Abstract
Two new species of Amphoriscidae Dendy, 1892 (Porifera Grant, 1836: Calcarea Bowerbank, 1862: Leucosolenida Hartman, 1958) from the northern coast of Peru, Tropical Eastern Pacific, are described integrating molecules and morphology. Leucilla mancoraensis sp. nov. is a tube-shaped sponge with apical osculum, sylleibid aquiferous system, and a skeleton composed of cortical triactines and giant tetractines, thick subatrial triactines, and atrial tetractines with short apical actines. Paraleucilla tarazonai sp. nov. is the first record of Paraleucilla Dendy, 1892 for the Eastern Pacific and presents a skeleton formed of cortical microdiactines, diactines, triactines, and tetractines, subatrial triactines, and atrial triactines and tetractines. These two species are provisionally endemic to the northern coast of Peru (Guayaquil ecoregion). Our phylogenetic analysis, which included the largest number of Amphoriscidae species, was congruent with previous studies. The family Amphoriscidae including an Amphoriscus Haeckel, 1870 species as well as the genera Leucilla Haeckel, 1872 and Paraleucilla were recovered as non-monophyletic groups. Despite being polyphyletic, information on species distribution of this family is relevant for diversity studies; consequently, we present the geographic distribution of each genus of Amphoriscidae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Eastern Pacific harbours a great variety of coastal ecosystems and submarine habitats with different sea currents and comprises the entire Tropical Eastern Pacific realm and the Eastern sides of the Temperate Northern Pacific and the Temperate South America realms (Spalding et al. 2007). Despite several sampling campaigns in this large area, subsequent published inventories (Breitfuss 1898; Wilson 1904; Thiele 1905; Lendenfeld 1910a, b, 1915; de Laubenfels 1930; Austin 1996; Azevedo et al. 2009, 2015; Willenz et al. 2009; Hajdu et al. 2013), and sponge species descriptions (Desqueyroux-Faúndez and Van Soest 1996; Díaz et al. 2005; Aguirre et al. 2011; Hajdu et al. 2015; Fernández et al. 2016; Lehnert and Stone 2017; Carballo et al. 2018; Gómez et al. 2018), the Eastern Pacific is still one of the least studied marine regions in terms of diversity and distribution of sponges of the class Calcarea (Porifera: Calcarea) (Van Soest et al. 2012).
Up to date, only 34 species of Calcaronea Bidder, 1998 (Porifera, Calcarea) are known from the Eastern Pacific, 11 from the Temperate Northern Pacific (Lambe 1893; Urban 1902, 1906; de Laubenfels 1930; Duplessis and Reiswig 2000; Lehnert and Stone 2017), three from the Tropical Eastern Pacific (Hôzawa 1940), and 20 from the Temperate South America (Haeckel 1872; Ridley 1881; Breitfuss 1898; Tanita 1942; Azevedo et al. 2009). The low number of species reported from the Tropical Eastern Pacific may not reflect the real diversity of the subclass Calcaronea in that area, but instead the lack of sampling effort and taxonomy expertise.
Amphoriscidae comprises Calcaronea diagnosed as “Leucosolenida with syconoid, sylleibid or leuconoid organisation, and a distinct cortex supported by tangential tetractines with centripetal apical actines crossing the outer part, or the whole choanosome. Tangential triactines and small tetractines may be also present in the cortex. The choanoskeleton is typically inarticulate, composed of the apical actines of cortical tetractines, and the unpaired actines of subatrial spicules. In species with a thick wall, scattered triactines and/or tetractines may also be present, either in the inarticulate choanoskeleton, or forming a distinct subatrial layer. An atrial skeleton is always present” (Borojević et al. 2002). Although molecular phylogenies reject the monophyly of this family (Voigt et al. 2012; Alvizu et al. 2018; Cóndor-Luján et al. 2018), it is still accepted in the systematics of Calcarea, as an alternative system could not yet been established (Hooper and Van Soest 2002).
Amphoriscidae comprises three genera, Leucilla Haeckel, 1872, Paraleucilla Dendy, 1892, and Amphoriscus Haeckel, 1870 (Borojevic et al. 2002), and includes 45 species distributed worldwide (Van Soest et al. 2018). Among those species, only Leucilla nuttingi (Urban, 1902) had been recorded from the Eastern Pacific (Temperate Northern Pacific, California).
In a previous study (Azevedo et al. 2015), we reported five new species and three new records of Calcinea (Porifera, Calcarea) from Peru, all of them from the Temperate South America and the Tropical Eastern Pacific realms. In this work, we present two new Amphoriscidae from the Tropical Eastern Pacific province (Guayaquil ecoregion).
Material and methods
Sample collection
All samples analysed in this study were collected in the northern coast of Peru, Guayaquil ecoregion, in the Tropical Eastern Pacific province. Specimens were collected in five localities (Fig. 1) at depths varying from the intertidal to 15 m, from 2007 to 2009, during the expeditions of projects ESPER (Esponjas del Perú) and EsponjAS (Esponjas da América do Sul).
Collection sites of Amphoriscidae in Peru. a Location of Peru in South America (grey); b Detail of Peru indicating the study area; c Sampled localities: (1) Punta Sal Resort, Tumbes; (2) Máncora Beach, Piura; (3) Máncora Pier, Piura; (4) El Ñuro site 4, north of Quebrada Verde, Piura; (5) El Ñuro site 7, south of Quebrada Verde, Piura
All specimens were preserved in ethanol 96% and were deposited in the sponge collections of the Museu Nacional of the Universidade Federal do Rio de Janeiro (MNRJ), Brazil; Colección de Zoologia Acuática, Laboratorio de Biología Marina/Universidad Peruana Cayetano Heredia (CZA), Peru; and the Royal Belgian Institute of Natural Sciences (RBINS), Belgium.
Morphological analyses
The external morphology and the internal anatomy of each specimen were assessed. Characteristics of the surface, presence of oscula, consistency, texture, and colour were analysed on the preserved specimens under a stereomicroscope and complemented with information retrieved from the in situ pictures and field notes.
Preparations of spicules and sections followed standard procedures (Wörheide and Hooper 1999; Klautau and Valentine 2003). To illustrate the species descriptions, micrographs were taken with a digital camera AxioCam MRC5 coupled to a Zeiss Imager A2 microscope. Additional spicule preparations were made for scanning electron microscopy (SEM) following procedures detailed in Azevedo et al. (2015). SEM micrographs were recorded at the Royal Belgian Institute of Natural Sciences on a FEI/Philips XL30 ESEM at 20 to 30 kv, or at the Institute of Biology of Universidade Federal do Rio de Janeiro (UFRJ) on a JSM-6510. Spicule measurements were made using an ocular micrometre. The length and width at the base of the actines of each spicule category were taken (n = 20). Identifications followed the Systema Porifera (Hooper and Van Soest 2002) and other appropriate literature (e.g. Cavalcanti et al. 2014; Cóndor-Luján et al. 2018).
