Abstract
A new species of caligid copepod, Lepeophtheirus azoricus n. sp., is described from the dusky grouper, Epinephelus marginatus (Lowe), caught in Atlantic waters off Faial Island in the Azores. Both sexes of the parasite were collected from the body surface of the host by the application of osmotic shock. The new species can be distinguished from its congeners by the following set of characters: (1) an obvious conical projection on the posterolateral corners of the genital complex, (2) the relative lengths of the second and third spines on the terminal exopodal segment of leg 4, (3) the relative lengths of the setal elements on legs 5 and 6 of the male, (4) the ornamentation, and lack of a myxal process, on the proximal segment of the maxilliped in both sexes, (5) the unusual structure of the male antenna.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the parasitic copepod families reported from both cultured and wild marine fishes, the family Caligidae Burmeister, 1835 is, by far, the most species-rich taxon within the order Siphonostomatoida (Dojiri and Ho 2013). The family consisted of 31 valid genera according to the recent revision by Dojiri and Ho (2013). However, the genus Sciaenophilus van Beneden, 1852 was recently placed into synonymy with the genus Caligus O.F. Müller, 1785, so the current number of valid genera within the Caligidae is now only 30 (Özak et al. 2017). Among these, the genus Lepeophtheirus von Nordmann, 1832 is the second largest (after Caligus), and it currently comprises 122 valid species (Boxshall and Walter 2015). The majority of these species has been reported from teleost hosts, although a few have been recorded from elasmobranchs (Kabata 1979; Dojiri and Ho 2013). Although there is no formal taxonomic subdivision within the genus Lepeophtheirus, Kabata (1973) divided the species of Lepeophtheirus into two groups based on the ratio of the abdominal length to genital complex length. One group consists of species with a short abdomen, i.e., one that is less than the half-length of the genital complex, and another group with a long abdomen, i.e., which is equal to or longer than the genital complex. The new species of Lepeophtheirus described herein from a dusky grouper, Epinephelus marginatus (Lowe), caught in Atlantic waters off Faial Island in the Azores (Portugal) has a short abdomen that is less than half the length of the genital complex, and, thus, can be included in the first of Kabata’s (1973) two groups. Here, we present descriptions of both sexes of the new species, supported by drawings, light microscope, and scanning electron microscope photographs.
Materials and methods
A dusky grouper, Epinephelus marginatus (total length: 93 cm), was captured during a research project on “Developing methods for capture, husbandry and live transportation of dusky grouper” conducted in oceanic waters off Faial Island, in the Azores archipelago. External parasites were removed from the fish by applying the freshwater treatment method of Wildgoose (2001) as follows: the fish was initially placed in a 750-L tank containing 36 ppt seawater; the salinity was then decreased to 9 ppt within 35 min; the fish was kept at 9 ppt for 30 min, after which time the salinity was increased slowly from 9 ppt to 36 ppt. After the osmotic shock, a total of 542 specimens of a parasitic copepod were collected from the bottom of by filtering the bath water through a plankton net (mesh size 100 μ). The copepods were immediately preserved in 70% ethanol. Specimens were cleared in lactic acid for 2 h prior to examination using an Olympus SZX16 dissecting microscope and Olympus BX51 compound microscope. Specimens were dissected on glass slides and mounted as temporary preparations in lactophenol. Measurements were made using an ocular micrometer and drawings were made with the aid of a drawing tube. All measurements in the text are given in millimeters (mm) unless stated otherwise, and are presented as the range followed by the mean in parentheses. The scientific and common names of fishes follow Froese and Pauly (2017), the morphological terminology for the copepods follows Huys and Boxshall (1991), and parasitological terms follow Bush et al. (1997). The type material is stored in the collection of the Natural History Museum, London and additional paratypes are stored in the Flying Sharks facilities, Faial (Azores). The protocols for preparing crustaceans for scanning electron microscopy (SEM) outlined by Felgenhauer (1987) were followed. Ethanol-fixed specimens were hydrated to distilled water and post-fixed in 1–2% osmium tetroxide (OsO4) in buffer for 2 h, washed in distilled water, dehydrated through graded acetone series, critical point dried using liquid carbon dioxide as the exchange medium, mounted on aluminum stubs, and sputter coated with platinum. Coated specimens were examined on a Zeiss Supra 55 (FE-SEM, Germany) field emission scanning electron microscope at 1–3 kV.
