Abstract
Studies that focus on meiofaunal assemblages of deep-sea mud volcanoes show an unpredictable abundance and diversity in a clear response to the different environmental conditions of the seeped sediment. The mud volcanoes Abzu, Tiamat and M. Ivanov (ATI), are located along the SWIM1 fracture zone, in front of the accretionary wedge of the Gulf of Cadiz (AWGC). The geological setting and the fluid geochemical characteristics of the ATI mud volcanoes are different from those located within the AWGC. The main aim of this study is to describe and compare the spatial and vertical distributions of the meiofauna and nematode assemblages from the ATI mud volcanoes, the Porto mud volcano located in the AWGC, and a non-seep site (Site 2) as reference. The pore-water on the uppermost sediment layers has compositions close to the near-bottom seawater. The meiofauna abundances were generally lower and the vertical distribution of the assemblages showed a typical pattern, gradually decreasing towards depth. The lack of spatial patterns of the standing stocks contrasts with the spatial variability of diversity and biomass, related to the differences in the nematode assemblages that are distinct between ATI, Site 2 and the Porto mud volcano. The ATI and Site 2 assemblages are similar to deep-sea non-seep habitats, and are clearly coupled with the environmental conditions of the bottom seawater. No evidence of seep conditions favouring the development of specialised fauna were found. The lower diversity and the presence of higher dominance species could be driven by distinct seepage conditions of the Porto mud volcano.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluid seepage and mud volcanism in marine environments occur in active and passive continental margins (Dimitrov 2002; Kopf 2002). Submarine mud volcanoes (MV) are geological structures driven by upward flow of fluidized sediments, intercalated with periods of hemipelagic sedimentation. Thousands of deep-water mud volcanoes are estimated to exist worldwide varying greatly in water depth, size, morphology, substrate and biogeochemical conditions (Milkov 2000; Kopf 2002). Furthermore, variations at a small scale in flow rates and sediment pore-water concentrations of both methane and sulphur compounds act as an additional source of habitat heterogeneity that strongly shapes local faunal standing stocks (abundance and biomass) and diversity (Sibuet and Olu 1998; Levin 2005; Cordes et al. 2010). Similarly to other seep habitats where free-living and symbiotic bacteria use methane oxidation and sulphate reduction as the main energy sources to fix organic carbon, faunal communities inhabiting MVs can depend largely on the chemosynthetic derived carbon production (Levin 2005). Patches of enhanced abundance of tolerant species are usually observed at the surface sediments where fluid seepage occurs (Sibuet and Olu 1998; Levin 2005; Cordes et al. 2010). Among the most relevant examples are the mat forming microbial assemblages (e.g. Beggiatoa) and the symbiotic-bearing bivalves and siboglinids (Sibuet and Olu 1998; Levin 2005).
The importance of meiofauna both in terms of abundance and diversity in deep-sea sediments is well established and benthic nematodes are among the most abundant metazoan taxa.
(Giere 2009; Vanreusel et al. 2010a, b). Nematodes are an useful tool to assess ecological environmental changes and the nematode community composition at genus level reflects macro-ecological patterns, providing an appropriate basis for comparison of communities between habitats on a large scale (Heip et al. 1985; Vincx et al. 1994; Vanreusel et al. 2010a, b). Studies on mud volcanoes meiofaunal assemblages, especially those on nematodes, showed an unpredictable variability not only on abundance and biomass but also in their composition and diversity, as a clear response to the different seep sediment conditions (e.g. Olu et al. 1997; Buck and Barry 1998; Robinson et al. 2004; Van Gaever et al. 2009a; Bright et al. 2010; Pape et al. 2011; Zeppilli et al. 2011; Lampadariou et al. 2013). For instance, nematode abundances can range between 116 and 11,364 ind. 10 cm−2 in MVs of the Barbados accretionary prism (Olu et al. 1997). Unusual high abundances were also observed at the Håkon Mosby MV with more than 11,000 ind. per 10 cm−2 associated with Beggiatoa bacterial mats (Van Gaever et al. 2006). Such abundances have been attributed to both the positive effects caused by the presence of habitat-forming organisms, food availability and structural heterogeneity, and the negative effects caused by harmful liquid and gaseous emissions.
(Van Gaever et al. 2009a; Vanreusel et al. 2010a). Patterns of nematode diversity at MVs are also very heterogeneous, displaying both very high or very low species richness and dominance. Usually, poor diversity is due to the dominance of few tolerant species such as Halomonhystera disjuncta, Terchellingia longicaudata, Sabatieria spp. and Desmodora spp. (e.g.
Van Gaever et al. 2006, 2009a, b, 2010; Zeppilli et al. 2011, 2012), although in some studies nematode genera composition showed similarities to communities of the non-seep sediments (Buck and Barry 1998; Lampadariou et al. 2013).
Before detailed geological and geophysical surveys were carried out, the boundary between the African and Eurasian lithospheric plates in the Atlantic Ocean was called the Azores-Gibraltar Fracture Zone (AGFZ). The Gulf of Cadiz (GoC) is the easternmost termination of the AGFZ where the African and Eurasian plates meet along a complex tectonic scenario including a slow right-lateral fracture zone, where the two plates move past each other at a speed of ~4 mm/ year (Argus et al. 1989) and a subduction complex, the Accretionary Wedge of the GoC (AWGC, Gutscher et al. 2002, 2012). The tectonic deformation is accommodated by the right-lateral southwest Iberian Margin (SWIM) strike-slip faults (Zitellini et al. 2009) and by the AWGC underlain by a strip of old (140 My) oceanic crust (Sallares et al. 2011). The subduction of old oceanic crust has driven the formation of the Gibraltar orogenic arc and of the AWGC. The AWGC with a surface of ~56,000km2 is one of the largest cold seep areas on the European margin with more than 30 MVs identified between 200 m and 4000 m water depths (Van Rensbergen et al. 2005; León et al. 2012).
Hensen et al. (2015) reported for the first time the occurrence of three mud volcanoes, Abzu, Tiamat, and Michael Ivanov (ATI MVs), at approximately 4500 m depth, along the Africa-Eurasia plate boundary off southwest Iberia out of the realm of the AWGC. The ATI MVs are manifestations of the expulsion of fluidized sediments at the seafloor along the seismogenic segment of one of the SWIM faults: the strike-slip SWIM1 fault. Hensen et al. (2015) also showed that the chemical constitution of the interstitial fluids in the ATI MVs indicate fluid exchange from the deep oceanic crust to the seafloor surface, across 5 km of sedimentary thickness.
Most of the current understanding on the GoC biodiversity arises from studies dealing with macrofaunal organisms from within the AWGC (e.g. Cunha et al. 2013; Génio et al. 2013; Hilário et al. 2010; Rodrigues et al. 2011, 2013). The only work on meiofauna diversity available for the GoC was carried in the Darwin MV at the shallower depth of 1100 m on the AWGC, where the diversity of nematode assemblages increase with increasing distance to the seepage area, while the sediments with high hydrogen sulphide levels presented overall a high dominance of the Sabatieria spp. (Pape et al. 2011). The present study analyses the spatial and vertical distribution of the abundance and composition of the meiofauna communities, focusing on nematode standing stocks, structural and functional biodiversity in the newly found ATI MVs. Additionally, these results are compared with nematode assemblages of the Porto MV located within the AWCG and a site located at the Horseshoe Abyssal Plain (HAP), without evidence of mud volcanism.
The working hypothesis of this study is that there is a response of the standing stocks, structural and functional biodiversity of meiofauna and nematodes to different geological setting and fluids geochemical characteristics of the newly found ATI MVs. The measured pore water environmental parameters were used to identify the influence of the upward flowing fluids in buffering their chemical composition at the depths below sea floor colonised by the meiofauna.
