Abstract
As part of an ongoing effort to monitor the fish diversity in Greenland waters using morphological and molecular taxonomy, and construct pragmatic identification keys, we here update distributions of all known halosaurs (Halosauridae) and notacanthids (Notacanthidae) in the subarctic Atlantic Ocean. New distributions are included for Aldrovandia oleosa, Halosauropsis macrochir, and Notacanthus bonaparte, all caught once in the Denmark Strait off SE Greenland. We also present the first observation of luminous tissues in halosaurs. Photo, illustrative and text identification keys are included for pragmatic identification corroborated by molecular barcoding data, produced as part of the Greenland Fishes Barcoding Project (GLF) introduced in this study. Barcoding data revealed five operational taxonomic units (OTUs) of Notacanthus cf. chemnitzii present, including three distinct haplotypes observed off SE Greenland alone, within this species complex currently described as one circumglobal species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subarctic Atlantic fishes
At present, the true species diversity of the fish fauna in the deeper parts of the pelagic North Atlantic is underestimated (e.g., Poulsen 2015a). Nevertheless, the benthic composition of fishes is believed to be more established due to extensive bottom-trawling over the last 20 years by both research-oriented and commercial fleets (Møller et al. 2010). However, extended species distributions into the subarctic province of also benthic-associated fishes are regularly observed, and the separation of habitat regarding sampling and known biodiversity is by no means as straightforward as often regarded. Large areas of the subarctic Atlantic are inaccessible to trawling due to bottom topography, and taxa showing clandestine or hiding behaviours may remain unknown at present. The large deep-sea pelagic realm and extensive areas of steep slopes in and around the Labrador Sea (SW Greenland) and the Irminger Sea (SE Greenland) lead to multiple unknowns in terms of distribution ranges, as little sampling has been carried out (Fig. 1). In addition, the Subpolar Front (SPF) is an important transition area concerning temperate and subarctic species distributions (Vecchione et al. 2010, 2015; Sutton et al. 2013), although probably having a different effect on benthic, epipelagic and pelagic species migration on a temporal scale. However, due to continuous findings of new benthic fish distributions around Greenland in recent years (J.Y. Poulsen, personal observation), it must be recognized that even distribution of the benthic deep-sea fish fauna in deep subarctic waters is incomplete at present, making predictions and monitoring of subarctic fish species somewhat trivial at present (Izzo et al. 2016). Furthermore, new species are still being described from subarctic Atlantic waters that are not correlated with increased water temperatures; however, being the result of an increased taxonomic effort (Knudsen and Møller 2008; Chernova 2014; Poulsen 2015a). New subarctic distributions based on single or few specimens are possibly associated with expatriate specimens extending to the north (e.g., Poulsen 2015b), possibly along the Mid-Atlantic Ridge (MAR) or transferred via the Gulf Stream and subsequent polar gyres (Fig. 1). General information on particular water masses and currents relating to biodiversity in the subarctic Atlantic, as observed in the southern Atlantic by Olivar et al. (2017), is scarce and speculative. Therefore, incomplete knowledge of taxonomy and real fish distributions are probably the primary factors determining both pelagic and benthic-associated fishes in large parts of the subarctic Atlantic, not changes in ocean temperature as often noted by intuition. It is important to recognize distributional limitations when establishing baselines for temporal changes in species diversity, especially considering the emphasis on global warming associated with species diversity in this region (Rose 2005; Fossheim et al. 2015; Wisz et al. 2015), and the intuitive assumption that new northern distributions are due to increased water temperatures. Therefore, new species distributions in subarctic waters are difficult to establish owing to multiple reasons (Byrkjedal et al. 2004; Møller et al. 2010; Vecchione et al. 2010). The three new notacanthiform distributions presented in this study are testaments to this fact, as taxonomic and sampling errors are associated with all three records (discussed later). Fluctuating ocean temperatures are also complicating the assessment of species distributions, as the fluctuations observed affect surface temperatures on a short temporal scale (MacKenzie et al., 2014; Palsson and Astthorsson 2016), making continuous yearly onboard monitoring of species diversity necessary to establish any trends or changes related to true distributional shifts. Finally, oceanic habitats for open-ocean species, versus coastal habitats that are more frequently sampled, are also an important caveat concerning the true biodiversity in subarctic waters (Poulsen 2015a).
