. mnazi Muthumbi & Vincx, 1999
A. ninae Portnova, 2009
A. papillatum Pastor de Ward, 1980
A. pontica Revkova, 2017
A. subtile Leduc & Wharton, 2008
A. torosum (Lorenzen, 1973) Jensen 1978
= Microlaimus torosus Lorenzen, 1973
Aponema pseudotorosum sp. nov. Fu & Leduc
Material examined. Holotype male (NIWA 115479), one paratype male and two paratype females (NIWA 115480) deposited in the NIWA Invertebrate Collection (Wellington). One paratype male and two paratype females (NNCNZ 3323 and 3324) deposited in the National Nematode Collection of New Zealand (Auckland). All specimens collected on 11 September 2017.
Type habitat and locality. Subsurface (1–5 cm) muddy sediments, 491 and 570 m water depth, Conway Trough, off the east coast of New Zealand’s South Island (42.7250° S, 173.6012° E and 42.6108° S, 173.5822° E, respectively).
Etymology. The species name refers to the similar species Aponema torosum.
Diagnosis
Aponema pseudotorosum sp. nov. is characterised by relatively short body (556–612 μm), presence of sparse and short somatic and caudal setae, cephalic region set-off by a constriction, male reproductive system monorchic, gubernaculum with dorso-caudal apophyses 6–10 μm long, precloacal supplements absent, and conico-cylindrical tail with swollen tip.
Description
Males
Body short, cylindrical, with tail curved slightly dorsally. Cephalic region set-off by constriction slightly posterior to cephalic setae. Cuticle transversely striated, striations beginning from constriction to near tail tip. Short, ca. 1-μm-long somatic setae present, sparsely distributed along entire body. Six minute inner labial papillae and 6 minute outer labial papillae in separate circles, four cephalic setae, 2 μm long, slightly anterior to constriction at the base of the cephalic region. Amphideal fovea cryptospiral, with a broken, cuticularised outline. Buccal cavity small, with weakly cuticularised walls; one small dorsal tooth and smaller two ventrosublateral teeth present. Pharynx muscular, with a well-developed, rounded posterior bulb, 17–19 μm long. Nerve ring located at middle or slightly posterior to middle of pharynx length from anterior. Ventral gland present posterior to the cardia, excretory pore not observed. Cardia small, not surrounded by intestine.
Reproductive system with one anterior outstretched testis located to the left of the intestine. Sperm cells large, rounded. Spicules strongly cuticularised, curved distally and bent proximally; gubernaculum with pair of straight, dorso-caudal apophyses tapering distally and most strongly cuticularised along the dorsal margin. Precloacal supplements not observed. Tail conico-cylindrical with swollen tip; short, sparsely distributed caudal setae present, terminal setae not observed. Caudal glands and spinneret present.
Females
Similar to males. Reproductive system with two opposed and genital branches; anterior ovary to the left or right of the intestine and posterior ovary to the opposite side. Spermatheca not observed. Pars proximalis vaginae surrounded by constrictor muscle; vulva located slightly pre-median.
Remarks
Aponema pseudotorosum sp. nov. is similar to A. ninae, A. papillatum, A. pontica, A. subtile, and A. torosum, which are all characterised by a male reproductive system with a single anterior testis. Aponema pseudotorosum sp. nov. differs from A. ninae by its much longer body length (556–612 versus 352–383 μm in A. ninae) and absence of terminal setae, from A. papillatum by the shorter body length (556–612 versus 1250–1300 μm in A. papillatum) and absence of precloacal papillae and spermatheca, from A. pontica by shorter spicules (23 versus 35–43 μm), presence of constriction at base of cephalic region, and shape of the gubernacular apophyses, and from A. subtile by the shorter body length (556–612 versus 781–894 μm), stouter body shape (a = 21–26 versus 31–39), absence of lateral grooves on the cuticle, and absence of short setae in the pre- and post-cloacal regions. The new species is most similar to A. torosum in the shape of the copulatory apparatus and overall body dimensions but differs from the latter in the shorter spicules (23 versus 28–31 μm) and somewhat shorter posterior pharyngeal bulb (17–19 versus 19–25 μm).
Molecular phylogenetic relationships
Near full-length SSU sequences were obtained for Molgolaimus kaikouraensis sp. nov. (1568 bp), but only partial SSU sequences could be obtained for the other four species (460–866 bp). Near full-length D2-D3 of LSU sequences (684–789 bp) were obtained for all species we investigated.