Results
Family Caligidae Burmeister, 1835
Genus Lepeophtheirus von Nordmann, 1832
Lepeophtheirus azoricus n. sp.
Type host:Epinephelus marginatus (Lowe) (Serranidae).
Type locality: Atlantic waters off Faial Island, Azores, Portugal; collected by Alfredo M.V. Rodrigues, Nina S.S. Vieira, and Rui M.G. Rosa.
Site on host: Body surface.
Type material: Holotype female, 3 female and 4 male paratypes stored in the collections of the Natural History Museum London, UK (NHMUK 2016.505-512); remaining paratype material stored in the Flying Sharks facilities and the personal collection of Alfredo M.V. Rodrigues.
Etymology: The species name refers to the sampling area.
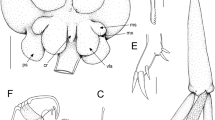
Adult female. Body (Fig. 1a) comprising caligiform cephalothorax, incorporating first to third pedigerous somites, free fourth pedigerous somite, genital complex and 1-segmented abdomen. Body length 3.9–4.7 (4.48) (n = 10) excluding caudal setae. Dorsal cephalothoracic shield slightly longer than wide, 3.3–3.74 × 3.0–3.6 (3.47 × 3.42) excluding marginal hyaline membranes, lateral margins convex and ornamented with array of about 36 small compound sensillae beneath marginal membrane along each side (Fig. 1b). Lunules absent. Free thoracic zone of shield comprising almost half-length of cephalothorax, wider than long, 1.14–2.19 × 2.1–2.35 (1.73 × 2.21). Posterior margin of free thoracic zone straight, extending beyond posterior ends of lateral zones. Fourth pedigerous somite 0.22–0.32 × 0.7–0.76 (0.26 × 0.73), distinctly separated from genital complex and ornamented with sensillae. Genital complex wider than long, 0.5–0.64 × 1.1–1.21 (0.57 × 1.16), with rounded anterior angles, slightly convex lateral margins, posterolateral corners with small, spiniform processes dorsally; posteroventral margin with two small, corrugated lobes near egg sac attachment area (Fig. 4). Length of genital complex c. 16.4% of length of cephalothorax. Abdomen (Figs. 1a and 4) 1-segmented, as long as wide, 0.23–0.28 × 0.24–0.27 (0.26 × 0.26), just less than half-length (c. 45.6%) of genital complex. Caudal rami (Fig. 1c), 0.13–0.17 × 0.08–0.13 (0.15 × 0.1), armed with 6 pinnate setae.
Antennule (Fig. 1d) 2-segmented, proximal segment distinctly wider than distal, armed with 25 plumose setae on anterior and antero-ventral surfaces plus 2 unarmed setae located dorsally; distal segment short, armed with 1 subterminal seta on posterior margin and 11 setae plus 2 aesthetascs on distal margin. Antenna (Figs. 1e and 5a) uniramous, 3-segmented; proximal segment produced into blunt spinous process (Fig. 5b) and bearing small corrugated pad; middle segment with dorsal corrugated pad on outer distal corner (Fig. 5b black arrow, c) and a small inner distal pad; distal segment forming sharply curved claw with small spine-like seta proximally and longer distal seta. Postantennal process (Figs. 1e and 5d) weakly curved with bifid tip (Fig. 5d inset) carrying 2 multi-sensillate papillae; similar multi-sensillate papilla located on body surface adjacent to process (Fig. 5d black arrows); additional single papilliform process (Fig. 5d white arrow) located close to base of postantennal process. Maxillule (Figs. 1f and 6a) with two unequal tines; outer tine shorter and more slender than inner tine: anterior papilla bearing 3 unequal setae. Sternal furca (Figs. 1g and 6b) with bluntly pointed divergent tines, inner margin of tines slightly convex. Maxilla (Fig. 2a) 2-segmented, brachiform; proximal segment (lacertus) large, unarmed; slender distal segment (brachium) bearing large subterminal flabellum (hyaline membrane) (Fig. 2b) on the inner margin plus short canna and long calamus distally. Maxilliped (Figs. 2c and 6c) comprising long, slender proximal segment (corpus) and distal subchela representing fused endopodal segments plus short claw; subchela armed with small seta at base of claw (Fig. 6d), corpus ornamented with two patches of scale-like denticles (Figs. 6e, inset).