Molecular analyses
The C-region of LSU (28S rDNA) was amplified using the primers fwd: 5′-GAAAAGCACTTTGAAAAGAGA-3′ (Voigt and Wörheide 2016) and rv: 5′-TCCGTGTTTCAAGACGGG-3′ (Chombard et al. 1998). DNA extraction, amplification, and sequencing followed Cóndor-Luján et al. (2018).
Sequences used in recent phylogenies (Van Soest and de Voogd 2015; Klautau et al. 2016; Voigt and Wörheide 2016; Alvizu et al. 2018; Cóndor-Luján et al. 2018) were retrieved from the GenBank database and are listed in Table 1, as well as those generated in this study. Sequences were aligned through the MAFFT v.7 online platform (Katoh and Standley 2013) using the strategy Q-INS-i (Katoh and Toh 2008). The nucleotide substitution model that best fitted the alignment was indicated by the Bayesian Information Criterion in MEGA 6 (Nei and Kumar 2000; Tamura et al. 2013): TN93 + G.
Phylogenetic reconstructions were performed under two approaches: Bayesian inference (BI) and maximum likelihood (ML). BI analyses were executed in MrBayes 3.1.2 (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003) under 106 generations and a 25% burn-in, generating a consensus tree of majority with clades supported with posterior probability values. ML analyses were conducted in MEGA 6 using an initial NJ tree (BIONJ) and bootstrap analysis of 1000 pseudo-replicates. To estimate the genetic interspecific distances, we calculated the uncorrected p-distance in MEGA 6. The obtained molecular phylogeny and p-distance values were used to confirm species identifications.
Results
Morphology
Phylum Porifera Grant, 1836
Class Calcarea Bowerbank, 1862
Subclass Calcaronea Bidder, 1898
Order Leucosolenida Hartman, 1958
Family Amphoriscidae Dendy, 1892
Genus Leucilla Haeckel, 1872
Diagnosis: Amphoriscidae with sylleibid or leuconoid organisation. Choanoskeleton formed primarily by apical actines of giant cortical tetractines, and unpaired actines of subatrial triactines or tetractines. Dispersed spicules may occur, but typical articulated choanoskeleton is always absent (Borojevic et al. 2002, emend.)
Type species: Leucilla amphora Haeckel, 1872 (by subsequent designation; Dendy and Row 1913)
Leucilla mancoraensis sp. nov. (Figs. 2 and 3, Table 2)
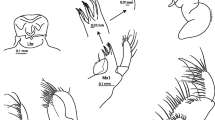
Leucilla mancoraensis sp. nov. (MNRJ 12948, holotype). a Specimen in vivo; b Specimen after fixation; c Oscular margin supported by tetractines; d Cross section of the skeleton; e Cortex with triactines and tetractines; f Cortex with trichoxeas; g Subatrial triactines; h Atrium with apical actines of tetractines. cx cortex, at atrium, tcx trichoxea, sbat subatrial triactine
Paraleucilla tarazonai sp. nov. (MNRJ 11448). a Specimen after fixation; b Cortical microdiactines (arrows); c Cross-section (arrows = broken diactines); d Cortical triactines (arrow) and tetractines; e Cross-section showing the outer (white arrowheads) and inner regions (black arrowheads); f Atrium with the apical actine of the tetractines. cx cortex, at atrium
Diagnosis: Leucilla with tubular to ovoid body, apical osculum, sylleibid aquiferous system, and skeleton composed of cortical triactines and giant tetractines, subatrial triactines with thick actines (10–40 μm), and atrial tetractines with short apical actines (18–65 μm). Trichoxeas can be added to the cortex.
Etymology: named after its type locality.
Type locality: Máncora Beach, Piura
Type material: (Holotype/ethanol): MNRJ 12948; Máncora Beach, Piura, Piura Region, Peru (04° 06′ 20.50″ S–81° 03′ 34.00″ W); collected by Y. Hooker, F. Menendez, and Ph. Willenz; intertidal; 17 November 2009. (Paratypes/ethanol): MNRJ 12967; Máncora Pier, Piura, Piura Region, Peru (04° 06′ 35.65″ S–81° 04′ 02.41″ W); collected by Y. Hooker, F. Menendez, and Ph. Willenz; 2.9 m depth; 18 November 2009. MNRJ 13075; Punta Sal Resort, Tumbes Region, Peru (03° 59′ 02.70″ S–80° 59′ 11.40″ W); collected by Y. Hooker and Ph. Willenz; intertidal; 2 December 2009
Additional material examined: MNRJ 12988; El Ñuro site 7, south of Quebrada Verde, Piura Region, Peru (04° 13′ 30.40″ S–81° 12′ 31.60″ W); collected by Y. Hooker and F. Menendez; 15.0 m depth; 19 November 2009. MNRJ 21304 and MNRJ 21305; El Ñuro site 4, north of Quebrada Verde, Piura Region, Peru (04° 13′ 22.30″ S–81° 12′ 24.10″ W; collected by Y. Hooker, M. Rios, and Ph. Willenz; 4.0–5.0 m depth; 14 October 2007
Colour: yellowish-beige in life (Fig. 2a) and white in ethanol (Fig. 2b)
Morphology and anatomy: sponge with a tubular to ovoid body with an apical osculum (Fig. 2a, b) supported by sagittal tetractines and with a feeble crown of trichoxeas (Fig. 2c). The holotype (MNRJ 12948) measures 1.5 × 4.5 × 4.0 mm (Fig. 2a, b). Surface rough and consistency friable. Aquiferous system sylleibid with several spherical chambers ranging, in the largest diameter, from 110 to 176 μm (Fig. 2d)
Skeleton: osculum ornamented with short, delicate trichoxeas, imperceptible under the stereomicroscope. Oscular margin composed only of tetractines (Fig. 2c). Cortical skeleton formed of triactines, the basal system of giant tetractines and few scattered trichoxeas. Triactines and tetractines are tangentially distributed on the cortex (Fig. 2e), whereas trichoxeas are arranged perpendicularly (Fig. 2f). Choanosomal skeleton inarticulate, formed by the apical actine of the giant cortical tetractines and the unpaired actine of the subatrial triactines (Fig. 2g). Apical actine of the tetractines crosses the choanosome. Atrial skeleton formed by tetractines bearing a very short apical actine projected into the atrium (Fig. 2h).