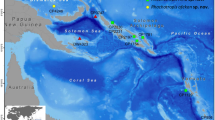
Sea floor topology of the Greenland subarctic zone and the North temperate zones. The four Greenland EEZ areas noted corresponding to NW, SW, SE, and NE regions. Note the bottom-topological restricted boundaries (Canadian–Greenlandic submarine ridge and Greenland–Iceland submarine ridge) separating the Greenland northern parts from the warmer southern areas. Northern Atlantic range extensions in fishes are often observed in the areas SW and SE Greenland, SW Iceland, and the Rockall Trough, all showing bottom topological features affecting depth and ocean currents. The six yearly legs surveyed with R/V Pâmiut (GINR) from June to September are noted, constituting a large continuous monitoring effort (maximum fishing depths 1,500 m). Note how the three new distributions observed — Halosauropsis macrochir (*), Notacanthus bonaparte (**) and Aldrovandia oleosa (***) — were caught on the deeper usually inaccessible slopes of the Irminger Sea off SE Greenland. Map modified after Ryan et al. (2009) and ocean currents after Masson-Delmotte et al. (2012)
Notacanthiformes
The fish order Notacanthiformes consists of two families: The halosaurs (Halosauridae) and the deep-sea spiny eels (Notacanthidae). Worldwide, the two families are deemed species-poor, with Halosauridae containing 16 species and Notacanthidae containing 11 species (Eschmeyer et al. 2017). Prior to this study, only three notacanthiforms were registered from the subarctic western Atlantic (Møller et al. 2010); one halosaur Hawaiian halosaurid fish, Aldrovandia phalacra (Vaillant 1888), and two notacanthids, Chemnitz’s spiny eel Notacanthus chemnitzii (Bloch 1788) and the Smallmouth spiny eel Polyacanthonotus rissoanus (De Filippi and Verany 1857). The notacanthid Shortfin spiny eel Notacanthus bonaparte (Risso 1840) was included by Sulak (1986) as distributed off SE Greenland, although omitted by Møller et al. (2010) who noted that it could not be confirmed; therefore, it was excluded in an annotated checklist of Greenland fishes. In the eastern subarctic Atlantic, only N. bonaparte, N. chemnitzii, P. rissoanus, and the Longnose tapirfish Polyacanthontus challengeri (Vaillant 1888) have been registered, the latter from only one 590 mm specimen caught off SE Iceland in 1995 at 2,265 m (Jónsson and Pálsson 2013). The phylogenetic association of Halosauridae and Notacanthidae is unambiguously supported from morphological and molecular characters. Both families possess unique long-filament type leptocephali larvae, “Tiluropsis” and “Tilurus” sensu Smith (1970), distinguishing the notacanthiforms from other elompomorph taxa (Inoue et al. 2004). However, the larvae of notacanthiform fishes are poorly known at present, and identification to species level practically impossible (Smith 1970; Miller and Tsukamoto 2004; Figueroa et al. 2008). It is of note that recent nuclear and mitochondrial combined data removed the albuliform fishes from a long-standing phylogenetic position as sister clade to the notacanthiforms, resulting in monophyletic notacanthiform and albuliform clades corresponding to the different types of leptocephali larvae found within the Elopomorpha (Chen et al. 2014).
This study is part of a larger effort to monitor fish distributions in Greenland waters, including the construction of pragmatic materials for onboard species identification. It includes: 1) new species distributions of notacanthiform fishes, 2) the first observation of luminescent tissue in halosaurs, 3) pragmatic materials for identification of notacanthiforms including taxonomic keys, photos and illustrations, 4) reference for the Greenland Fishes Barcoding Project (GLF), a publicly open Barcode of Life (BOLD) project, that aims to barcode all present and future fish species in Greenland waters, and 5) molecular evidence showing multiple OTUs in the species complex Notacanthus cf. chemnitzii, including several sympatric units in the subarctic North Atlantic.
Materials and methods
New species records
Three new notacanthiform observations caught in Greenland waters provide new data presented in this study: Oily halosaurid fish Aldrovandia oleosa (Sulak 1977), Abyssal halosaur Halosauropsis macrochir (Günther 1878), and Notacanthus bonaparte. They all provide new records and range extensions in the North Atlantic, and were all collected onboard R/V Pâmiut, Greenland Institute of Natural Resources (GINR). The specimens were digitally X-rayed at the Australian Museum (AMS) and examined using a stereomicroscope. The morphological measurements were obtained using a digital calliper to the nearest 0.1 mm and compared to previous specimens (Table 1).
Greenland Fishes (GLF) barcoding project
The GINR is barcoding all Greenland fishes, as a service to the taxonomic community, albeit many deep-sea taxa are rare and only known from single specimens. The ongoing project is here introduced as the BOLD project “Greenland Fishes”. Voucher specimens are usually deposited at the Zoological Museum University of Bergen (ZMUB) in Norway or alternatively at the Zoological Museum University of Copenhagen (ZMUC) in Denmark. Metadata and collection/field/tissue numbers are provided in BOLD or may be obtained by contacting the corresponding author.