Material and methods
Study area
The GoC straddles the Africa-Eurasia lithospheric plate boundary in the Atlantic Ocean in a geologically and tectonically complex area, the evolution of which includes Mesozoic rifting and oceanic drifting of the Atlantic and Tethyan oceans, subduction and almost complete consumption of the Tethys during the Alpine orogeny (Frizon de Lamotte et al. 2011) and the present day right-lateral oblique collision of Eurasia with respect to Africa (Gutscher et al. 2012). The present day tectonics are responsible for i) important moderate and high magnitude seismicity, ii) existence of morphologic features in the deep-sea floor such as mud volcanoes and the SWIM1 fault trace along which the ATI MVs were emplaced (Hensen et al. 2007).
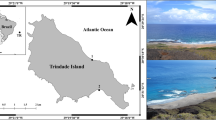
The studied sites include the ATI and Porto MVs, as well as a reference location in the HAP. The ATI MVs are located in the Horseshoe Valley, an area that lies between the HAP and the AWGC (Fig. 1a; Duarte et al. 2010), ~90 km west of the deformation front of the AWGC. Noteworthy, the ATI MVs were emplaced at the intersection of the main SWIM plate boundary strike-slip fault and the Horseshoe thrust fault (Rosas et al. 2015) on top of a cluster of seismicity (Geissler et al. 2010). These faults cut through lithospheric mantle and most of the crust, respectively, and are main pathways for fluid percolation in the area, leading to the development of seepage areas with associated mud volcanism (Hensen et al. 2015).
a) Overview of the sampling sites: Site 2, ATI and Porto MVs located in the Gulf of Cadiz and b) detailed side-scan sonar mapping showing the local geomorphological setting of the M. Ivanov MV and the sampled stations. AWGC: Accretionary Wedges of the Gulf of Cadiz; CPR: Coral Patch Ridge; GoB: Gorringe Bank; SWIMF: SWIM faults; HAP: Horseshoe Abyssal Plain; HF: Horseshoe Fault; HV: Horseshoe Valley. (Bathymetry data compiled from Zitellini et al., 2009)
During the RV Meteor cruise M86/5 the three discovered ATI MVs were extensively sampled (Fig. 1a). The M. Ivanov MV, located at approximately 4500 m water depth, is a complex of several cone-shaped elevations with a maximum of 50 m in diameter that extends 480 m in length approximately (Fig. 1b). The Abzu and the Tiamat MVs are located in an ESE-WNW bathymetric high at a distance of less than 4 km of each other; the Abzu MV has a circular shape with a diameter of approximately 100 m and a small depression at the top; the Tiamat MV (4650 m water depth) has an elliptical shape with the major axis (striking NW-SE) with 600 m and the minor axis with 420 m length. In the vicinities of both mud volcanoes the seafloor has abundant small depressions, resulting from fluid seepage (Hensen 2014). The sediments collected on the three mud volcanoes are mud-breccias overlain by a hemipelagic silty clay layer of variable thickness, with abundant forams. In addition, two other locations were sampled: at the Porto MV (a sub-circular mud volcano with a diameter of about 500 m located within the AWGC at 3880 m water depth), and from the Horseshoe Abyssal Plain (HAP) in the SWIM1 fault area without evidences of mud volcanism (4864 m water depth). The sedimentary cover in the Porto MV is similar to the one found in the ATI MVs (mud-breccia overlain by hemipelagic silty clay); the HAP sedimentary cover is made of an intercalation of turbidite deposits with silty clay hemipelagic sediments.
Sampling and sample processing
Samples were collected for the study of the mud volcanoes and the fluid migration along the SWIM1 fault using the following gears: multi-corer, box corer and gravity corer. Sampling sites were selected based on the previously available swath-bathymetry data and side-scan sonar coverage information. Five sites were sampled: the Porto MV, located on the AWGC, three MVs (Abzu, Tiamat and Ivanov) located outside the AWCG along the SWIM1 fault and Site 2, located in the HAP on the SWIM1 fault, without evidence of seepage. A total of nine stations were sampled without replication for meiofauna (Fig. 1a; Table 1). The largest number of samples was collected at the M. Ivanov MV, including three stations (st.) from an apparently active seepage crater (st. 329, 407 and 388) and one station from an apparently inactive (AUV observations) crater (st. 348; Fig1b). Abzu (st. 369 and 349), Tiamat (st. 339), Porto (st. 308) MVs samples were collected from apparently active seepage craters, while at Site 2 (st. 366) no seepage activity was observed. The sediment samples for meiofauna studies were obtained using a boxcorer at the three ATI MVs and Site 2 and a multicorer was used (MUC, Plexiglas tubes with 10 cm internal diameter) at Porto MV. Sediments of the boxcorer were then sub-sampled using a single MUC Plexiglas tube in order to obtain comparable sampled areas. Each sample was sliced into four sediment depths (0–1; 1–3; 3–5; 5–10 cm) subsequently fixed in borax-buffered 4% formalin. In the laboratory, meiofauna was extracted from the sediment using a density gradient solution in a centrifugation procedure (Heip et al. 1985). The fixed samples were rinsed through a 1000-μm mesh sieve followed by sieving through a 32-μm mesh. The retained 32–1000 μm fraction was washed and centrifuged three times using the colloidal silica polymer LUDOX HS-40 (specific gravity 1.19). The supernatant of each washing cycle was again collected on a 32-μm sieve. After extraction, the samples were kept in buffered 4% formalin and stained with Rose Bengal. All metazoan meiobenthic organisms were counted and classified to higher taxon level following.
(Higgins and Thiel 1988; Giere 2009) under a stereomicroscope (40× magnification). Subsequently, for nematode identification, 100 to 120 nematodes (or all nematodes when abundances were lower than 120 per sample) were picked out randomly and mounted on permanent glycerine slides after stepwise dehydration in a graded series of ethanol: glycerine mixtures (Seinhorst 1959). The nematodes were identified down to genus level using the pictorial keys (Platt and Warwick 1983, 1988; Warwick et al. 1998), online identification keys and relevant literature available on the Nemys Database Guilini et al. 2017). Nematode length (excluding tail tips; L (μm)) and maximum body width (W (μm)) were measured under a Olympus BX-50 compound microscope (1000× magnification) with Olympus Cell^D software. Biomass of all specimens was estimated by applying Andrassy’s formula and expressed as wet weight (wwt; Andrassy 1956) and a ratio of 0.25 was assumed to convert nematode wet weight into dry weight (dwt; Wieser 1960).
Characterisation of the environmental setting of the studied sites was performed through the analysis of pore water geochemistry as described in Hensen et al. (2015) on a total of five gravity cores obtained at Site 2, and at ATI and Porto MVs at the same locations of the biological sampling. Each core was sliced at several sediment depths (between 2 cm, 20 cm until the end of the core) and the pore water was extracted from the sediments at ambient temperature using a squeezing device that is operated with argon gas at a pressure up to 5 bar. Upon extrusion from the sediment matrix, the pore water was filtered through 0.2 μm cellulose acetate membrane filters. The chlorine concentration (measured as Cl−) was immediately measured on board by ion chromatography. Total alkalinity (TA) was determined by titration with 0.02 M HCl (Ivanenkov and Lyakhin 1978). Both chlorine concentration and total alkalinity were used to identify the influence of upward flowing fluids in the buffering of pore-water composition at depths colonised by the meiofauna.