Spicules:
Cortical triactines: sagittal. Actines are conical, straight with sharp tips. Paired actines can be longer than the unpaired ones (Fig. 3(a–d)). Size: 130–378/11–32 μm (paired) and 118–410/11–27 μm (unpaired)
Cortical tetractines: sagittal. Actines are conical, straight with sharp tips. The apical actine is the longest actine, and it can be distally undulated (Fig. 3(e–g)). Size: 140–583/16–54 μm (paired), 313–658/11–54 μm (unpaired), and 184–994/16–54 μm (apical)
Subatrial triactines: sagittal. Actines are conical, straight with sharp tips. The unpaired actine is frequently longer than the paired ones (Fig. 3(h–l)). Size: 118–389/11–43 μm (paired) and 238–616/11–43 μm (unpaired)
Atrial tetractines: sagittal. Actines are conical with sharp tips. Paired actines are often shorter than the unpaired ones, and they are curved (Fig. 3(m)). Size: 84–227/10–18 μm (paired) and 103–284/10–19 μm (unpaired) and 19–59/7–12 μm (apical)
Ecology: this species was found living from the intertidal down to 15 m depth. Some specimens (MNRJ 11448 and 12948) were associated with bryozoans and algae, in a highly silted area.
Geographical distribution: provisionally endemic to the northern coast of Peru (Piura and Tumbes regions). Corresponding MEOW: Guayaquil ecoregion, Tropical East Pacific Province (TEP)
Remarks: the species that most resemble Leucilla mancoraensis sp. nov. are L. micropilosa Cóndor-Luján, Louzada, Hajdu & Klautau, 2018 from Curaçao and L. nuttingi from California, as they have sylleibid aquiferous system and share similar spicule composition (cortical triactines and tetractines, subatrial triactines, and atrial tetractines). Although Lanna et al. (2017) had showed that specimens of Leucilla could be young paraleucillas, Leucilla mancoraensis sp. nov. has sylleibid aquiferous system, a type of aquiferous system known only in Leucilla.
Leucilla micropilosa is well-characterised by the presence of cortical microdiactines, which are absent in L. mancoraensis sp. nov. In addition, the skeleton of the new species present thicker subatrial triactines (26–28 μm) compared to L. micropilosa (14 μm).
Unlike L. mancoraensis sp. nov., L. nuttingi has a stem and microdiactines protruding through the cortex. Moreover, in L. nuttingi, the cortical triactines and tetractines have similar width (40 μm) and are located in the same layer (see Urban 1902, Plate XIV, Fig. 2), whereas in L. mancoraensis sp. nov. (holotype measurements), the cortical triactines (14 μm) are thinner than the tetractines (31–37 μm), which are located in a subjacent layer.
Genus Paraleucilla Dendy, 1892
Diagnosis: “Amphoriscidae with leuconoid organisation. The thick wall is divided into two regions. The outer region is supported by the skeleton, which remains essentially inarticulate, with the apical actines of cortical tetractines pointed inwards, and a layer of triactines and/or tetractines with the unpaired actine pointed outwards. The inner region of the choanoskeleton is intercalated between the original subatrial skeleton and the atrial one, and it is supported by large triactines and/or tetractines, that are scattered in disarray, and whose form is similar to the spicules found in the outer layer of the choanoskeleton, or inside the atrial skeleton. Because the original subatrial layer still remains in the outer part of the choanosome, facing the cortical tetractines, there are no typical subatrial spicules adjacent to the atrial skeleton” (Borojevic et al. 2002).
Type species: Leucandra cucumis Haeckel, 1872 (by monotypy)
Paraleucilla tarazonai sp. nov. (Figs. 4 and 5, Table 3)
Diagnosis: Paraleucilla with massive body, osculum without crown, skeleton composed of rare large diactines (> 1100/27 μm), cortical spined thin microdiactines (54–189/1–3 μm), cortical triactines and tetractines, subatrial triactines, and atrial triactines and tetractines with short apical actines (33–135 μm)
Etymology: named after the ecologist Juan Tarazona Barboza who greatly contributed to the understanding of the Peruvian marine ecosystem
Type locality: El Ñuro site 4, north of Quebrada Verde, Piura.
Type material: (Holotype/ ethanol): MNRJ 11448; El Ñuro site 4, north of Quebrada Verde, Piura Region, Peru (04° 13′ 22.30″ S–81° 12′ 24.10″ W); collected by Y. Hooker, M. Rios, and Ph. Willenz; 4.0–5.0 m depth; 14 October 2007. (Paratypes/ethanol): MNRJ 21306 and MNRJ 21307; same data as the holotype
Colour: unknown in life and beige in ethanol (Fig. 4a).
Morphology and anatomy: the holotype (MNRJ 11448) is massive and ramified, with two oscula without crown. It measures 12.0 × 10.0 × 4.0 mm (Fig. 4a). Consistency firm but friable. Osculum supported by sagittal triactines and tetractines, the same spicules as the body wall. Surface rough and scarcely hispid because of the presence of few diactines. Aquiferous system leuconoid with subspherical choanocyte chambers, ranging from 98 to 130 μm.
Skeleton: cortical skeleton composed of microdiactines, few large diactines, rare triactines, and a basal system of tetractines. Microdiactines and diactines are perpendicular to the cortex. The microdiactines are frequently organised in tufts (Fig. 4b) and the diactines can cross the choanosome (arrows in Fig. 4c). The triactines and the basal system of the tetractines lay tangentially to the surface (Fig. 4d). Choanosomal skeleton typical of the genus, being inarticulate near the surface (outer region) and without organisation below the subatrial skeleton (inner region). The outer region is formed by the diactines, the apical actine of the cortical tetractines, and the unpaired actine of subatrial triactines (Fig. 4e). The apical actine of the cortical tetractines crosses the choanosome and can even reach the atrium. The inner region is evident only when the body wall of the sponge is thick. Triactines similar to those of the subatrial skeleton and subregular triactines are scattered in this region. Atrial skeleton formed by triactines and tetractines. The apical actine of the atrial tetractines is not conspicuous (Fig. 4f).