The order Desmodorida was recovered as a well-supported, monophyletic clade (100% posterior probability and bootstrap support) in the broad-level consensus SSU tree of the orders Chromadorida, Microlaimida, and Desmodorida (Fig. 4). The placement of Pseudonchus virginiae within the Desmodorida clade supports its current classification with this order. Phylogenetic relationships of Onepunema enigmaticum, however, could not be resolved due to lack of support in Bayesian and maximum likelihood analyses.
Bayesian tree of the orders Chromadorida, Microlaimida, and Desmodorida inferred from SSU sequences, aligned using the MUSCLE alignment algorithm under the general time-reversible (GTR) + proportion of invariable sites (I) + gamma distribution (G) model. New sequences provided in the present study are shown by grey background. Posterior probability (left) and bootstrap values (right) are given on corresponding clades. Dashes (−) indicate less than 50% support. The scale stands for substitutions per site. **Previously labelled Molgolaimus demani by Cook et al. (2005); however, this species had previously been synonymised with Microlaimus tenuispiculum by Lorenzen (1981)
The Chromadorida did not form a monophyletic clade and included the two Molgolaimus sequences; this placement of Molgolaimus also resulted in the paraphyly of the order Microlaimida. The Microlaimidae, which included sequences of Microlaimus de Man, 1880 and Calomicrolaimus Lorenzen, 1976 as well as Aponema pseudotorosum sp. nov., formed a well-supported monophyletic clade (100% posterior probability and bootstrap support). The Microlaimidae and Monoposthiidae (the latter is comprised of Monoposthia de Man, 1889 and Nudora bipapillata Platt, 1973) formed a poorly supported monophyletic clade (59 posterior probability and < 50% bootstrap support).
More detailed SSU phylogenetic analyses focusing on the order Chromadorida and Molgolaimus show that the two Molgolaimus sequences comprised a well-supported monophyletic clade (100% posterior probability and bootstrap support), which formed poorly to a well-supported clade with the family Chromadoridae (95% posterior probability and 50% bootstrap support; Fig. 5). This Molgolaimus + Chromadoridae clade formed a larger, monophyletic clade with the Cyatholaimidae, Ethmolaimidae, Achromadoridae, and Neotonchidae with moderate to strong support (98% posterior probability and 70% bootstrap support). The Selachinematidae and Paramicrolaimus sequences formed a separate clade with weak or no support (52% posterior probability and < 50% bootstrap support).
Bayesian tree of the order Chromadorida inferred from SSU sequences, aligned using the MUSCLE alignment algorithm under the general time-reversible (GTR) + proportion of invariable sites (I) + gamma distribution (G) model. New sequences provided in the present study are shown by grey background. Posterior probability (left) and bootstrap values (right) are given on corresponding clades. Dashes (−) indicate less than 50% support and asterisks (*) indicate no support. The scale stands for substitutions per site
Discussion
The present study provides the first molecular sequences for Molgolaimus, Aponema, Onepunema, and Pseudonchinae (Pseudonchus). Whilst the results of our SSU phylogenetic analyses provide support for the current classification of Aponema within the family Microlaimidae and of Pseudonchus within the Desmodorida, we could not confirm relationships for Onepunema enigmaticum with either the Desmodorida, Microlaimida, or Chromadorida. It is likely that the relatively short length of the SSU sequence we obtained for this species limited our ability to resolve phylogenetic relationships.
Molgolaimus was originally classified with the Microlaimidae (see Gerlach and Riemann 1973/1974), presumably based on similarities to the genus Microlaimus de Man, 1880 in head and amphideal fovea shape, arrangement of head sensilla, and buccal cavity structure. Jensen (1978) later erected the family Molgolaimidae distinguished from the Microlaimidae mainly based on the presence of a single anterior testis in males and two reflexed ovaries in females. Lorenzen (1981) then placed Molgolaimus in its own single-genus subfamily within the Desmodoridae and established the monophyly of the Desmodoroidea (order Desmodorida) based on the presence of only one (anterior) testis in males. Most recently, Molgolaimus was included in the Microlaimida by Leduc et al. (2018) based on SSU phylogenetic analyses of Molgolaimus demani Jensen, 1978. Molgolaimus demani, however, was synonymised with Microlaimus tenuispiculum De Man, 1922 by Lorenzen (1981) based on the presence of two outstretched ovaries and two testes, which are both characteristics of the Microlaimoidea, whereas the Desmodorida is characterised by two reflexed ovaries and (with the exception of Onepunema) a single anterior testis.