Lepeophtheirus azoricus n. sp. Paratype female, scanning electron microscopy (SEM) micrographs. a General view of appendages on antero-ventral part of cephalothorax. b Antenna, with black arrow indicating corrugated pad on outer distal corner of middle segment. c Detail of corrugated pad on antenna. d Postantennal process (pap), with black arrows indicating multi-sensillate papillae, white arrow showing a papilliform process at the base of the pap, and the inset showing the bifid tip of the pap. Scale bars: a 100 μm; b 50 μm; c 10 μm; d 20 μm; d inset: 5 μm
Lepeophtheirus azoricus n. sp. Paratype female, SEM micrographs. a Maxillule. b Sternal furca. c Maxilliped. d Seta on proximal part of maxilliped claw. e Patches of scale-like denticles on the surface of the protopodal segment of the maxilliped, with the inset showing detail of scale-like denticles. Scale bars: a 50 μm; b 25 μm; c 100 μm; d 30 μm; e 20 μm; e inset: 3 μm
Leg 1 (Figs. 2d and 7a) biramous, with 2-segmented exopod and unsegmented, vestigial endopod (Fig. 7a black arrow). Sympod armed with lateral plumose seta and inner seta. First exopodal segment ornamented with row of setules along posterior margin (Fig. 7a) and bearing small spine at outer distal corner. Distal exopodal segment (Figs. 2e and 7b) with 3 plumose setae posteriorly plus 4 terminal elements; outermost element (spine 1) finely serrated, middle 2 elements (spines 2 and 3) each bearing single prominent accessory process and ornamented with fine serrations along inner and outer margins (Figs. 7b, c), innermost element (seta 4) (Figs. 2e and 7b arrow) with setules along inner and outer margins. Endopod (Fig. 7d) vestigial, with bifid spiniform processes at apex. Arthrodial membrane ornamented with small swellings near vestigial endopod (Fig. 7a, e). Leg 2 (Figs. 2f and 8a) biramous, with 3-segmented rami. First exopodal segment c. 4.3 times longer than second; both segments with pinnate seta on inner margin and long oblique spine at outer distal corner. Each spine fringed with hyaline membrane. Third exopodal segment with 3 outer spines plus 5 pinnate setae. The first two spines fringed with hyaline membrane; the third spine bearing an outer marginal membrane and row of setules on inner margin. First endopodal segment with inner pinnate seta; second endopodal segment with 2 inner pinnate setae and ornamented with rows of long setules on outer margin; third segment with 6 pinnate setae. Leg 3 (Figs. 2g and 8b) with coxa and basis fused into flattened apron-like sympod (not figured). Exopod 3-segmented (Figs. 2g and 8b, c); first segment with 3 lateral setules and an outer spine extending beyond second exopodal segment; spine with finely serrated lateral margin and pecten at base, plus an inner plumose seta. Second exopodal segment with outer spine (Fig. 8b black arrow) and inner plumose seta. Third exopodal segment with 3 outer spines (Fig. 8b white arrows) and 4 short pinnate setae. Endopod (Figs. 2g and 8b) 2-segmented; proximal segment with long inner pinnate seta and setules along outer margin; compound distal segment with 6 pinnate setae and ornamented with rows of long setules along outer margin. Leg 4 (Figs. 3a and 9a) uniramous, extending (including terminal spines) beyond caudal rami. Protopodal segment with outer seta derived from basis. Exopod 3-segmented; first segment with 1 tiny distal spine (Fig. 9b); middle segment with longer bilaterally serrate spine at apex (Fig. 9c); terminal segment with 3 apical spines decreasing in size laterally; whip-like longest spine with serrate membrane along inner margin (Fig. 9d), middle and outermost spines with serrate membranes along inner and outer margins. Middle spine less than half-length of the longest spine, and the outermost spine smallest. All spines on exopodal segment with pecten at base (Fig. 9e). Spine (Roman numerals) and seta (Arabic numerals) formula of legs 1–4 as follows:
Exopod | Endopod | |
Leg 1 | I-0; III,1,3 | vestigial |
Leg 2 | I-1; I-1; II,I,5 | 0–1; 0–2; 6 |
Leg 3 | I-1; I-1; III,4 | 0–1; 6 |
Leg 4 | I-0; I-0; III | absent |