Spicules:
Diactines: fusiform with sharp tips (Fig. 5(a)). Size: > 1100/27 μm
Cortical microdiactines: straight, spined, with one sharp and one lanceolated tip (Fig. 5(b)). Size: 54–189/1–3 μm
Cortical triactines: sagittal. Actines are conical, straight, with sharp tips (Fig. 5(c, d)). Size: 70–170/4–12 μm (unpaired) and 86–265/4–14 μm (paired)
Cortical tetractines: sagittal. Basal actines are slightly conical to conical, straight, with sharp tips. The apical actine is slightly undulated and longer than the other actines (Fig. 5(e, f)). Size: 32–230/11–27 μm (unpaired actine); 118–400/11–43 μm (paired); and 178–745/13–65 μm (apical)
Subatrial triactines: sagittal. Actines are conical, straight, with sharp tips. Paired actines are shorter than the unpaired one (Fig. 5(g–k)). Size: 150–535/11–38 μm (unpaired) and 89–300/11–38 μm (paired)
Atrial triactines: sagittal. Actines are slightly conical, with sharp tips. Size: 78–338/4–12 μm (unpaired) and 103–262/7–13 μm (paired)
Atrial tetractines: sagittal. Actines are conical, straight with sharp tips. The apical actine is the shortest one (Fig. 5(l, m)). Size: 127–263/5–16 μm (unpaired), 89–300/5–16 μm (paired), and 33–135/5–11 μm (apical)
Ecology: the specimens were found on an oyster shell at 4–5 m depth.
Geographical distribution: provisionally endemic to the northern coast of Peru (Piura region). Corresponding MEOW: Guayaquil ecoregion, Tropical East Pacific Province (TEP)
Remarks: Paraleucilla comprises 13 species (Van Soest et al. 2017). Among them, P. crosslandi (Row, 1909) from the Red Sea and P. proteus (Dendy, 1913) from the Indian Ocean present a similar skeleton composition as that of P. tarazonai sp. nov. The skeleton of these three species is composed of triactines and tetractines in the cortex, triactines in the inner region, and triactines and tetractines in the atrium. However, differently from the new species, P. crosslandi and P. proteus do not have diactines, nor microdiactines.
As a juvenile Paraleucilla can be easily misidentified as Leucilla (see P. magna, Lanna et al. 2017), we compared our specimens also with other Leucilla species with similar skeleton composition. Among them, the skeleton of P. tarazonai sp. nov. most resembles L. endoumensis Borojevic & Boury-Esnault, 1986 from the Mediterranean Sea, L. oblata Row & Hôzawa, 1931 from Western Australia, and L. schauinslandi (Preiwisch, 1904) from the Chatham Islands (New Zealand). Nonetheless, they can be easily distinguished by the absence of diactines in the latter three species. Additionally, L. oblata possesses tetractines in the subatrial skeleton, and L. schauinslandi has tetractines scattered in the choanosome, whereas P. tarazonai sp. nov. lacks tetractines in those regions.
Paraleucilla tarazonai sp. nov. is the first Paraleucilla reported from the Eastern Pacific and can be easily distinguished from the other Paraleucilla and Leucilla species because of its unique skeleton composition (diactines, microdiactines, triactines, and basal system of tetractines in the cortex, subatrial triactines, and triactines and tetractines supporting the atrium).
As the inner region of the skeleton of Paraleucilla is only evident when the wall is thick enough, specimens with thin wall (i.e. in early stages of growth) can be erroneously identified as a Leucilla (Lanna et al. 2017). Thus, descriptions of new Paraleucilla and Leucilla must be made with caution. To avoid misidentifications, the study should be based, when possible, in more than one specimen of different ages (or sizes). In this study, it was possible to analyse specimens of L. mancoraensis sp. nov. and P. tarazonai sp. nov. with different growth stages, which allowed us to unequivocally allocate these specimens in the correct genera.
Molecular taxonomy
In this study, we provide sequences for the new species Leucilla mancoraensis sp. nov. and Paraleucilla tarazonai sp. nov., as well as for two known Amphoriscidae species, Amphoriscus pedunculatus Klautau, Cavalcanti & Borojevic, 2017 and P. perlucida Azevedo & Klautau, 2007.
The final alignment included 52 sequences (Table 1) and had a total length of 430 bp (including gaps), 262 conservative sites, 140 variable sites, and 31 singletons.
Both phylogenetic methods (BI and ML) recovered trees with similar topologies. The obtained ML tree is shown in Fig. 6. Neither Amphoriscidae nor Leucilla or Paraleucilla were recovered as monophyletic, instead Amphoriscidae sequences appeared in three different clades.
Leucilla mancoraensis sp. nov. appeared as sister species of L. micropilosa, with a p-distance of 2.2% and branch support of 0.87 (BI). Both species grouped with another Amphoriscidae, Amphoriscus pedunculatus, with high support (1 in BI and 97% in ML analyses). Paraleucilla tarazonai sp. nov. grouped with a clade formed by Anamixilla torresi and A. singaporensis, with p-distances of 1.8% and 1.4%, respectively, and supported by 0.86 in BI and 55% in ML analyses.
Discussion
Phylogenetic affinities in Amphoriscidae
This is the first study that included all the available sequences of the three accepted Amphoriscidae genera, Amphoriscus, Leucilla, and Paraleucilla, in a molecular tree. As observed in previous studies (Voigt et al. 2012; Alvizu et al. 2018; Cóndor-Luján et al. 2018), Amphoriscidae was not recovered as a monophyletic group. Moreover, Leucilla and Paraleucilla were polyphyletic, as recently shown by Cóndor-Luján et al. (2018) and Alvizu et al. (2018), respectively.
Although not being monophyletic in our molecular tree species of Leucilla with similar skeleton composition (cortical triactines and tetractines, subatrial triactines and atrial tetractines) and sylleibid aquiferous system, L. mancoraensis sp. nov. and L. micropilosa grouped in a monophyletic clade. Interestingly, L. antillana, which presents different skeleton composition (cortical tetractines, subatrial triactines, and atrial tetractines) and leuconoid aquiferous system, appeared in another clade. Whether skeleton composition and type of aquiferous system are characters with phylogenetic/evolutionary signal in certain species of Leucilla should be revised in future studies.
Klautau et al. (2016) obtained a highly supported clade formed by two species of Paraleucilla (P. magna and P. dalmatica); however, this genus was recovered as polyphyletic in further molecular analyses (Alvizu et al. 2018; Cóndor-Luján et al. 2018). Herein, we added new sequences of Paraleucilla (P. erpenbecki and P. tarazonai sp. nov.) and our results supported again the non-monophyly of this genus.