The results of our SSU phylogenetic analyses based on sequences of two Molgolaimus species from the continental slope of New Zealand provided no support for the placement of Molgolaimus with either the Desmodorida (as suggested by Lorenzen 1981) or Microlaimidae/Microlaimida (as suggested by Gerlach and Riemann 1973 and Leduc et al. 2018); instead, Molgolaimus was classified with the Chromadorida with moderate and strong support in maximum likelihood and Bayesian analyses, respectively (see Fig. 5). This placement is unexpected as a relationship between Molgolaimus and Chromadorida has never been suggested previously, likely as a result of the difference in cuticle ornamentation between the Chromadorida (punctated) and Molgolaimus (smooth or striated). However, an exception was recently suggested by Leduc et al. (2018), who found evidence for a close relationship between Paramicrolaimus Wieser, 1954 (characterised by striated cuticle) and Chromadorida based on SSU and D2-D3 of LSU phylogenetic analyses. Although they differ in cuticle ornamentation, Molgolaimus and Chromadorida share the same female reproductive system structure which consists of two opposed and reflexed ovaries, a feature which differs from the Microlaimoidea (two outstretched ovaries).
Molecular phylogenetic analyses conducted to date suggest that the structure of the female reproductive system does not vary within each of the three basal Chromadorean orders, and therefore provides a taxonomically informative character for the classification of taxa. This character, however, has only two character states within these orders (outstretched or reflexed), and additional characters therefore need to be used to differentiate among the orders. The structure of the male reproductive system is not always taxonomically informative because of the limited number of character states, and because more than one character state can be present within a single order. In Molgolaimus, the male reproductive system consists of a single anterior testis, which would be consistent with a classification with either the Chromadorida or Microlaimida (both have either a single anterior testis or two opposed testes) or Desmodorida (single anterior testis except for Onepunema with two opposed testes). The cuticle is widely regarded as a fundamental trait for high-level nematode classification; however, this trait is also characterised by only few character states which often overlap among orders. Most marine taxa, for example, are characterised by the plesiomorphic character state ‘non-punctated cuticle’ (i.e., striated, annulated, and/or smooth), and only a few have the synapomorphic character state ‘punctated cuticle’. Moreover, molecular phylogenetic analyses suggest that the genera Paramicrolaimus and Molgolaimus constitute two exceptions within Chromadorida. Similarly, variation in cuticle ornamentation occurs within the Areaolaimida De Coninck & Schuurmans Stekhoven, 1933, which currently includes taxa with punctated and others with non-punctated cuticle (Fonseca and Bezerra 2014). The current composition of the latter order was changed following SSU analyses showing that the Comesomatidae Filipjev, 1918, which is characterised by punctated cuticle and female reproductive system with outstretched ovaries, does not belong to the Chromadorida (Meldal et al. 2007). Thus, molecular phylogenies suggest that the structure of the female reproductive system is a more informative trait than cuticle ornamentation for the classification of Comesomatidae. Based on our results, we postulate that this is also the case for Molgolaimus, which we suggest should be classified with the Chromadorida and not the Desmodorida or Microlaimida.