Data analysis
Meiofauna abundances (individuals per 10 cm2) of each major taxon and their relative contribution was calculated for each sampling station. Since nematodes represent the major part of the metazoan meiofauna, this group was thoroughly investigated. A nematode genera dataset was used to examine community composition and diversity using Hill’s (1973) diversity numbers (N0, N1, N2 and Ninf). Furthermore, rarefaction curves were calculated from the Hurlbert expected number of genera at each station (EG(n); Hurlbert 1971). PRIMER v6 software (Clarke and Gorley 2006) was used to compute all the diversity measures. To investigate the trophic composition and diversity of the nematode assemblages, nematodes genera were assigned to one of the four functional feeding groups, designated by Wieser (1953), based on the buccal cavity morphology: selective (1A) and non-selective deposit feeders (1B), epigrowth feeders (2A), and predators/scavengers (2B). An additional feeding group, Parasitic, was considered in our study attributed to the adult parasitic nematodes belonging to the family Benthimermithidae (Miljutin 2004). The index of trophic diversity (Heip et al. 1984) was then calculated as: ITD = Σ θ2, where θ is the relative contributions of each trophic group to total nematode abundance. The reciprocal value of the trophic index (θ−1) was used, so that the higher values of the index correspond to higher trophic diversity, ranging from 1 (lowest) to 5 (highest value), considering the five feeding groups included. Nematode genera were assigned a value on a coloniser–persister scale (c–p scale) from 2 (colonisers) to 5 (persisters). Taxa with rapid growth and reproduction and usually high tolerance to disturbance are considered colonisers, whereas persisters are slow-growing and often more sensitive taxa which thrive well in fairly undisturbed environmental conditions (Bongers 1990; Bongers et al. 1991). The MI was then calculated as the weighted average of the individual coloniser–persister (c–p) scores as MI = Σν(i).f(i), where ν(i) is the c–p value of the taxon i and f(i) is the frequency of that taxon.
PRIMER v6 software package (Clarke and Gorley 2006) was used to i) perform multivariate analysis in order to detect the spatial and vertical distribution patterns of the meiofauna and nematode assemblages following the procedures presented by Clarke (1993) and ii) compute Hill’s diversity numbers and the rarefaction curves EG (n). The similarity in communities among sampling sites and along sediment depths were analysed by non-metric multidimensional scaling (nMDS) ordination. Prior to analysis, the meiofauna and nematode abundance data were fourth-root transformed for the calculation of the resemblance matrix based on the Bray-Curtis similarity measure. One-way analysis of similarity (ANOSIM) was performed to test for differences in the assemblages between the sediment depths across the sampling stations.
Results
Characterisation of the environment
The sediment recovered from the ATI and Porto mud volcanoes were similar, characterised by an uppermost layer of hemipelagic light olive brown to olive brown silty clay with abundant forams overlaying a mud-breccia (Hensen 2014; Hensen et al. 2015). The hemipelagites showed a variable thickness with 7 cm at Porto MV, 118 cm and 190 cm at Abzu MV, 32 cm and 270 cm at the Tiamat MV and 46 cm to close to 0 cm at the M. Ivanov MV. At Site 2 (the control station), the cover sediment is also an olive brown silty clay intercalated with turbidite deposits. The chemical composition of the fluids driving the mud volcanism along the SWIM1 fault resulted from a complex mix of clay-minerals dehydration, limestone recrystallization and fluid-rock interactions with the underlying oceanic crust (Hensen et al. 2015). The Cl− concentrations of pore water at 2 cm depth (the only analysed depth compatible with the meiofauna vertical distribution) show that the different sampling stations have similar values ranging between 551 and 561 mM and also the TA values were similar at the different sampling stations, ranging between 2.1 and 2.3 m eql−1. Pore water Cl− vertical profiles at the mud volcanoes show a mixing trend between deep-seated fluids and bottom seawater (Hensen et al. 2015), whereas at Site 2 no such mixing relationship is observed (Fig. 2a). The peak in TA at the mud volcanoes (Fig. 2b) is a result of anaerobic oxidation of methane (AOM) coupled to sulphate reduction. At Site 2, TA increases throughout the sampled depth interval suggesting that the depth of AOM is located at greater sediment depth. Due to downward mixing and diffusive exchange, Cl− and TA show near-bottom seawater concentrations in the uppermost tens of centimetres at both the mud volcanoes and the control station.
Metazoan meiofauna
Metazoan meiofauna composition and abundances at each sampled station is presented in Table 2. Considering all sampling stations, sixteen major taxa were identified and nematode genera were always the dominant taxon (85.4–96.7%), followed by harpacticoid copepods (2.3–8.5%) and nauplli larvae (0.4–4%). The remaining taxa comprised less than 1% of the total meiofauna abundance. Meiofauna abundance generally presented low values, ranging between 125.5 ind.10 cm−2 (st. 329) and 364.3 ind.10 cm−2 (st. 348), both located at M. Ivanov MV (Fig. 3). The vertical distribution of the meiofauna abundance across all sampling stations showed a typical vertical distribution pattern of meiofaunal assemblages with the upper 5 cm sediment depth harbouring most of the total abundances (70% – 91%), whereas the highest abundances were found at the surface layer (0–1 cm; Fig. 4).
Nematodes
Nematode assemblages: density
A total of 69 nematode genera belonging to 28 families were identified (Table 3). The most abundant families were Chromadoridae (14–27.0%), Desmoscolecidae (9–19%), Monhysteridae (9–18%), Xyalidae (5–15%), Leptolaimidae (3–13%) and Comesomatidae (0–14%). The 15 most common nematode genera encountered (72% of all individuals) were: Acantholaimus, Monhystrella, Halalaimus, Leptolaimus, Thalassomonhystera, Syringolaimus, Sabatieria, Metadesmolaimus, Aegialoalaimus, Metadesmolaimus, Desmoscolex, Aegialoalaimus, Metasphaerolaimus, Microlaimus, Tricoma, Daptonema and Diplopeltula with abundances ranging between 3% and 23% (Table 3). The remaining genera (28% of all individuals) contributed with <3% to the total abundances each. The multidimensional scaling analysis ordination (nMDS) of the nematodes dataset reveals a high variability among sampling stations; nevertheless, a distinct assemblage was identified at Porto MV, based on the composition and abundance of the nematode populations (Fig. 5a). At the Porto MV the families Comesomatidae and Linhomoeidae, together with Monhysteridae and Chromadoridae contributed to 58% of the total nematode assemblages and the higher abundances were of the genera Sabatieria (13%), Linhomoeus (12%) and Halichoanolaimus (6%), the genus Syringolaimus was absent. Despite the variability of nematode assemblages within the ATI MVs and Site 2, these sites were mostly represented by the families Chromadoridae, Desmoscolecidae and Monhysteridae with 59% at ATI MVs and 63% at Site 2. The dominant nematode genera at these sites included Acantholaimus (16–23%), Monhystrella (3–10%), Halalaimus (3–10%), Thalassomonhystera (2–7%) and Syringolaimus (3–13%). The ordination also identified some variation in nematode abundance and composition within the sampling stations of the M. Ivanov MV, explained by the genus Sabatieria (Table 3); however, no obvious spatial pattern was detected reflecting the distance to the active crater.
On the other hand, there was a clear vertical pattern of the nematode assemblage composition and abundance. The nMDS ordinations and the ANOSIM test allowed distinguishing between the nematode assemblages of the uppermost and the deeper sediment layers because significant differences were obtained between the sediments depths (R = 0.383; p = 0.001%; Fig. 5b). The detected vertical pattern is mainly explained by the genera Acantholaimus and Metadesmolaimus, that decrease in their relative abundance with depth and by the genera Monhystrella, which is the most abundant genus at 3–5 cm sediment depth.