Recently, Klautau et al. (2017) pointed out the possible phylogenetic signal of attachment structures after describing A. pedunculatus, an Amphoriscidae with peduncle. Those authors suggested that certain Calcaronean species provided with peduncle or root-like structures could be congeneric as they appeared in a monophyletic group within a molecular phylogeny. In our analysis, A. pedunculatus, Leucilla micropilosa, and L. mancoraensis sp. nov., which present similar skeleton composition, formed a monophyletic group. However, differently from A. pedunculatus and L. mancoraensis sp. nov., L. micropilosa does not bear any peduncle. Considering that convergent evolution and secondary loss can have a relevant role in the evolutionary history of Calcaronea (Alvizu et al. 2018), the absence of peduncle in L. micropilosa could be a case of secondary loss.
Nonetheless, an ecological response cannot be discarded. While L. mancoraensis sp. nov. and A. pedunculatus were found attached to organisms, L. micropilosa was collected underneath boulders. Considering this, the attachment structures may be related to the sponge habitat more than to ancestrality, as previously suggested by Klautau et al. (2017). Although very incipient, our results may be the starting point to understand the evolution of attachment structures within Amphoriscidae.
Until now, the 28S (including the C-region of LSU) is being a good marker for alpha systematics in Calcaronea (e.g. Klautau et al. 2016; Alvizu et al. 2018; Cóndor-Luján et al. 2018). However, the phylogenetic relationships among genera are not yet clearly solved. We agree with previous authors (e.g. Klautau et al. 2016; Alvizu et al. 2018) that it is necessary to find an additional molecular marker to really unveil the phylogeny of this subclass.
Geographic distribution of Amphoriscus, Leucilla, and Paraleucilla
Although the three genera of Amphoriscidae are probably not monophyletic, as our and others’ results showed (Alvizu et al. 2018; Cóndor-Luján et al. 2018), they are still considered valid. Therefore, we show here their geographic distribution.
Amphoriscus comprises 17 accepted species (Van Soest et al. 2018). Among them, 13 were recorded from tropical or subtropical waters (Atlantic Ocean, Mediterranean Sea, and Indo-Pacific) and four from temperate waters (South Atlantic and sub-Antarctic) (Fig. 7). Until now, Amphoriscus has not been reported from the Eastern Pacific, probably because of the poor sampling effort on Calcarea in this region.
World distribution of Amphoriscus. (1) A. ancora; (2) A. bucchichii; (3) A. chrysalis; (4) A. cyathiscus; (5) A. cylindricus; (6) A. dohrni; (7) A. elongatus; (8) A. gastrorhabdifer; (9) A. gregorii; (10) A. kryptoraphis; (11) A. oviparus; (12) A. pedunculatus; (13) A. salfii; (14) A. semoni; (15) A. synapta; (16) A. testiparus; (17) A. urna
With this work, Leucilla now includes 16 valid species. Six species are exclusive of temperate waters (East Pacific, North Atlantic, South Atlantic, and Indo-Pacific), seven occur in tropical or subtropical waters (Atlantic, Caribbean, Mediterranean, East Pacific, and Indo-Pacific), two are found in both temperate and tropical waters, and only one is present in cold waters (Arctic) (Fig. 8). Leucilla uter shows a wide geographic range, being found in both sides of the Atlantic (Poléjaeff 1883; Borojevic 1967; Muricy et al. 2011) and in the Indo-Pacific (Poléjaeff 1883). Leucilla mancoraensis sp. nov. is the second species recorded from the Eastern Pacific, and it constitutes the first record of this genus for the Southeastern Pacific.
World distribution of Leucilla. (1) L. amphora; (2) L. antillana; (3) L. australiensis; (4) L. capsula; (5) L. echina; (6) L. endoumensis; (7) L. hirsuta. (8) L. leuconoides; (9) L. mancoraensis sp. nov; (10) L. micropilosa; (11) L. minuta; (12) L. nuttingi; (13) L. oblata; (14) L. sacculata; (15) L. schauinslandi; (16) L. uter
Paraleucilla groups now 14 valid species. Nine of them were recorded exclusively from tropical or subtropical waters (East Pacific, Western Atlantic, Mediterranean Sea, Red Sea, and Indo-Pacific). Three species are present in both tropical and temperate waters, and only one occurs exclusively in temperate waters (Fig. 9). Paraleucilla magna is one of the most widespread species of this genus. This species was described from the tropical Atlantic, although it is probably not originally from that region (Klautau et al. 2004). Some years later, it was registered from the Eastern Atlantic and from different parts of the Mediterranean Sea, mainly in harbours and mussel farms (Longo et al. 2007; Dailianis et al. 2016; Klautau et al. 2016; Topaloğlu et al. 2016; Gerovasileiou et al. 2017; Mačić and Petović 2017). Most recently, it was found in St. Helena Island in the South Atlantic and in the southern coast of Portugal (Alvizu et al. 2018). Paraleucilla magna has been categorised an alien-invasive species because of its ability to rapidly colonise different environments and for being responsible for economic losses in mussel farms in the Mediterranean Sea (Longo et al. 2007). Paraleucilla cucumis and P. saccharata also present wide distributions, being recorded throughout the Indo-Pacific (Cavalcanti et al. 2014).
Distributional patterns of calcareous sponges from Peru
Azevedo et al. (2015) described three biogeographical patterns for Peruvian species belonging to the subclass Calcinea: (1) endemic to the northern part of Peru, (2) widespread, and (3) discontinuous. The new Calcaronean species, Leucilla mancoraensis sp. nov. and Paraleucilla tarazonai sp. nov., may also be restricted to the northern coast of Peru as they have not been found in southernmost localities so far. However, the presence of some demosponges originally reported from Peru (Clathria aculeofila Aguirre, Hooker, Willenz & Hajdu, 2011 and Acarnus peruanus Van Soest, Hooper & Hiemstra, 1991) in localities farther north, in Ecuador and Mexico (personal observations and Aguilar-Camacho et al. 2014, respectively), suggests that provisionally endemic Peruvian calcareous species may also turn out in those latitudes from the Tropical Eastern Pacific, once these get enhanced taxonomic effort. The widespread distribution of Hamacantha hyaloderma (de Laubenfels, 1932), which occurs from British Columbia (Austin et al. 2012) down to Peru (Hajdu et al. 2015), also supports this hypothesis.