Lepeophtheirus azoricus n. sp. Paratype female, SEM micrographs. a Leg 1, with small swellings (white arrow) near vestigial endopod (black arrow). b Terminal elements on the distal exopodal segment of leg 1, with the white arrow indicating innermost spine. c Spine 2 with accessory process. d Vestigial endopod of leg 1. e Minute surface swellings on arthrodial membrane of leg 1. Scale bars: a 100 μm; b, c 10 μm; d, e 2 μm
Lepeophtheirus azoricus n. sp. Paratype female, SEM micrographs. a Leg 4. b Outer spine on first exopodal segment. c Bilateral serrations on lateral, outer terminal spine. d Lateral serrations on innermost terminal spine. e Terminal spines on distal exopodal segment. f Leg 5 (asterisk), dorsal spinous projection on corner of genital complex (black arrow) and corrugated lobe near egg sac attachment area (inset). Scale bars: a 100 μm; b 10 μm; c 2 μm; d, e 20 μm; f 30 μm; e inset: 5 μm
Leg 5 (Figs. 3b and 9f) represented by 2 ventral papillae at posterolateral corner of genital complex; outer papilla small and bearing single plumose seta; inner (exopodal) papilla conical, distinctly longer, ornamented with sensillae and carrying 3 unequal plumose setae and tiny denticle at outer distal corner. Egg-sac attachment area with small, rounded corrugated lobe close to leg 5 (Fig. 9f, inset).
Adult male. Body (Fig. 10a) 2.85–3.2 (3.07) long, excluding caudal setae. Dorsal cephalothoracic shield subcircular, longer than wide, 1.97–2.21 × 1.87–2.09 (2.1 × 1.96) excluding marginal hyaline membranes; lateral margins slightly convex and ornamented with array of 33 (vs. 36 in female) small compound sensillae adjacent to and beneath marginal membrane along each side; surface of cephalothorax ornamented with sensillae. Free thoracic zone of shield wider than long, 0.97–1.12 × 1.18–1.3 (1.06 × 1.24), about half-length of cephalothorax, with posterior margin extending beyond posterior ends of lateral zones. Fourth pedigerous somite 0.19–0.31 × 0.29–0.41 (0.24 × 0.34), distinctly divided from genital complex. Genital complex (Fig. 10a) elongate, 0.65–0.78 × 0.27–0.38 (0.72 × 0.34), c. 34.28% of length of cephalothorax, with slightly convex to parallel sides and triangular posterolateral lobes. Abdomen 1-segmented, 0.09–0.16 × 0.17–0.23 (0.13 × 0.2), about 18% of length of genital complex. Caudal rami 0.09–0.16 × 0.06–0.13 (0.13 × 0.1), c.1.3 times longer than wide, bearing 6 pinnate setae.
Appendages as in female except antenna, maxillule, maxilliped, and fifth and sixth legs. Antenna (Figs. 10b and 11a) 3-segmented; proximal segment long, narrow, with corrugated adhesion pad on mid-outer surface; middle segment with corrugated pads on medial and distal surfaces (Fig. 11b) and carrying a large lateral projection consisting of 3 to 4 overlapping scale-like plates decreasing in size from ventral to dorsal (Fig. 11b inset); distal segment of antenna consisting of two parts combined into a single recurved segment; proximal (first) part subrectangular, carrying two basal seta (Figs. 10b and 11b inset) plus large based, tapering claw ventrally (Fig. 10b); surface of dorsal distal half ornamented with scratches and a prominent suture line present ventrally between two parts (Fig. 11a, b). Distal (second) part, directed posteriorly and carrying overlapping flaps aligned with outer margin and another long flap on inner margin (Fig. 11a); triangular, tapering ventral claw extending beyond large, outer, terminal flap (Fig. 11a, b). Maxillule (Fig. 11c) with two unequal tines and additional process plus an anterior papilla carrying 3 unequal setae, outer tine tapering and longer; inner tine shorter and about equal to length of additional process on medial surface. Small, elongate corrugated pad (post oral process) present on ventral cephalothoracic surface just posterior to maxillule. Maxilliped (Figs. 10c and 12a) with elongate corpus ornamented with patch of spinules on median surface (Fig. 12b, inset). Leg 5 (Figs. 10d) represented by conical process on lateral margin of genital complex, bearing 1 lateral and 3 terminal plumose setae; 1 terminal setae shorter than other 2. Leg 6 (Figs. 10d and 12c) represented by small process armed with 1 lateral and 2 terminal plumose setae; lateral seta slightly smaller than terminal setae.