Nematode assemblages: structural and functional diversity
Table 4 shows the nematode assemblages structural and functional diversity in each sampling station. Genera richness and structural diversity based on the values of the Hill’s diversity were generally high. The highest values were obtained at Abzu MV sampling stations and Site 2, while the Porto MV registered the lowest diversity (N0, N1) and the higher dominance (Ninf). The expected number of genera (EG) (Hurlbert 1971), determined by the rarefaction curves, confirmed the diversity distribution of the sampling stations: the Abzu MV and Site 2 presented the highest expected diversity, followed by the Tiamat and M. Ivanov MVs, while the Porto MV registered the lowest expected diversity (Fig. 6).
The Index of Trophic Diversity, ITD (Θ−1) ranged from 3.31 to 3.60 (Tiamat MV and M. Ivanov MV respectively), indicating high trophic diversity and low variability between the locations regarding trophic composition, as the assemblages were characterised by high abundances of selective-deposit feeders (1A) (Table 4; Fig. 7a). The Tiamat MV registered the lowest value of ITD (Θ−1), while the M. Ivanov MV presented the highest values (Table 4). At Site 2, Tiamat and Abzu MVs selective-deposit feeders (1A) represented the highest abundance followed by epigrowth feeders (2A), non-selective deposit feeders (1B) and predator/scavenger (2B). At the sampling stations 329 and 388 of the Ivanov MV the nematode assemblages were also characterised by high abundances of selective-deposit feeders (1A), although at station 407 (of the same MV) non-selective deposit feeders (1B) have similar proportion; while at station 348 epigrowth feeders (2A) had the higher relative abundance. At Porto MV the non-selective deposit feeders (1B) presented the highest relative abundances followed by the epigrowth feeders (2A). The predator/scavenger (2B) were characterised by lower abundances in all stations. Parasitic nematodes were found at Tiamat (st. 339) and M. Ivanov MVs (st. 407 and st 348) representing <0.1% of the nematode abundance.
The Maturity Index (MI) ranged from 2.57 (Porto MV) to 3.01 (Site 2). Most of the nematodes species showed a c-p score of 2, described as “general opportunists” followed in abundance by species with a c-p score of 4, described as “persisters” (Table 4). At Site 2, the “general opportunists” (c-p score 2) and “persisters” (c-p score 4) registered similar proportions, 36 and 33%, respectively. The ATI MVs were characterised by “general opportunists”, with the abundance of species with c-p score 2 ranging between 41 and 58%, followed by “persisters”, with an abundance of c-p score 4 species varying between 21 to 32%. Porto MV showed the lowest values of MI, with the “general opportunists” species with c-p score 2 also representing the highest proportion (64%); “opportunist” nematode species with c-p score 1 represented 15% of the assemblage and the “persisters” with c-p score 4 represented 11%.
Nematodes assemblages: morphometrics and biomass
The lowest values of total and average individual nematode biomass were obtained at the Site 2 (13.57 μg C.10 cm−2), followed by the Abzu MV, with 18.87 μg C.10 cm−2 (st. 369) and 19.33 μg C.10 cm−2 (st. 349) (Table 2). The highest value of total biomass of 97.65 μg C. 10 cm−2 was found at M. Ivanov (st. 407), mostly attributed to the presence of large epigrowth feeders (2A) and predator/scavengers (2B) despite the low relative abundances of these trophic groups. Still, Porto MV showed the highest value of the average individual biomass (Table 2). Tiamat MV (st. 339) presented also high biomass values and low abundance of the predator/scavenger (2B), indicating a high proportion of larger nematodes (Fig. 7). These observations are supported by a scatter plot of the length versus width measures presented in Fig. 8 where it is clear that the longest and largest individuals are found at the mud volcanoes particularly the M. Ivanov, Tiamat and Porto MVs.
Discussion
The GoC includes several mud volcanoes within and outside of the AWGC with a complex tectonic setting and considerable variation in their morphology, biogeochemical composition and origin of the percolating fluids associated (e.g. Pinheiro et al. 2003; Hensen et al. 2007, 2015; Medialdea et al. 2009; Nuzzo et al. 2009; Scholz et al. 2009, 2010). Notably, the fluid seepage activity associated with the mud volcanism is moderate in this area, with advection velocities up to 15 cm year.− 1 (Hensen et al. 2007) and consequently, there are few and scattered large habitat forming aggregations associated with chemosynthetic processes (Cunha et al. 2013; Rodrigues et al. 2013; Vanreusel et al. 2009).
Although the chemical composition of the fluids (e.g. strontium, lithium, oxygen and hydrogen isotopic ratios and boron content) indicate different provenances when comparing the ATI MVs and the Porto MV (Scholz et al. 2009), as shown by Hensen et al. (2015), the chemical composition of the sediment pore-water of the upper 50 to 80 cm contain fluids whose composition is close to the near-bottom seawater namely on Cl− and TA. Although SO4 2−, NH4 +, PO4 4− and B contents were also determined and the results are consistent with the results of Cl− concentrations and TA they are not used in this discussion since the values are less reliable. More, Cl− is considered to be geochemically conservative and a useful indicator of fluid sources and migration patterns (Aloisi et al. 2004; Haese et al. 2006; Martin et al. 1996). The depth below which the upward flowing fluids buffers the pore-water chemical composition is considerably deeper than the depth colonised by the meiofauna (Giere 2009). Similar observations of the upper portion of the sedimentary column having the chemical composition of pore-waters similar to the near-bottom sea water conditions were obtained at the Darwin MV where an overall low seepage activity occurs (Vanneste et al. 2012). However, the change of Cl−content and TA with depth shows the differences in pore-water chemical composition of the fluids among the studied MVs (Fig. 2.). The evident differences between the gradients of the vertical profiles are related to variations in the pore-water composition at depth and different upward advection velocities at the different locations.
The present work focus on the meiofauna abundances in the newly ATI MVs determined by the nematode abundances. The high proportion of the nematodes in meiofauna assemblages is frequently observed in marine and deep-sea sediments, including in the submarine mud volcanoes (e.g. Vanreusel et al. 2010a, 2010b; Pape et al. 2011; Lampadariou et al. 2013). However, in this study the abundances registered are lower when compared with previous studies in mud volcanoes. For instance in MVs located about 5000 m depth in Barbados accretionary wedge, the meiofauna abundances ranged between 116 and 6541 ind. 10 cm−2 (Olu et al. 1997), in the Hakon Moby MV located around 1280 m depth (West Barent Sea) average abundances were 968 ± 154 ind. 10 cm−2 (Van Gaever et al. 2009a) and in the Darwin MV, located at approximately 1100 m depth in the GoC, the abundances ranged between 248 and 3227 ind. 10 cm−2 (Pape et al. 2011). In Site 2, located in the Horseshoe Abyssal Plain where morphological and chemical evidences of seepage are absent, the meiofauna abundances obtained were similar to the ATI MVs; however, the total and individual nematode biomasses were lower, falling within the range of the observed abundances in other deep-sea sediments of the Iberian Margin with the same bathymetric range (Rachor 1975; García et al. 2007; Ingels et al. 2011). Nematode densities and biomass generally decrease with increasing water depth (Levin et al. 2001; Giere 2009; Vanreusel et al. 2010b), as the availability of food decreases, an important driving factor to the nematode standing stocks. On the contrary, it is expected that the in situ chemosynthetic production may lead to higher nematode densities and biomass when compared to non-seep sediments (Vanreusel et al. 2010a), since they can benefit from the increased bacterial production compared to adjacent sites (Olu et al. 1997; Van Gaever et al. 2009a). Nevertheless, in some cases, very low abundances can also be observed (Shirayama and Ohta 1990; Levin 2005; Vanreusel et al. 2010a). It is unclear what the main drivers are of these variations, but it has been argued that a combination of food availability, variable seep intensity, and the presence of soft substrates influence the observed variability (Vanreusel et al. 2010a). The general observation of the low densities at the ATI MVs, Porto MV and Site 2 suggest that nematode assemblages respond to low bioavailability of the organic matter (OM) usually associated with great water depths (Vanreusel et al. 2010b), in these sites where, as evidenced by the environmental factors, the sediment offers similar conditions to the near-bottom seawater with no evidence of the seepage influence.