Abbreviations
- GW:
-
Gert Wörheide
- IRB:
-
Institut Ruđer Bošković, Zagreb, Croatia
- MNRJ:
-
Museu Nacional of Universidade Federal do Rio de Janeiro, Brazil
- PMR:
-
Prirodoslovni Muzej Rijeka, Croatia
- QM:
-
Queensland Museum, Australia
- RMNH:
-
Rijksmuseum van Natuurlijke Histoire Leiden, Netherlands
- SAM:
-
South Australian Museum, Australia
- UFRJPOR:
-
Porifera collection of the Biology Institute of Universidade Federal do Rio de Janeiro, Brazil
- WAMZ:
-
Zoological collection of the Western Australian Museum, Perth, Australia
- ZMA.POR:
-
Zoölogisch Museum, Instituut voor Systematiek en Populatiebiologie, Amsterdam, The Netherlands
References
Aguilar-Camacho JM, Carballo JL, Cruz-Barraza JA (2014) Acarnidae Porifera: Demospongiae: Poecilosclerida) from the Mexican Pacific Ocean with the description of six new species. Sci Mar 77(4):677–696
Aguirre LK, Hooker Y, Willenz P, Hajdu E (2011) A new Clathria (Demospongiae, Microcionidae) from Peru occurring on rocky substrates as well as epibiontic on Eucidaris thouarsii sea urchins. Zootaxa 3085:41–54
Austin WC (1996) Sponges from Rocas Alijos. In: Schmieder RW (ed) Monographiae Biologicae. Rocas Alijos. Scientific Results from the Cordell Expeditions. Kluwer Academic Publishers, Dordrecht/Boston/London, pp 237–256
Austin WC, Ott BS, McDaniel NG, Reiswig HM, Romagosa P (2012) Sponges of the cold temperate NE Pacific. http://mareco.org/KML/Projects/NESponges.asp. Accessed 18 November 2017
Alvizu A, Eilertsen MH, Xavier JR, Rapp H (2018) Increased taxon sampling provides new insights into the phylogeny and evolution of the subclass Calcaronea (Porifera, Calcarea). Org Divers Evol (2018) 18: 279–290
Azevedo F, Cóndor-Luján B, Willenz P, Hajdu E, Hooker Y, Klautau M (2015) Integrative taxonomy of calcareous sponges (subclass Calcinea) from the Peruvian coast: morphology, molecules, and biogeography. Zool J Linnean Soc 173:787–817
Azevedo F, Hajdu E, Willenz P, Klautau M (2009) New records of calcareous sponges (Porifera, Calcarea) from the Chilean coast. Zootaxa 2072:1–30
Azevedo F, Klautau M (2007) Calcareous sponges (Porifera, Calcarea) from Ilha Grande Bay, Brazil, with description of three new species. Zootaxa 1402(1):1–22
Bidder GP (1898) The skeleton and the classification of calcareous sponges. Proc R Soc Lond 64:61–76
Borojević R (1967) Spongiaires d’Afrique du Sud. (2) Calcarea. Trans R Soc S Afr 37(3):183–226
Borojević R, Boury-Esnault N (1986) Description d'une éponge calcaire calcaronéenne du genre Leucilla Haeckel en Méditerranée: Leucilla endoumensis n. sp. Bull Mus Hist Nat Paris 8A(1):3–7
Borojević R, Boury-Esnault N, Manuel M, Vacelet J (2002) Order Leucosolenida Hartman, 1958. In: Hooper JNA, Van Soest RWM (eds) Systema Porifera: a guide to the classification of sponges. Kluwer Academic/Plenum Publishers, New York, pp 1157–1184
Bowerbank JS (1862) On the anatomy and physiology of the Spongiadae. Part II Philos Trans R Soc Lond 152(2):747–829
Breitfuss LL (1898) Die Kalkschwämme der Sammlung Plate. Zool Jahrb Suppl Band 4:455–470
Carballo JL, Bautista-Guerrero E, Cruz-Barraza JA (2018) Description and molecular phylogeny of Axinella nayaritensis n. sp. (Porifera: Axinellida) from East Pacific and remarks about the polyphyly of the genus Axinella. Zootaxa 4482(1):111–124
Cavalcanti F, Menegola C, Lanna E (2014) Three new species of the genus Paraleucilla Dendy, 1892 (Porifera, Calcarea) from the coast of Bahia state, northeastern Brazil. Zootaxa 3764(5):537–554
Chombard C, Boury-Esnault N, Tillier S (1998) Reassessment of homology of morphological characters in tetractinellid sponges based on molecular data. Syst Biol 47(3):351–366
Cóndor-Luján B, Louzada T, Hajdu E, Klautau M (2018) Morphological and molecular taxonomy of calcareous sponges (Porifera: Calcarea) from Curacao, Caribbean Sea. Zool J Linnean Soc 183(3):459–525
Dendy A (1892) Synopsis of the Australian Calcarea Heterocoela; with a proposed classification of the group and descriptions of some new genera and species. Proc R Soc Vic 5:69–116
Dendy A (1913) Report on the calcareous sponges collected by H. M. S. ‘Sealark’ in the Indian Ocean. Trans Linn Soc Lond 16:1–29
Dendy A, Row H (1913) The classification and phylogeny of the calcareous sponges, with a reference list of all the described species, systematically arranged. Proc Zool Soc Lond 47:704–813
de Laubenfels MW (1930) The sponges of California. (Abstracts of dissertations for the degree of doctor of philosophy). Stanford University Bulletin 5(98):24–29
de Laubenfels MW (1932) The marine and fresh-water sponges of California. Proc USNM 81(2927):1–140
Dailianis D, Akyol O, Babali N, Bariche M, Crocetta F, Gerovasileiou V, Ghanem R, Gökoğlu M, Hasiotis T, Izquierdo-Muñoz A, Julian D, Katsanevakis S, Lipej L, Mancini E, Mytilineou C, Ounifi Ben Amor K, Özgül A, Ragkousis M, Rubio-Portillo E, Servello G, Sini M, Stamouli C, Sterioti A, Teker S, Tiralongo F, Trkov D (2016) New Mediterranean biodiversity records (July 2016). Mediterr Mar Sci 17(2):608–626
Desqueyroux-Faúndez R, Van Soest RWM (1996) A review of Iophonidae, Myxillidae and Tedaniidae occurring in the South East Pacific (Porifera: Poecilosclerida). Rev Suisse Zool 103(1):3–79
Díaz MC, Van Soest RWM, Rützler K, Guzman HM (2005) Aplysina chiriquiensis, a new pedunculate sponge from the Gulf of Chiriqui, Eastern Pacific (Aplysinidae, Verongida). Zootaxa 1012:1–12
Duplessis K, Reiswig HM (2000) Description of a new deep-water calcareous sponge (Porifera: Calcarea) from Northern California. Pac Sci 54(1):10–14