Lepeophtheirus azoricus n. sp. Paratype male, SEM micrographs. a Antenna. b Antennae, showing corrugated pads on proximal and middle segments, basal seta on proximal part of distal segment (white arrows) and lateral projection on middle segment (inset). c Maxillule including anterior papilla. Scale bars: a 30 μm; b 50 μm; b inset: 10 μm; c 20 μm
Remarks
Lepeophtheirus is a large genus and the morphology of the adult female is relatively homogeneous. For example, the great majority of the 122 valid species (Boxshall and Walter 2015) have an adult female characterized by a short abdomen, i.e., it is 1-segmented and less than half as long as the genital complex (measured along the midline of the body in dorsal view), and have a 3-segmented exopod on the fourth leg armed with I, I, III spines. Similarly, the vast majority of species have simple tines on the sternal furca and have a bifid posterior process on the maxillule in the adult female, as found in the new species. There is another cluster of species, most of which were formerly placed in the genus Dentigryps Wilson, 1913, which is characterized by an extremely elongate and highly conspicuous fifth leg in the female. These too can be eliminated since the new species has a short conical fifth leg. For a more detailed comparison, it is, therefore, necessary to combine several such characters in order to identify the nearest relatives of the new species. The combination of the following five character states eliminates most species: (1) female abdomen 1-segmented, less than half the length of the genital complex, (2) female maxillule bifid, (3) sternal furca with simple (non-bifid) tines, (4) leg 4 elongate, extending posteriorly beyond the caudal rami, armed with 3 decreasingly longer spines (from inner to outer margin) on the third exopodal segment and with the lateral spine on the second exopodal segment about half as long as the outer margin of the third exopodal segment, and (5) female leg 5 conical, not elongate. There are 38 species that share this set of characters or which cannot be eliminated from the comparison because of incomplete descriptions. Only six of these species also possess a genital complex that is about 3 times longer than the abdomen (0.79:0.26). These remaining six species are: Lepeophtheirus furcatus (Capart, 1953), L. goniistii Yamaguti, 1936, L. kabatai Ho & Dojiri, 1977, L. mugiloidis Villalba & Duran, 1986, L. muraenae Shiino, 1960, and L. plectropomi Nuñes-Ruivo & Fourmanoir, 1956. However, the character (4) given above could not be compared with the last four species (L. kabatai, L. mugiloidis, L. muraenae, L. plectropomi), as these characters related to leg 4 were not discussed and described in detail in previous studies.
Lepeophtheirus furcatus was described from two females collected from an elasmobranch host, Mobula rochebrunei (Vaillant), caught off Dakar (Senegal) and the female is very similar to the new species in its body proportions. Unfortunately, it is incompletely described, so our comparisons are necessarily restricted to certain characters. The most significant difference is the body size: the ovigerous female of L. furcatus has a total body length of 2.54 mm, whereas the new species has a mean length of 4.48 mm, with a range of 3.90 to 4.70 mm. In addition, in L. furcatus, the genital complex is about half the width of the dorsal cephalothoracic shield, whereas in the new species, the genital complex is relatively narrower, equal to about one-third of the shield width. Finally, the longest (apical) spine on leg 4 is about equal in length to the outer margin of the apical segment (measured from Capart 1953; Fig. 2e), whereas in the new species, that spine is markedly longer than the margin of the segment.