The diversity of benthos is generally high in deep-sea sediments, with low dominance and high number of genera and species. In opposition, seep sediments are often dominated by a single or few species, representing 50–90% of the total community (Vanreusel et al. 2010a). Despite the lack of clear spatial patterns of nematode abundances, biodiversity displayed clear differences throughout the sampling sites, particularly at the Porto MV located at the AWGC, which showed the lowest diversity and highest dominance of genera classified as “general opportunists” (lower MI index). At the Porto MV, the high proportion of Comesomatidae and Linhomoedae families occurred with the presence of Chromadoridae and Monhysteridae. Higher proportions of Sabatieria at the Porto MV, are also well represented in known seep sediments and are known to be tolerant to seep toxic conditions (e.g.; Pape et al. 2011; Zeppilli et al. 2011; Zeppilli et al. 2012; Lampadariou et al. 2013).
In addition, it is noteworthy that cold seeps have low nematode diversity and high dominance of single species usually represented by large body sized nematodes (Lampadariou et al. 2013; Vanreusel et al. 2010a). This is also observed at the Porto MV, where the highest individual biomasses were found. In contrast, at the ATI MVs and at Site 2, located outside the AWGC, we observed an overall higher diversity and higher proportion of genera classified as “persisters” (highest MI index),and a dominance of the genera Acantholaimus, Monhystrella, Halalaimus, Leptolaimus and Thalasomonhystera, which typically are present in high relative abundances in several other deep-sea sediments along the Iberian Margin (e.g. Vanreusel et al. 2010b; Ingels et al. 2011; Ramalho et al., 2014). The lower genera richness and diversity, as well as the presence of higher dominance species considered “general opportunistics” could be driven by the distinct seepage conditions of the Porto MV with respect to the ATI MV and Site 2, where the composition is comparable with other deep-sea floor habitats.
The Trophic Diversity (ITD) of the nematode assemblages are high, but failed to discriminate nematode communities between sampling stations, as most of them belong to deposit feeders. The large majority of benthic nematodes in deep-sea sediments are deposit feeders while the predators are usually less abundant, as also happens in seeps and vents (Vanreusel et al. 2010a). Deep-sea benthic organisms must adapt to a food-poor environment where food sources are essentially provided by hemipelagic detritus whose influx rapidly decreases with depth. The use of photosynthetic and chemosynthetic derived carbon sources coupled with their spatial and trophic specialisation lead to the high diversity and biomass patterns of meiofauna (nematodes) distribution (Van Gaever et al. 2009).
The vertical distribution of meiofauna assemblages showed a typical pattern with gradually decreasing densities at deeper layers. The significant differences in the nematode community composition through the sediment depth layers in all sampled stations are the typical response to low oxygen concentration, high concentration of the reduced chemical compounds, and a decrease of food availability (Giere 2009; Vanreusel et al. 1997; Pape et al. 2011; Lampadariou et al. 2013).
The higher number of samples collected at the M. Ivanov MV provides further information on the faunal spatial distribution for this specific mud volcano. Meiofauna abundances and nematode composition at M. Ivanov MV were highly variable, when compared to the other studied mud volcanoes. Meiofauna abundances increased more than 50% at the station located in the inactive crater, and the highest proportion was found in the top sediment layer. The different nematode composition in the four studied stations of the M. Ivanov MV result from the variable abundance proportion of the tolerant species genus Sabatieria reflecting the high variable conditions among stations. The main environmental factors and their interactions can drive small-scale variability at seeps, such as the microhabitat heterogeneity, the food availability, the presence of larger infaunal organisms as ecosystems engineering, predation effects and the negative influence of the fluid flow in more active areas that comprised the dominance of the tolerant and opportunistic genus (Van Gaever et al. 2009a, 2009b; Vanreusel et al. 2010a; Zeppilli et al. 2011; Pape et al. 2011; Zeppilli et al. 2012; Lampadariou et al. 2013).
It should be, however, born in mind that the physical environmental conditions at the seafloor in this study area are barely known. Interpretation of multibeam data shows that the Horseshoe Valley is an area of high bottom hydrodynamics with juxtaposed areas of sediment aggradation, erosion and progradation (Duarte et al. 2010). Numerical modelling of seawater bottom velocities also shows the existence of important gradients near morphologic reliefs in the study area (Argus et al. 1989; Hernandez-Molina et al. 2011). The physical conditions can vary significantly and affect the nature of sediments, namely the sediment grain size, which has profound effects on standing stocks, and structural and functional diversity of meiobentic communities, directly and indirectly via the regulation of the chemical environment (Schratzberger & Ingels, 2017). More sediment sampling for biology, physical analysis of the sediments and chemical analysis from deep water is needed to provide important information on what drivers diversity in these environments.
Conclusions
The results from this study support previous observation that meiofauna and nematodes assemblages at cold seeps and mud volcanoes is strongly variable at the local scale. The great variety of geological, chemical, hydrodynamic and ecological conditions generate many different complex habitats in the deep-sea sediments. As a result, the heterogeneity of these habitats harbours specific nematode assemblages. The spatial and vertical distribution of the meiofauna and nematode assemblages of the uppermost 10 cm of the sediment of the newly discovered ATI MVs showed similarities with assemblages collected in the other deep-sea environments at comparable water depths. These communities were mainly coupled with characteristics of the bottom seawater and showed little evidence of specialised fauna benefiting from high “in situ” primary production.
Nevertheless, it is possible, based on the nematodes assemblages standing stocks (particularly biomass), structural and functional biodiversity, to speculate that the seep activity of the ATI MVs (out of the AWGC) is less intense than in the Porto MV located in the AWGC, where the communities are characterised by lower abundance and diversity, but higher proportion of “opportunistic species”, high dominance of single species and high individual biomass, typical from deep-sea cold seeps.