References
Armenteros M, Ruiz-Abierno A, Decraemer W (2014) Revision of Desmodorinae and Spiriniinae (Nematoda: Desmodoridae) with redescription of eight known species. Eur J Taxon 96:1–32
Bik HM, Lambshead PJD, Kelley Thomas W, Hunt DH (2010) Moving towards a complete molecular framework of the Nematoda: a focus on the Enoplida and early–branching clades. BMC Evol Biol 10:353
Bradford-Grieve JM, Chang FH, Gall M, Pickmere S, Richards F (1997) Size–fractioned phytoplankton standing stocks and primary production during austral winter and spring 1993 in the subtropical convergence region near New Zealand. N Z J Mar Freshw Res 31:201–224
Carter L, Carter RM, Griggs GB (1982) Sedimentation in the Conway Trough, a deep–near–shore marine basin at the junction of the Alpine transform and Hikurangi subduction plate boundary, New Zealand. Sedimentology 29:475–497
Chitwood BG (1933) A revised classification of the Nematoda. J Parasitol 20:1–130
Cook AA, Badhury P, Debenham NJ, Meldal BHM, Blaxter ML, Smerdon GR, Austen MC, Lamnshead PJD, Rogers AD (2005) Denaturing gradient gel electrophoresis (DGGE) as a tool for identification of marine nematodes. Mar Ecol Prog Ser 291:103–113
Coomans A (1979) A proposal for a more precise terminology of the body regions in the nematode. Ann Soc Roy Zool Bel 108:115–117
De Coninck LA (1965) Systématique des Nématodes. In: Grassé PP (ed) Traité de Zoologie: Anatomie, Systématique, Biologie. Nemathelminthes (Nematodes). Masson et Cie, Paris, pp 586–531 731 pp
De Coninck LA, Schuurmans Stekhoven JH (1933) The freeliving marine nemas of the Belgian coast. II. With general remarks on the structure and the system of nemas. Mém Mus R Hist Nat Belg 58:3–163
De Leo FC, Smith CR, Rowden AA, Bowden DA, Clark MR (2010) Submarine canyons: hotspots of benthic biomass and productivity in the deep sea. Proc R Soc B 1695:2783–2792
De Ley P, Blaxter ML (2002) Systematic position and phylogeny. In: Lee DL (ed) The biology of nematodes. Taylor & Francis, London, pp 1–30
De Man JG (1889) Espèces et genres nouveaux de Nématodes libres de la mer du Nord et de la Manche. Mém Soc Zool France 2:1–10
de Man JG (1922) Neue freilebende Nematoden aus der Zuidersee. Tijdschr ned dierk Vereen 2:124–134
Ditlevsen H (1921) Papers from Dr. Th. Mortensens Pacific Expedition 1914-16. III Marine free-living Nematodes from the Auckland and Campbell Islands. Vidensk Med f Dansk natur Fori Kjøbenhavn 73:1–39
Edgar RC (2004a) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Edgar RC (2004b) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 19:5–113
Filipjev IN (1917) Un nématode libre nouveau de la mer Caspienne, Chromadorissa gen. nov. (Chromadoridae, Chromadorini). Zool Zhurnal 2:24–30
Filipjev IN (1918) Free-living marine nematodes of the Sevastopol area. Transactions of the Zoological Laboratory and the S evastopol Biological Station of the Russian Academy of Sciences 2:1–203
Filipjev IN (1922) New data on free nematodes of the Black Sea (Novye dannye o svobodnykh nematodakh Chernogo Moria.). Trudy Stavropol'skogo Sel'skokhoziaistvennogo Instituta 1:13–184
Filipjev IN (1929) Classification of free-living Nematoda and relations to parasitic forms. J Parasitol 15:281–282
Filipjev IN (1934) The classification of the free-living nematodes and their relation to the parasitic nematodes. Smithson Misc Coll 89:1–63
Fonseca G, Bezerra TN (2014) Order Araeolaimida De Coninck & Schuurmans Stekhoven, 1933. In: Shmidt-Rhaesa A (ed) Handbook of zoology, Gastrotricha, Cyclioneura and Gnathifera Volume 2: Nematoda. De Gruyter, Hamburg, pp 467–486
Fonseca G, Vanreusel A, Decraemer W (2006) Taxonomy and biogeography of Molgolaimus (Ditlevsen, 1921 (Nematoda: Chromadoria)) with reference to the origins of deep–sea nematodes. Antarct Sci 18:23–50
Furstenberg J, Vincx M (1992) Two new species of the family Microlaimidae (Nematoda: order Chromadorida) from South-Africa. Cah Biol Mar 33:245–251
Gerlach SA (1950) Die Nematoden-Gattung Microlaimus. Zoologische Jahrbücher. Abteilung für Systematik, Ökologie und Geographie der Tiere 79:188–208
Gerlach SA (1959) Neue Meeres-Nematoden aus dem Supralitoral der Deutschen Küsten. Internationele Revue der Gemsamten Hydrobiologie 44:463–467