Fernandez JCC, Cárdenas CA, Bravo A, Lôbo-Hajdu G, Willenz P, Hajdu E (2016) Lissodendoryx (Ectyodoryx) Lundbeck, 1909 (Coelosphaeridae, Poecilosclerida, Demospongiae) from Southern Chile: new species and a discussion of morphologic characters in the subgenus. Zootaxa 4092(1):69–89
Gerovasileiou V, Akel EHK, Akyol O, Alongi G, Azevedo F, Babali N, Bakiu R, Bariche M, Bennoui A, Castriota L, Chintiroglou CC, Crocetta F, Deidun A, Galinou-Mitsoudi S, Giovos L, Gökoğlu M, Golemaj A, Hadjioannou HJ, Insacco G, Katsanevakis S, Kleitou P, Korun J, Lipej L, Malegue M, Michailidis N, Mouzai Tiforua A, Ovalis P, Petović S, Piraino S, Rizkalla SI, Rousou M, Savva I, Sen H, Spinelli A, Vougioukalou KG, Xharahi E, Zava B, Zenetos A (2017) New Mediterranean biodiversity records (July 2017). Mediterr Mar Sci 18(2):355–384
Gómez P, González-Acosta B, Sánchez-Ortíz C, Hoffman Z, Hernández-Guerrero CJ (2018) Amended definitions for Aplysinidae and Aplysina (Porifera, Demospongiae, Vergongiida): on three new species from a remarkable population in the Gulf of California. Zootaxa 4455(2):322–342
Grant RE (1836). Animal kingdom. In: Todd RB (ed) The cyclopaedia of anatomy and physiology. Sherwood, Gilbert, and Piper, London, pp 107–118
Haeckel E (1870) Prodromus eines Systems der Kalkschwämme. Jenaische Zeitschrift 5:176–119
Haeckel E (1872) Die Kalkschwämme. Eine Monographie in zwei Bänden Text und einem Atlas mit 60 Tafeln Abbildungen. Verlag von Georg Reimer, Berlin
Hajdu E, Desqueyroux-Faúndez R, Carvalho MDS, Lôbo-Hajdu G, Willenz P (2013) Twelve new Demospongiae (Porifera) from Chilean fjords, with remarks upon sponge-derived biogeographic compartments in the SE Pacific. Zootaxa 3744(1):1–64
Hajdu E, Hooker Y, Willenz P (2015) New Hamacantha from Peru, and resurrection of Zygherpe as subgenus (Demospongiae, Poecilosclerida, Hamacanthidae). Zootaxa 3926(1):87–99
Hartman W (1958) A re-examination of Bidder’s classification of the Calcarea. Syst Zool 7:97–110
Hooper J, Van Soest RWM (2002) Systema Porifera: a guide to the classification of sponges. Kluwer Academic/Plenum Publishers, New York
Hôzawa S (1940) Reports on the calcareous sponges obtained by the Zoological Institute and Museum of Hamburg. Sci Rep Tohoku Imp Univ 15:131–163
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17:754–755
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30(4):772–780
Katoh K, Toh H (2008) Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform 9(4):286–298
Klautau M, Cavalcanti FF, Borojevic R (2017) The new sponge species Amphoriscus pedunculatus (Porifera, Calcarea). Zootaxa 4341(1):105–112
Klautau M, Imešek M, Azevedo F, Pleše B, Nikolić V, Ćetković H (2016) Adriatic calcarean sponges (Porifera, Calcarea), with the description of six new species and a richness analysis. Eur J Taxon 178:1–52
Klautau M, Monteiro L, Borojevic R (2004) First occurrence of the genus Paraleucilla (Calcarea, Porifera) in the Atlantic Ocean: P. magna sp. nov. Zootaxa 710:1–8
Klautau M, Valentine C (2003) Revision of the genus Clathrina (Porifera, Calcarea). Zool J Linnean Soc 139:1–62
Lambe LM (1893) Sponges from the Pacific coast of Canada. Proceed Trans R Soc Can 11(4):25–43
Lanna E, Rattis L, Cavalcanti F (2017) The presence of the diagnostic character of the genus Paraleucilla (Amphoriscidae, Calcarea, Porifera) may depend on the volume and body wall thickness of the sponges. Invertebr Biol 136(3):321–329
Lehnert H, Stone R (2017) Description of a new species of Trichogypsiidae (Porifera, Calcarea) and first record of the genus in the Pacific Ocean. Zootaxa 4312(2):394–400
Longo C, Mastrototaro F, Corriero G (2007) Occurrence of Paraleucilla magna (Porifera: Calcarea) in the Mediterranean Sea. J Mar Biol Assoc U K 87:1749–1755
Mačić V, Petović S (2017) New data on the distribution of the alien sponge Paraleucilla magna Klautau, Monteiro & Borojević, 2004 in the Adriatic Sea. Studia Marina 29(1):63–68
Muricy G, Lopes DA, Hajdu E, Carvalho MS, Moraes FC, Klautau M, Menegola C, Pinheiro U (2011) Catalogue of Brazilian Porifera. Museu Nacional, Rio de Janeiro
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, New York
Poléjaeff N (1883) Report on the Calcarea dredged by H.M.S.‘ Challenger’, during the years 1873-1876. Report on the scientific results of the voyage of H.M.S. ‘Challenger’, 1873-1876. Zoology 8(2):1–76
von Preiwisch J (1904) Kalkschwämme aus dem Pacific. Ergebnisse einer Reise nach dem Pacific, Schauinsland 1896/97. Zool Jb Syt 19:9–26
Ridley SO (1881) Spongida. Horny and siliceous sponges of Magellan Straits, S.W. Chili, and Atlantic off S.W. Brazil. Account of the zoological collections made during the survey of H.M.S. ‘Alert” in the straits of Magellan and on the coast of Patagonia. XI. Proc Zool Soc Lond 107–137, 140–141
Ronquist F, Huelsenback JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Row RWH (1909) Reports on the marine biology of the Sudanese Red Sea. XIX. Report on the sponges collected by Mr Cyril Crossland in 1904–05. Zool J Linnean Soc 31:182–214
Row RWH, Hôzawa S (1931) Report on the Calcarea obtained by the Hamburg South-West Australian Expedition of 1905. Sci Rep Tohoku Imp Univ 6:727–809
Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson M, Halpern BS, Jorge MA, Lombana A, Lourie SA, Martin KD, McManus E, Molnar J, Recchia CA, Robertson J (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 57:573–583
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30(12):2725–2729
Tanita S (1942) Report on the calcareous sponges obtained by the Zoological Institute and Museum of Hamburg. Part II. Sci Rep Tohoku Imp Univ (4) 17(2):105–135
Thiele J (1905) Die Kiesel- und Hornschwämme der Sammlung Plate. Zool Jahrb Suppl 6 (Fauna Chilensis III): 407–496