Abbreviations
- ATI MVs:
-
Abzu, Tiamat and M. Ivanov mud volcanoes
- AGFZ:
-
Azores-Gibraltar Fracture Zone
- AWGC:
-
Accretionary Wedge of the Gulf of Cadiz
- SWIM:
-
South-West Iberia Margin strike-slip faults
- HAP:
-
Horseshoe Abyssal Plain
References
Andrassy I (1956) Die Rauminhalts- und Gewichtsbestimmung der Fadenwürmer (Nematoden). Acta Zool 2:1–15
Aloisi G, Wallmann K, Bollwerk SM, Derkachev A, Bohrmann G, Suess E (2004) The effect of dissolved barium on biogeochemical processes at cold seeps. Geochim Cosmochim Acta 68(8):1735–1748. https://doi.org/10.1016/j.gca.2003.10.010
Argus DF, Gordon RG, DeMets C, Stein S (1989) Closure of the Africa-Eurasia-North America plate motion circuit and tectonics of the Gloria fault. J Geophys Res: Solid Earth 94:5585–5602. https://doi.org/10.1029/JB094iB05p05585
Bongers T (1990) The maturity index: an ecological measure of environmental disturbance based on nematode species composition. Oecologia 83:14–19. https://doi.org/10.1007/BF00324627
Bongers T, Alkemade R, Yeates GW (1991) Interpretation of disturbance-induced maturity decrease in marine nematode assemblages by means of the maturity index. Mar Ecol Prog Ser 76:135–142
Bright M, Plum C, Riavitz LA, Nikolov N, Arbizu PM, Cordes EE, Gollner S (2010) Epizooic metazoan meiobenthos associated with tubeworm and mussel aggregations from cold seeps of the northern Gulf of Mexico. Deep-Sea Research Part II: Topical Studies in Oceanography 57:1982–1989. https://doi.org/10.1016/j.dsr2.2010.05.003
Buck KR, Barry JP (1998) Monterey Bay cold seep infauna: quantitative comparison of bacterial mat meiofauna with non-seep control sites. Cah Biol Mar 39:333–335
Clarke KR (1993) Nonparametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Clarke KR, Gorley RN (2006) PRIMER version 6: user manual/tutorial. PRIMER-E, Plymouth, UK, p 192
Cordes EE, Cunha MR, Galéron J, Mora C, Olu-Le Roy K, Sibuet M, Van Gaever S, Vanreusel A, Levin LA (2010) The influence of geological, geochemical, and biogenic habitat heterogeneity on seep biodiversity. Mar Ecol 31:51–65. doi: https://doi.org/10.1111/j.1439-0485.2009.00334.x
Cunha MR, Rodrigues CF, Génio L, Hilário A, Ravara A, Pfannkuche O (2013) Macrofaunal assemblages from mud volcanoes in the Gulf of Cadiz: abundance, biodiversity and diversity partitioning across spatial scales. Biogeosciences 10:2553–2568. https://doi.org/10.5194/bg-10-2553-2013
Dimitrov LI (2002) Mud volcanoes - the most important pathway for degassing deeply buried sediments. Earth-Sci Rev 59:49–76. https://doi.org/10.1016/S0012-8252(02)00069-7
Duarte JD, Terrinha P, Rosas FM, Valadares V, Pinheiro LM, Matias L, Magalhães V, Roque C (2010) Crescent-shaped morphotectonic features in the Gulf of Cadiz (offshore SW Iberia). Mar Geol 271:236–249. https://doi.org/10.1016/j.margeo.2010.02.017
Frizon de Lamotte D, Raulin C, Mouchot N, Wrobel-Daveau J-C, Blanpied C, Ringenbach J-C (2011) The southernmost margin of the Tethys realm during the Mesozoic and Cenozoic: initial geometry and timing of the inversion processes. Tectonics 30(3). https://doi.org/10.1029/2010TC002691
García R, Koho KA, de Stigter HC, Epping E, Koning E, Thomsen L (2007) Distribution of meiobenthos in the Nazare canyon and adjacent slope (western Iberian margin) in relation to sedimentary composition. Mar Ecol Prog Ser 340:207–220. https://doi.org/10.3354/meps340207
Geissler WH, Matias L, Stich D, Carrilho F, Jokat W, Monna SIbenBrahim A, Mancilla F, Gutscher MA, Sallàres, V, Zitellini N (2010) Focal mechanisms for sub-crustal earthquakes in the Gulf of Cadiz from a dense OBS deployment. Geophys Res Lett, 37:L18309. https://doi.org/10.1029/2010GL044289
Génio L, Warén A, Matos FL, Cunha MR (2013) The snails tale in deep-sea habitats in the Gulf of Cadiz (NE Atlantic). Biogeosciences 10:5159–5170. https://doi.org/10.5194/bg-10-5159-2013
Giere O (2009) Meiobenthology: the microscopic motile Fauna of aquatic sediments. Springer-Verlag, Berlin, Heidelberg
Guilini K, Bezerra T N, Eisendle-Flöckner U, Deprez T, Fonseca G, Holovachov O, Leduc D, Miljutin D, Moens T, Sharma J, Smol N, Tchesunov A, Mokievsky V, Vanaverbeke J, Vanreusel A, Venekey V, Vincx M (2017) NeMys: world database of free-living marine nematodes. Accessed at http://nemys.ugent.be on 2017-06-12
Gutscher MA, Dominguez S, Westbrook G, Le Roy P, Rosas, F, Duarte J, Terrinha P, Miranda JM, Graindorge D, Gailler A, Sallares V (2012). The Gibraltar subduction: a decade of new geophysical data. Tectonophysics 574:72–91. https://doi.org/10.1016/j.tecto.2012.08.038
Gutscher MA, Malod J, Rehault J-P, Contrucci I, Klingelhoefer F, Mendes-Victor L, Spakman W (2002) Evidence for active subduction beneath Gibraltar. Geology 30:1071–1074. https://doi.org/10.1130/0091-7613
Haese RR, Hensen C, de Lange GJ (2006) Pore water geochemistry of eastern Mediterranean mud volcanoes: implications for fluid transport and fluid origin. Mar Geol 225(1):191–208. https://doi.org/10.1016/j.margeo.2005.09.001
Heip C, Herman R, Vincx M (1984) Variability and productivity of meiobenthos in the southern bight of the North Sea. Rapports et procès-verbaux des réunions / Conseil permanent international pour l'exploration de la mer 183:51–56
Heip C, Vincx M, Vranken G (1985) The ecology of marine nematodes. Oceanogr Mar Biol 23:399–489
Hensen C (2014). TransFlux-tracing for active dewatering sites along deep-reaching transform faults in the western gulf of Cadiz: METEOR cruise M86/5, February 23–march 16, 2012, Palma de Mallorca (Spain) – Lisbon (Portugal), DFG Senatskommission für Ozeanographie
Hensen C, Nuzzo M, Hornibrook E, Pinheiro LM, Bock B, Magalhães VH, Brückmann W (2007) Sources of mud volcano fluids in the Gulf of Cadiz—indications for hydrothermal imprint. Geochim Cosmochim Acta 71:1232–1248. https://doi.org/10.1016/j.gca.2006.11.022
Hensen C, Scholz F, Nuzzo M, Valadares V, Gràcia E, Terrinha P, Liebetrau V, Kaul N, Silva S, Martinez-Loriente S, Bartolome R, Pinero E, Magalhães VH, Schmidt M, Weise SM, Cunha M, Hilário A, Perea H, Rovelli L, Lackschewitz K (2015) Strike-slip faults mediate the rise of crustal-derived fluids and mud volcanism in the deep sea. Geology 43:339–342. https://doi.org/10.1130/G36359.1
Hernandez-Molina FJ, Serra N, Dorik AVS, Llave E, Ercilla G, VanRooij D (2011) Along-slope oceanographic processes and sedimentary products around the Iberian margin. Geo-Mar Lett 31(5–6):315–341. https://doi.org/10.1007/s00367-011-0242-2