Yamaguti (1936) described both sexes of L. goniistii in detail based on material from the cheilodactylid Cheilodactylus zonatus Cuvier (as Goniistius zonatus) caught in Japanese waters. This well-characterized species has subsequently been reported from a range of other hosts, including representatives of the Serranidae, Mullidae, and Siganidae, from Sri Lanka, Korea, and Japan (Shiino 1952; Kirtisinghe 1964; Kim 1998). It has a relatively large genital complex: the dorsal cephalothoracic shield is only 2.5 times longer than the genital complex, whereas in L. azoricus n. sp., it is about 4.5 times longer. Another possible difference is the form of the outer spine on the first exopodal segment of leg 3, which was shown as being strongly bifid in female L. goniistii by Yamaguti (1936, fig. 130), but is simple in the new species. There are also differences in the relative lengths of the spines on the distal segment of leg 4. In addition, the female of L. goniistii lacks the pair of spiniform processes on the genital complex, and has a thinner outer tine on the maxillule and an oblique leg 5; the male of L. goniistii has a less modified antenna and a myxal process on the maxilliped (Kim 1998).
The adult female of L. muraenae was described by Shiino (1960) based on material from Enchelycore pardalis (Temminck & Schlegel) (as Muraena pardalis) in Japan. It can be distinguished from the new species by its caudal rami, which are wider than long, and the wide separation of the tines of the bifid posterior process of the maxillule. In L. azoricus n. sp., the caudal rami are longer than wide and the tines of the maxillule are adjacent at the base and diverge distally.
Lepeophtheirus mugiloidis occurs off the Chilean coast in the eastern Pacific on a host given as Mugiloides chilensis (Molina), but which cannot be found in FishBase (Froese and Pauly 2017). This large species has female mean body length of 5.33 mm, ranging from 5.02 to 5.61 mm (Villalba and Duran 1986). It differs from the new species in having caudal rami which are wider than long.
The remaining two species, L. kabatai and L. plectropomi, both occur on serranid hosts belonging to the subfamily Epinephelinae. The former was established by Ho and Dojiri (1977) based on material from a species of Epinephelus collected in Australia, but also including material from two species of Epinephelus from Hawaii, described by Lewis (1968) under the name L. plectropomi. This latter species was originally described from Plectropomus maculatus (Bloch) caught in the Indian Ocean off Madagascar, but Ho and Dojiri (1977) also provided supplementary information on this species based on new specimens collected from Epinephelus megachir (Richardson) accepted as E. quoyanus (Valenciennes) and Plectropomus leopardus (Lacepède) caught off the Great Barrier Reef. The abdomen of L. kabatai has markedly convex lateral margins, while those of L. azoricus n. sp. are straight to slightly convex and the caudal rami are shorter than wide in the former but longer than wide in the latter.
The inadequate original description of L. plectropomi makes comparisons with L. azoricus n. sp. problematic, but using Ho and Dojiri (1977) as a basis for comparison, a number of differences can be identified that serve to distinguish L. plectropomi and L. azoricus. The most striking difference between females is the presence of only 5 plumose setae on the compound distal endopodal segment of leg 3 in the former, whereas the new species has the full complement of 6 setae. The males can be readily separated by the presence of a prominent recurved claw-like process on the second segment of the antenna in L. plectropomi; no such process is present in L. azoricus. The differences between the species collected from E. marginatus caught off the Azores and these closely related species of Lepeophtheirus are sufficient to justify the establishment of the new species, L. azoricus.
In addition to the species compared above, Lepeophtheirus erecsoni Thomson G.M., 1891, which was reported from the fish species within the families Latridae and Labridae, also reveals close similarity to L. azoricus in having a genital complex that is about 3 times longer than the abdomen. However, L. erecsoni can be distinguished from the new species by having prominent, lobate posterolateral corners on genital complex, conspicuous barb (vs. no barb) on the convex margin of canna on the maxilla, and a male antenna that is less modified in comparison to the male antenna of L. azoricus.