Topaloğlu B, Evcen A, Cinar ME (2016) Sponge fauna in the Sea of Marmara. Turk J Fish Aquat Sci 16(1):51–59
Urban F (1902) Rhabdodermella nuttingi, nov. gen. et nov. spec. Z Wiss Zool 71:268–275
Urban F (1906) Kalifornische Kalkschwämme. Arch Naturgesch 72(1):33–76
Van Soest RWM, Boury-Esnault N, Hooper JNA, Rützler K, de Voogd NJ, Alvarez de Glasby B, Hajdu E, Pisera AB, Manconi R, Schoenberg C, Janussen D, Tabachnick KR, Klautau M, Picton B, Kelly M, Vacelet J, Dohrmann M, Díaz MC, Cárdenas P (2018) World Porifera database. In: World Porifera Database. http://www.marinespecies.org/porifera/. Accessed 09 September 2018
Van Soest RWM, Boury-Esnault N, Vacelet J, Dohrmann M, Erpenbeck D, De Voogd N, Santodomingo N, Vanhoorne B, Kelly M, Hooper JNA (2012) Global diversity of sponges (Porifera). PLoS One 7(4):e35105
Van Soest RWM, Hooper JNA, Hiemstra F (1991) Taxonomy, phylogeny and biogeography of the marine sponge genus Acarnus (Porifera: Poecilosclerida). Beaufortia 42(3):49–88
Van Soest RWM, de Voogd NJ (2015) Calcareous sponges of Indonesia. Zootaxa 3951(1):1–105
Voigt O, Wörheide G (2016) A short LSU rRNA fragment as a standard marker for integrative taxonomy in calcareous sponges (Porifera: Calcarea). Org Divers and Evol 16:53–64
Voigt O, Wülfing E, Wörheide G (2012) Molecular phylogenetic evaluation of classification and scenarios of character evolution in calcareous sponges (Porifera, class Calcarea). PLoS One 7(3):e33417
von Lendenfeld R (1910a) The sponges. 1. The Geodidae. In: Reports on the Scientific Results of the Expedition to the Eastern Tropical Pacific, in charge of Alexander Agassiz, by the U.S. Fish Commission Steamer ‘Albatross’, from October, 1904, to March, 1905, Lieut. Commander L.M. Garrett, U.S.N., Commanding, and of other Expeditions of the “Albatross”, 1888-1904. (21). Mem Mus Comp Zoology Harv Coll 41(1):1–259
von Lendenfeld R (1910b) The sponges. 2. The Erylidae. In: Reports on the Scientific Results of the Expedition to the Eastern Tropical Pacific, in charge of Alexander Agassiz, by the U.S. Fish Commission Steamer ‘Albatross’, from October, 1904, to March, 1905, Lieut. Commander L.M. Garrett, U.S.N., Commanding, and of other Expeditions of the “Albatross”, 1888-1904. (21). Mem Mus Comp Zoology Harv Coll 41(2):261–324
von Lendenfeld R (1915) The sponges. 3. Hexactinellida. In: Reports on the Scientific Results of the Expedition to the Eastern Tropical Pacific, in charge of Alexander Agassiz, by the U.S. Fish Commission Steamer ‘Albatross’, from October, 1904, to March, 1905, Lieut. Commander L.M. Garrett, U.S.N., Commanding, and of other Expeditions of the “Albatross”, 1891-1899 (29). Mem Mus Comp Zoology Harv Coll 42(2):1–396
Willenz P, Hajdu E, Desqueyroux-Faúndez R, Lôbo-Hajdu G, Carvalho MS, Azevedo F, Klautau M (2009) Porifera. In: Häusserman V, Försterra G (eds) Fauna Marina Bentónica de la Patagonia Chilena, 1st edn. Nature in Focus, Santiago, pp 93–170
Wilson HV (1904) Reports on an exploration off the West Coasts of Mexico, Central and South America, and off the Galapagos Islands, in charge of Alexander Agassiz, by the U.S. Fish Commission Steamer ‘Albatross” during 1891, Lieut. Commander Z.L. Tanner, U.S.S., commanding. XXX. The sponges. Mem Mus Comp Zool̈ogy Harv Coll 30(1):1–164
Wörheide G, Hooper JNA (1999) Calcarea from the Great Barrier Reef. 1: cryptic Calcinea from Heron Island and Wistari Reef (Capricorn-Bunker Group). Queensland Mus Mem 43:859–891
Acknowledgements
We thank the field staff (F. Menendez and M. Rios) for assistance in collecting samples. We also thank Gisele Lôbo-Hajdu (UERJ), students, and biologists from both Peru and Brazil (B. Ibañez, F. Menendez, M. Rios, C. Segami, and W. Vieira) for assistance in collecting samples on different expeditions.
Funding
ESPER Project granted to RBINS was funded by the Global Taxonomy Initiative from the Belgian Development Cooperation (ESPER Project, 2419JVG2, 2431JVG2, and 2433FRK2); Proyecto EsponjAS was funded by the National Council of Technological and Scientific Development (CNPq/PROSUL –490425/2007-0; CNPq/Universal 476558/2012-3; CNPq/Universal 476597/2013-7) and by the Coordination for the Improvement of Higher Education Personnel (CAPES/Protax – 561782/2010-5). The collection of samples was performed thanks to RBINS diving gear. BCL and FA received scholarships from CAPES. PhW was supported by travel grant #18834 from the FNRS (Fonds National de la Recherche Scientifique) to conduct part of this study at MNRJ and UFRJ. YH obtained travel funds from the GTIBDC. MK received a fellowship from CNPq (302459/2014-6) and CAPES (440628/2015-6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities.
Data availability
The datasets generated during and/or analysed during the current study are available in the GenBank repository, https://www.ncbi.nlm.nih.gov/genbank/
Additional information
Communicated by D. Janussen
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Leucilla mancoraensis sp. nov. is registered in ZooBank under urn:lsid:zoobank.org:act:F7BA6F47-9205-4F93-950B-43A96970DDFD Paraleucilla tarazonai sp. nov. is registered in ZooBank under urn:lsid:zoobank.org:act:03448075-D3C5-445B-AFC5-5DCA800EE07E
This article is registered in ZooBank under urn:lsid:zoobank.org: pub:2E7AA716-8B23-41B5-AA63-EDB985C4E5F0
Rights and permissions
About this article
Cite this article
Cóndor-Luján, B., Azevedo, F., Hajdu, E. et al. Tropical Eastern Pacific Amphoriscidae Dendy, 1892 (Porifera: Calcarea: Calcaronea: Leucosolenida) from the Peruvian coast. Mar Biodiv 49, 1813–1830 (2019). https://doi.org/10.1007/s12526-019-00946-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-019-00946-y