Higgins RP, Thiel H (1988) Introduction to the study of Meiofauna. Smithsonian Institution Press, London, UK
Hilário A, Johnson SB, Cunha MR, Vrijenhoek RC (2010) High diversity of frenulates (Polychaeta: Siboglinidae) in the Gulf of Cadiz mud volcanoes: a DNA taxonomy analysis. Deep-Sea Research Part I: Oceanographic Research Papers 57:143–150. https://doi.org/10.1016/j.dsr.2009.10.004
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432. https://doi.org/10.2307/1934352
Hurlbert SH (1971) The nonconcept of species diversity: a critique and alternative parameters. Ecology 52(4):577–586. https://doi.org/10.2307/1934145
Ingels J, Billett DSM, Kiriakoulakis K, Wolff GA, Vanreusel A (2011) Structural and functional diversity of Nematoda in relation with environmental variables in the Setúbal and Cascais canyons, western Iberian margin. Deep-Sea Res II Top Stud Oceanogr 58:2354–2368. https://doi.org/10.1016/j.dsr2.2011.04.002
Ivanenkov VN, Lyakhin YI (1978) Determination of total alkalinity in seawater. In: Bordovsky OK, Ivanenkov VD (eds) Methods of Hydrochemical investigations in the ocean. Nauka Publishing House, Moscow, pp 110–114 (in Russian)
Kopf AJ (2002) Significance of mud volcanism. Rev Geophys 40(2):1–52. https://doi.org/10.1029/2000RG000093
Lampadariou N, Kalogeropoulou V, Sevastou K, Keklikoglou K, Sarrazin J (2013) Influence of chemosynthetic ecosystems on nematode community structure and biomass in the deep eastern Mediterranean Sea. Biogeosciences 10(8):5381–5398. https://doi.org/10.5194/bg-10-5381-2013
León R, Somoza L, Medialdea T, Vázquez JT, González FJ, López-González N, Casas D, Pilar Mata M, Fernández-Puga MC, Giménez-Moreno CJ, Díaz-del-Río V (2012) New discoveries of mud volcanoes on the Moroccan Atlantic continental margin (gulf of Cádiz): morpho-structural characterization. Geo-Mar Lett 32:473–488. https://doi.org/10.1007/s00367-012-0275-1
Levin LA, Etter RJ, Rex MA, Gooday AJ, Smith CR, Pineda J, Stuart CT, Hessler RR, Pawson D (2001) Environmental influences on regional deep-sea species diversity. Annu Rev Ecol Syst 32:51–93. https://doi.org/10.1146/annurev.ecolsys.32.081501.114002
Levin LA (2005) Ecology of cold seep sediments: interactions of fauna with flow, chemistry and microbes. Oceanogr Mar Biol 43:1–46. https://doi.org/10.1201/9781420037449.ch1
Martin JB, Kastner M, Henry P, Le Pichon X, Lallement S (1996) Chemical and isotopic evidence for sources of fluids in a mud volcano field seaward of the Barbados accretionary wedge. J Geophys Res: Solid Earth 101(B9):20325–20345. https://doi.org/10.1029/96JB00140
Medialdea T, Somoza L, Pinheiro LM, Fernández-Puga MC, Vázquez JT, León R, Ivanov MK, Magalhães V, Díaz-del-Río V, Vegas R (2009) Tectonics and mud volcano development in the Gulf of Cádiz. Mar Geol 261:48–63. https://doi.org/10.1016/j.margeo.2008.10.007
Miljutin DM (2004) New findings of deep-sea nematodes of genus Benthimermis Petter, 1980 (Nematoda, Benthimermithidae) with description of seven new species. Zoosystema 26:21–48
Milkov AV (2000) Worldwide distribution of submarine mud volcanoes and associated gas hydrates. Mar Geol 167:29–42. https://doi.org/10.1016/S0025-3227(00)00022-0
Nuzzo M, Hornibrook ERC, Gill F, Hensen C, Pancost RD, Haeckel M, Reitz A, Scholz F, Magalhães VH, Brückmann W, Pinheiro LM (2009) Origin of light volatile hydrocarbon gases in mud volcano fluids, gulf of Cadiz — evidence for multiple sources and transport mechanisms in active sedimentary wedges. Chem Geol 266:350–363. https://doi.org/10.1016/j.chemgeo.2009.06.023
Olu K, Lance S, Sibuet M, Henry P, Fiala-Medioni A, Dinet A (1997) Cold seep communities as indicators of fluid expulsion patterns through mud volcanoes seaward of the Barbados accretionary prism. Deep-Sea Res I Oceanogr Res Pap 44:811–841. https://doi.org/10.1016/S0967-0637(96)00123-9
Pape E, Nara Bezerra T, Vanneste H, Heeschen K, Moodley, L, Leroux F, van Breugel P, Vanreusel A (2011) Community structure and feeding preference of nematodes associated with methane seepage at the Darwin mud volcano (gulf of Cádiz). Mar Ecol Prog Ser 23:71–83. https://doi.org/10.3354/meps09278
Pinheiro LM, Ivanov MK, Sautkin A, Akhmanov G, Magalhães VH, Volkonskaya A, Monteiro JH, Somoza L, Gardner J, Hamouni N, Cunha MR (2003) Mud volcanism in the Gulf of Cadiz: results from the TTR-10 cruise. Mar Geol 195:131–151. https://doi.org/10.1016/j.margeo.2014.06.006
Platt HM, Warwick RM (1983) Freeliving marine nematodes. Part 1: British enoplids. Pictorial key to world genera and notes for the identification of British species. Cambridge University press, for the Linnean Society of London and the estuarine and brackish-water sciences association
Platt HM, Warwick RM (1988) Free-living marine nematodes. Part II: British chromadorids. Brill/Backhuys, for the Linhe Linnean Society of London and the Estuarine and Brackish-Water Sciences Association
Rachor E (1975) Quantitative Untersuchungen über das Meiobenthos der nordostatlantischen Tiefsee. Meteor Forschungsergeb. Reihe D 21:1–10
Ramalho SP, Adao H, Kiriakoulakis K, Wolff GA, Vanreusel A, Ingels J (2014) Temporal and spatial variation in the Nazare canyon (western Iberian margin): inter-annual and canyon heterogeneity effects on meiofauna biomass and diversity. Deep Sea Res I Oceanogr Res Pap 83:102-114. https://doi.org/10.1016/j.dsr.2013.09.010
Robinson CA, Bernhard JM, Levin LA, Mendoza GF, Blanks JK (2004) Surficial hydrocarbon seep infauna from the Blake ridge (Atlantic Ocean, 2150 m) and the Gulf of Mexico (690–2240 m). Mar Ecol 25:313–336. https://doi.org/10.1111/j.1439-0485.2004.00034.x
Rodrigues CF, Hilário A, Cunha MR (2013) Chemosymbiotic species from the Gulf of Cadiz (NE Atlantic): distribution, life styles and nutritional patterns. Biogeosciences 10:2569–2581. https://doi.org/10.5194/bg-10-2601-2013
Rodrigues CF, Paterson GP, Cabrinovic A, Cunha MR (2011) Deep-sea ophiuroids (Echinodermata) from mud volcanoes in the Gulf of Cadiz (NE Atlantic). Zootaxa 2754:1–26. https://doi.org/10.5281/zenodo.276727
Rosas FM, Duarte JC, Schellart WP, Tomás R, Grigorova V, Terriinha P (2015) Analogue modelling of different angle thrust-wrench fault interference in a brittle medium. J Struct Geol 74:81–104. https://doi.org/10.1016/j.jsg.2015.03.005
Sallarès V, Gailler A, Gutscher MA, Graindorge D, Bartolomé R, Gracia E, Diaz J, Dañobeitia JJ, Zitellini N (2011) Seismic evidence for the presence of Jurassic oceanic crust in the central gulf of Cadiz (SW Iberian margin). Earth Planet Sci Lett 311(1):112–123. https://doi.org/10.1016/j.epsl.2011.09.003