References
Boxshall G, Walter TC (2015) Lepeophtheirus von Nordmann, 1832. In: Walter TC, Boxshall G (2017) World of Copepods database. Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=135568. Accessed 13 Mar 2017
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Capart A (1953) Quelques Copépodes parasites de poissons marins de la région de Dakar. Bull Inst Fr Afr Noire 15(2):647–671
Dojiri M, Ho JS (2013) Systematics of the Caligidae, copepods parasitic on marine fishes. Crustaceana monographs, 18. Brill, Boston
Felgenhauer BE (1987) Techniques for preparing crustaceans for scanning electron microscopy. J Crustac Biol 7:71–76
Froese R, Pauly D (eds) (2017) FishBase. World Wide Web electronic publication. http://www.fishbase.org. Accessed 13 Mar 2017
Ho JS, Dojiri M (1977) Parasitic copepods on the fishes of the great barrier reef, Australia. Part II. Caligoida: Dissonus, Lepeophtheirus, and Dentigryps. Publ Seto Mar Biol Lab 24(1–3):77–97
Huys R, Boxshall GA (1991) Copepod evolution. The Ray Society, London
Kabata Z (1973) The species of Lepeophtheirus (Copepoda: Caligidae) from fishes of British Columbia. J Fis Res Board Can 30(6):729–759
Kabata Z (1979) Parasitic Copepoda of British fishes. The Ray Society, London
Kim IH (1998) Illustrated encyclopedia of fauna and flora of Korea. Vol. 38. Cirripedia, Symbiotic Copepoda, and Pycnogonida. Ministry of Education in Korea, Seoul
Kirtisinghe P (1964) A review of the parasitic copepods of fish recorded from Ceylon with description of additional forms. Bull Fish Res Stn Ceylon 17(1):45–132
Lewis AG (1968) Copepod crustaceans parasitic on fishes of Eniwetok atoll. Proc U S Nat Mus 12(366):1–78
Özak AA, Yanar A, Boxshall GA (2017) The discovery of Caligus macrurus Heller, 1865 (Copepoda: Caligidae) in the Mediterranean Sea, and the recognition of Sciaenophilus van Beneden, 1852 as a junior synonym of Caligus Müller, 1785. Syst Parasitol 94(1):97–109. https://doi.org/10.1007/s11230-016-9682-4
Shiino SM (1952) Copepods parasitic on Japanese fishes. 1. On the species of Caligus and Lepeophtheirus. Rep Fac Fish Prefect Univ Mie 1:79–113
Shiino SM (1960) Copepods parasitic on the fishes collected on the coast of province Shima, Japan. Rep Fac Fish Prefect Univ Mie 3(3):471–500
Villalba C, Durán L (1986) Lepeophtheirus mugiloidis sp. n. (Copepoda: Caligidae), parasito de Mugiloides chilensis (Molina, 1782) (Pisces: Mugiloididae). Chile Boln Soc Biol Concepción 56:59–66
Wildgoose WH (2001) BSAVA manual of ornamental fish. Wiley, Gloucester
Yamaguti S (1936) Parasitic copepods from fishes of Japan. Part 2. Caligoida, I. Kyoto Imperial University. Published by author
Acknowledgements
The authors gratefully acknowledge Flying Sharks and MARE for their technical and logistic support, and all the colleagues who assisted in the field and laboratory work. Special thanks go to Luís Silva and Nelson Campino, from Flying Sharks, for their valuable technical support on planning and collecting the dusky grouper and to António Godinho, from the University of Azores, for the materials and facilities provided for this work. The research conducted complies with the current Portuguese Law. We would also like to thank Dr. Masahiro Dojiri from California, USA for his comments on the identification of the new species and also for providing valuable documents. We would also like to thank Assoc. Prof. Dr. Kasım Ocakoglu, Head of Advanced Technologies Research & Application Center (MEITAM) of the University of Mersin, Turkey, and Prof. Suphan Karaytug and Ms. Seher Kuru from Mersin University for their administrative and technical support during the SEM and LM studies.
Funding
This study was partially supported by Flying Sharks—Collections, Consulting, Conservation and Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national, and international guidelines for the care and use of animals were followed.
Additional information
Communicated by P. Martinez Arbizu
This article is registered in ZooBank under urn:lsid:zoobank.org:pub:350807BE-1C26-4AD6-B995-8C71A71DD3CA
Rights and permissions
About this article
Cite this article
Özak, A.A., Rodrigues, A.M.V., Vieira, N.S.S. et al. Lepeophtheirus azoricus n. sp. (Copepoda: Caligidae) parasitic on dusky grouper, Epinephelus marginatus, from Atlantic waters off the Azores, Portugal. Mar Biodiv 48, 1045–1055 (2018). https://doi.org/10.1007/s12526-017-0797-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-017-0797-x