Gerlach SA (1963) Freilebende meeresnematoden von den Malediven II. Kiel Meeresfosch 19:67–103
Gerlach A, Riemann F (1973) The Bremerhaven checklist of aquatic nematodes. A catalogue of Nematoda Adenophorea excluding the Dorylaimida. Part 1. Veröff Inst Meer Bremerhaven 4:1–736
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321
Holterman M, Van Der Wurff A, Van Den Elsen S, Van Megen H, Bongers T, Holovachov O, Bakker J, Helder J (2006) Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol Biol Evol 13:1792–1800
Holterman M, Holovachov O, van den Elsen S, van Megen H, Bongers T, Bakker J, Helder J (2008) Small subunit ribosomal DNA-based phylogeny of basal Chromadoria (Nematoda) suggests that transitions from marine to terrestrial habitats (and vice versa) require relatively simple adaptations. Mol Phylogenet Evol 48:758–763
Jensen P (1978) Revision of Microlaimidae, erection of Molgolaimidae fam. N., and remarks on the systematic position of Paramicrolaimus (Nematoda, Desmodorida). Zool Scr 7:159–173
Jensen P (1988) Four new nematode species, abundant in the deep-sea benthos of the Norwegian Sea. Sarsia 73:149–155
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Mentjies P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649
Leduc D, Verschelde D (2013) One new genus and two new free–living nematode species (Desmodorida, Desmodoridae) from the continental margin of New Zealand, Southwest Pacific. Zootaxa 3609:274–290
Leduc D, Verschelde D (2015) New Spirinia and Stygodesmodora species (Nematoda, Spiriniinae) from the Southwest Pacific, and a revision of the related genera Spirinia, Chromaspirina and Perspiria. Eur J Taxon 118:1–25
Leduc D, Wharton DA (2008) Three new species of free-living nematodes from inter-tidal sediments in southern New Zealand. Nematology 10:743–755
Leduc D, Rowden AA, Nodder SD, Berkenbusch K, Probert PK, Hadfield MG (2014) Unusually high food availability in Kaikoura canyon linked to distinct deep–sea nematode community. Deep–Sea Res II 104:310–318
Leduc D, Verdon V, Zhao ZQ (2018) Phylogenetic position of the Paramicrolaimidae, description of a new Paramicrolaimus species and erection of a new order to accommodate the Microlaimoidea (Nematoda: Chromadorea). Zool J Linnaean Soc 183:52–69
Lorenzen S (1971) Die Nematodenfauna im Verklappungsgebiet für Industrieabwässer nordwestlich von Helgoland: I. Araeolaimida und Monhysterida. Zool Anz 187:223–248
Lorenzen S (1973) Freilebende Meeresnematoden aus dem sublittoral der Nordsee und der Kieler Bucht. Veröff Inst Meer Bremerhaven 14:103–130
Lorenzen S (1981) Entwurf eines phylogenetischen Systems der freilebenden Nematoden. Veröff Inst Meer Bremerhaven 7:472S
Meldal BHM, Debenham NJ, De Ley P, De Ley IT, Vanfleteren JR, Vierstraete AR, Bert W, Borgonie G, Moens T, Tyler PA, Austen MC, Blaxter ML, Rogers AD, Lambshead PJD (2007) An improved molecular phylogeny of the Nematoda with special emphasis on marine taxa. Mol Phylogenet Evol 42:622–636
Micoletzky H (1922) Die freilebenden Erdnematoden. Archiv für Naturgeschichte 87A:1–650
Micoletzky H (1923) Freilebende Nematoden der Wolga mit besonderer Berücksichtigung der Umgebung von Saratow. Arbeiten der Biologischen Wolga-Station 7:3–29
Miljutin DM, Miljutina MA (2009) Deep-sea nematodes of the family Microlaimidae from the Clarion-Clipperton Fracture Zone (North-Eastern Tropic Pacific), with the descriptions of three new species. Zootaxa 2096:137–172
Murphy DG (1966) An initial report on a collection of Chilean marine nematodes. Mitteilungen der Hamburger Zoologische Museum Institut 63:29–50
Murphy RJ, Pinkerton MH, Richardson KM, Bradford-Grieve JM (2001) Phytoplankton distributions around New Zealand derived from SeaWiFS remote–sensed ocean colour data. N Z J Mar Freshw Res 35:343–362
Muthumbi AW, Vincx M (1996) Nematodes from the Indian Ocean: description of six new species of the genus Molgolaimus Ditlevsen, 1921 (Nematoda: Desmodoridae). Bull Inst R Sciences Nat Belg Bio 66:17–28
Muthumbi AW, Vincx M (1999) Microlaimidae (Microlaimoidea: Nematoda) from the Indian Ocean: description of nine new and known species. Hydrobiologia 397:39–58
Nunn GB (1992) Nematode molecular evolution. Ph.D. Thesis, University of Nottingham, UK