Scholz F, Hensen C, Reitz A, Romer RL, Liebetrau V, Meixner A, Weise SM, Haeckel M (2009) Isotopic evidence (87Sr/86Sr, d7Li) for alteration of the oceanic crust at deep-rooted mud volcanoes in the Gulf of Cadiz, NE Atlantic ocean. Geochim Cosmochim Acta 73:5444–5459. https://doi.org/10.1016/j.gca.2015.01.001
Scholz F, Hensen C, Lu Z, Fehn U (2010) Controls on the 129I/I ratio of deep-seated marine interstitial fluids: 'Old' organic versus fissiogenic 129-iodine. Earth and Planetary Science Letters 294:27–36. https://doi.org/10.1016/j.epsl.2010.02.034
Schratzberger M, Ingels J (2017) Meiofauna matters: the roles of meiofauna in benthic ecosystems. J Exp Mar Biol Ecol. https://doi.org/10.1016/j.jembe.2017.01.007
Seinhorst JW (1959) A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica 4:67–69
Shirayama Y, Ohta S (1990) Meiofauna in a cold-seep community off Hatsushima, central Japan. J Oceanograph Soc Jap 46:118–124. https://doi.org/10.1007/BF02123438
Sibuet M, Olu K (1998) Biogeography, biodiversity and fluid dependence of deep-sea cold-seep communities at active and passive margins. Deep-Sea Res II Top Stud Oceanogr 45:517–567. https://doi.org/10.1016/S0967-0645(97)00074-X
Van Gaever S, Moodley L, De Beer D, Vanreusel A (2006) Meiobenthos at the Arctic Hakon Mosby mud volcano, with a parental-caring nematode thriving in sulphide-rich sediments. Mar Ecol Prog Ser 321:143–155. https://doi.org/10.3354/meps321143
Van Gaever S, Galéron J, Sibuet M, Vanreusel A (2009a) Deep-sea habitat heterogeneity influence on meiofaunal communities in the Gulf of Guinea. Deep-Sea Res II Top Stud Oceanogr 56:2259–2269. https://doi.org/10.1016/j.dsr2.2009.04.008
Van Gaever S, Olu K, Derycke S, Vanreusel A (2009b) Metazoan meiofaunal communities at cold seeps along the Norwegian margin: influence of habitat heterogeneity and evidence for connection with shallow-water habitats. Deep-Sea Res I Oceanogr Res Pap 56:772–785. https://doi.org/10.1016/j.dsr.2008.12.015
Van Gaever S, Raes M, Pasotti F, Vanreusel A (2010) Spatial scale and habitat-dependent diversity patterns in nematode communities in three seepage related sites along the Norwegian Sea margin. Mar Ecol 3:66–77. https://doi.org/10.1111/j.1439-0485.2009.00314.x
Van Rensbergen P, Depreiter D, Pannemans B, Moerkerke G, Van Rooij D, Marsset B, Akhmanov G, Blinova V, Ivanov M, Rachidi M, Magalhaes V, Pinheiro L, Cunha M, Henriet J-P (2005) The el Arraiche mud volcano field at the Moroccan Atlantic slope, gulf of Cadiz. Mar Geol 219:1–17. https://doi.org/10.1016/j.margeo.2005.04.007
Vanneste H, Kastner M, James RH, Connelly DP, Fisher RE, Kelly-Gerreyn BA, Heeschen K, Haeckel M, Mills RA (2012) Authigenic carbonates from the Darwin mud volcano, gulf of Cadiz: a record of palaeo-seepage of hydrocarbon bearing fluids. Chem Geol 300:24–39. https://doi.org/10.1016/j.chemgeo.2012.01.006
Vanreusel A, Andersen AC, Boetius A, Connelly D, Cunha MR, Decker C, Hilário A, Kormas KA, Maignien L, Olu K, Pachiadaki M, Ritt, B, Rodrigues, C, Sarrazin, J, Tyler, P, Van Gaever, S, Vanneste H (2009) Biodiversity of cold seep ecosystems along the European margins. Oceanography 22:110–127. https://doi.org/10.5670/oceanog.2009.12
Vanreusel A, De Groote A, Gollner S, Bright M (2010a) Ecology and biogeography of free-living nematodes associated with chemosynthetic environments in the deep sea: a review. PLoS One 5:e12449–e12449. https://doi.org/10.1371/journal.pone.0012449
Vanreusel A, Fonseca G, Danovaro, RC, Da Silva MC, Esteves AM, Ferrero T, Gad G, Galtsova V, Gambi C, Da Fonsêca-Genevois V, Ingels J, Ingole B, Lampadariou N, Merckx B, Miljutin D, Miljutina M, Muthumbi A, Netto S, Portnova D, Radziejewska T, Raes M, Tchesunov A, Vanaverbeke J, Van Gaever S, Venekey V, Bezerra TN, Flint H, Copley J, Pape E, Zeppilli D, Martinez PA, Galéron J (2010b) The contribution of deep-sea macrohabitat heterogeneity to global nematode diversity. Mar Ecol 31:6–20. https://doi.org/10.1111/j.1439-0485.2009.00352.x
Vanreusel A, Van den Bossche I, Thiermann F (1997) Free-living marine nematodes from hydrothermal sediments: similarities with communities from diverse reduced habitats. Mar Ecol Prog Ser 157:207–219. https://doi.org/10.3354/meps157207
Vincx M, Bett BJ, Dinet A, Ferrero T, Gooday AJ, Lambshead P, Pfannkuche O, Soltwedel T, Vanreusel A (1994) Meiobenthos of the deep Northeast Atlantic. Adv Mar Biol 30:1–88. https://doi.org/10.1016/S0065-2881(08)60061-9
Warwick RM, Platt HM, Somerfield PJ (1998) Freeliving marine nematodes: part III. Monhysterida. Synopses of the British Fauna no. 53. Field studies council
Wieser W (1960) Benthic studies in Buzzards Bay II: the Meiofauna. Limnol Oceanography 5:121–137
Wieser W (1953) Die Beziehung zwischen Mundhöhlengestalt, Ernährungsweise und Vorkommen bei freilebenden marinen Nematoden. Arkiv Zoologi 4:439–484
Zeppilli D, Canals M, Danovaro RC (2012) Pockmarks enhance deep-sea benthic biodiversity: a case study in the western Mediterranean Sea. Divers Distrib 18:832–846. https://doi.org/10.1111/j.1472-4642.2011.00859.x
Zeppilli D, Mea M, Corinaldesi C, Danovaro RC (2011) Mud volcanoes in the Mediterranean Sea are hot spots of exclusive meiobenthic species. Prog Oceanogr 91:260–272. https://doi.org/10.1016/j.pocean.2011.01.001
Zitellini N, Gràcia E, Matias L, Terrinha P, Abreu MA, DeAlteriis G, Henriet JP, Dañobeitia JJ, Masson DG, Mulder T, Ramella R, Somoza L, Diez S (2009) The quest for the Africa–Eurasia plate boundary west of the strait of Gibraltar. Earth Planet Sci Lett 280:13–50. https://doi.org/10.1016/j.epsl.2008.12.005
Acknowledgements
We thank the Captain and the crew of the R/V Meteor M86/5 cruise for excellent support at sea. Cruise was funded by the German Research Foundation (TransFlux grant) with additional support by FCT with the SWIMGLO project (FCT PTDC/MAR/100522/2008 grant), UID/MAR/04292/2013, and MODELINK, and FLOWS (EU-COST, ES 1301) projects. NemaLab of the University of Evora is acknowledge for the use of the facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Y. Shirayama
Rights and permissions
About this article
Cite this article
Ramalho, S.P., Ribeiro, C., Hensen, C. et al. Benthic nematode biodiversity of the Abzu, Tiamat and Michael Ivanov mud volcanoes located along the SWIM fracture zone (Gulf of Cadiz). Mar Biodiv 48, 423–438 (2018). https://doi.org/10.1007/s12526-017-0809-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-017-0809-x