Nylander JAA (2004) MrModeltest 2.3. Program distributed by the author. Evolutionary Biology Centre. Uppsala University, Uppsala
Pastor de Ward CT (1980) Aponema papillatum sp. nov., nueva especie de nematode marino de puerto deseado, (Santa Cruz, Argentina). Centro de Investigacion de Biologia Marina Contribucion Cientifica 160:1–11
Platt HM (1973) Freeliving marine nematodes from Strangford Lough, Northern Ireland. Cah Biol Mar 14:295–321
Portnova D (2009) Free-living nematodes from the deep-sea Hakon Mosby mud volcano, including the description of two new and three known species. Zootaxa 2096:197–213
Rambaut A, Drummond AJ (2007) Tracer v 1.4, Available from http://beast.bio.ed.ac.uk/Tracer
Revkova TN (2017) Two new species of free–living nematode genera Microlaimus de Man, 1880 and Aponema Jensen, 1978 (Nematoda: Microlaimidae) from the Black Sea. Zootaxa 4344:387–394
Shi B, Xu K (2017) Spirobolbolaimus undulatus sp. nov. in intertidal sediment from the East China Sea, with transfer of two Microlaimus species to Molgolaimus (Nematoda, Desmodorida). J Mar Biol Assoc U K 97:1335–1342
Somerfield PJ, Warwick RM (1996) Meiofauna in marine pollution monitoring Programmes: a laboratory manual. Ministry of Agriculture, Fisheries and Food, Lowestoft
Swofford DL (2002) PAUP*. Phylogentic analysis using parsimony (* and other methods). Version 4.0b10. Sinauer associates, Sunderland, MA
Tchesunov AV (2014) Order Chromadorida Chitwood, 1933. In: Scmidt-Rhaesa A (ed) Handbook of Zoology, Gastrotricha, Cyclioneuralia and Gnathifera. Volume 2: Nematoda. CABI Publishing, Cambridge
Timm RW (1961) The marine nematodes of the bay of Bengal. Proc Pak Academy Science 1:25–88
van Megen H, van den Elsen S, Holterman M, Karssen G, Mooyman P, Bongers T, Holovachov O, Bakker J, Helder J (2009) A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences. Nematology 11:927–950
Vitiello P (1970) Nématodes libres marins des vases profondes du Golfe du Lion. II Chromadorida. Téthys 2:449–500
Vitiello P (1973) Nouvelles espèces de Desmodorida (Nematoda) des côtes de Provence. Téthys 5:137–146
Warwick RM (1970) Fourteen new species of free-living marine nematodes from the Exe estuary. Bull Br Mus Nat Hist 19:137–177
Wieser W (1954) Free-living marine nematodes II. Chromadoroidea. Acta Universitets Lunds 50:1–148
Zheng JW, Subbotin SA, He SS, Gu JF, Moens M (2002) Molecular characterisation of some Asian isolates of Bursaphelenchus xylophilus and B. mucronatus using PCR-RFLPs and sequences of ribosomal DNA. Russ J Nematol 11:17–22
Acknowledgements
We thank the crew and scientific personnel of RV Tangaroa (voyages TAN1701 and TAN1708). We thank two anonymous reviewers for providing constructive criticisms on the manuscript.
Funding
This research was funded by the NIWA under Coasts and Oceans Research programme 2 (2018/19 SCI) and supported by core funding for Crown Research Institutes from the Science and Innovation Group of the Ministry of Business, Innovation and Employment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for animal testing, animal care and use of animals were followed by the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements, if applicable. The study is compliant with CBD and Nagoya protocols.
Data availability
All data sources on which this manuscript is based are either provided in the manuscript or available in GenBank.
Additional information
Communicated by M. Schratzberger
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is registered in ZooBank under urn:lsid:zoobank.org:pub: D17A8CBD-01EB-4B38-A954-E91E7FF1788F.
Aponema pseudotorosum is registered under urn:lsid:zoobank.org:act: C77EECD9-EB73-45D7-AABA-EFA2C0D23BFC.
Molgolaimus kaikouraensis is registered under urn:lsid:zoobank.org:act:7EF1D501-92E7-4CF5-BBE2-C099FB73B591.
Rights and permissions
About this article
Cite this article
Leduc, D., Fu, S. & Zhao, Z.Q. New nematode species from the continental slope of New Zealand (Chromadorea, Microlaimida, and Chromadorida), and unexpected placement of the genus Molgolaimus Ditlevsen, 1921. Mar Biodiv 49, 2267–2280 (2019). https://doi.org/10.1007/s12526-019-00961-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-019-00961-z