Molecular work was carried out at the Biodiversity Laboratories (BDL, DNA Section) at the University Museum of Bergen/Department of Biology, University of Bergen, Norway. Total DNA extraction was performed on muscle tissue using standard extraction kit protocols such as the DNeasy Blood & Tissue kit (Qiagen GmbH, Hilden, Germany). Primers used for the Cox1 barcode are forward FishF2 (5′-TCGACTAATCATAAAGATATCGGCAC-3′) and reverse FishR2 (5′-ACTTCAGGGTGACCGAAGAATCAGAA-3′) (Ward et al. 2005). PCR was performed according to standard thermal cycle protocols (e.g., Ward et al. 2005) with the protocol as follows: total reaction mixture volume was 25 ml, including 18.75 ml of ultrapure water, 2.25 ml buffer, 1.25 ml of MgCl2 (50 mM), 0.25 ml of each primer (0.01 mM), 0.125 ml dNTP (0.05 mM), 0.625 U Taq polymerase, and 1 ml of DNA template. Thermal cycling consists of an initial step of 2 min at 95 °C followed by 35 cycles of 30 s at 94 °C, 30 s at 50 °C, and 60 s at 72 °C, followed by 10 min at 72 °C, and then held at 4 °C. PCR products were UV-visualized on 1% agarose gels stained with either Gel Red or ethidium bromide. Successful PCR products were purified using the EXO-SAP method with Exonuclease 1 (EXO 10 units/μl) and Shrimp Alkaline Phosphatase (SAP 1 unit/μl, USB©) in 10-μl reactions. Reactions were carried out on a thermal cycler at 37 °C (incubation) for 30 min followed by 15 min at 80 °C (enzyme inactivation). Subsequently, 25 rounds of direct cycle-sequencing with dye-labelled terminators BigDye terminator 3.1 (Thermo Fisher Scientific, Inc., Waltham, MA, USA) were performed following the manufacturer’s protocol. Sequence reads were performed with an automated ABI 3730XL DNA Analyser (Thermo Fisher Scientific, Inc.) in the Department of Molecular Biology at the University of Bergen (UIB). All Cox1 DNA sequences for the GLF project were meticulously examined for ambiguities and trimmed for primer attachment sites in order to upload unambiguous base pairs to BOLD.
Little molecular data on subarctic notacanthiforms was available prior to this study, and in order to compare the new records molecularly, we have included CoxI barcodes for all notacanthiform taxa in the Atlantic Ocean except Polyacanthonotus merretti (Sulak et al. 1984), Aldrovandia rostrata (Günther 1878), and Aldrovandia gracilis (Goode and Bean 1896), as no tissue or barcodes were available for these taxa. Polyacanthonotus africanus (Gilchrist and von Bonde 1924) is presently treated as a junior synonym of P. challengeri according to Sulak et al. (1984), although see findings by Barros-García et al. (2015).
A distance-based Kimura-2-parameter (K2P) substitution model (Kimura 1980) was used in order to obtain a Cox1 molecular taxonomic cladogram. We have not included support values as analyses in a molecular taxonomy context, as the present use of few single-locus base pairs, such as the barcoding Cox1 fragment, should not be confused with phylogeny reconstruction (phylogenetic systematics), which on the contrary, should be corroborated with support values. However, we recognize that the K2P is not necessarily an appropriate model choice for barcoding data (Srivathsan and Meier 2012).
Newly determined notacanthiform barcodes were deposited in the BOLD database (prefix GLF) and in the DNA Databank of Japan, European Molecular Biology Laboratory and GenBank repositories with the accession numbers LC163599–606. In addition, the complete mitochondrial genome DNA sequence (mitogenome) was determined for H. macrochir (ZMUC P3369) according to laboratory works presented in Poulsen et al. (2016) and deposited in the same repositories with the accession number AP018130. Specimens newly barcoded or downloaded for this study are listed in Table 2, including the associated metadata.
Results
New species records
Three subarctic notacanthiform observations; Aldrovandia oleosa, Halosauropsis macrochir and Notacanthus bonaparte provide new records for the Greenland fish fauna and novel range extensions in the North Atlantic. The new records are shown in Figs. 1 and 2. Photo and illustration material are included for all subarctic Atlantic species of notacanthiforms in Figs. 3 and 4, corresponding to a taxonomic key presented below. Each species present in the subarctic Atlantic is discussed below, with notes on morphology, distribution, and/or observations relevant to taxonomy, development or abundance.
New notacanthiform distributions in the subarctic Atlantic, newly caught. a Notacanthus bonaparte. b Halosauropsis macrochir. c Aldrovandia oleosa. Note the newly discovered bioluminescent structures on the head of A. oleosa. Morphometrics and meristics for all specimens presented in Table 1. Photos, ID, and sampling by J.Y. Poulsen (GINR)
Photo identification keys for subarctic Atlantic halosaurs and notacanthids. Drawings included correspond to the characters listed in the taxonomic key showing defining characters for species separation. Photo credit: A. phalacra by T. Wenneck (IMR), H. macrochir, N. bonaparte, P. rissoanus and P. challengeri by A. Orlov (VNIRO), A. oleosa and N. chemnitzii by J. Nielsen (GINR), P. rissoanus (insert), N. bonaparte (insert), N. chemnitzii (insert) and H. macrochir (insert) by J.Y. Poulsen (GINR)
Illustrations of all notacanthiform taxa presently registered in the subarctic Atlantic Ocean. Illustrations correspond with the taxonomic key presented in the text and with the photo key in Fig. 3. Aldrovandia oleosa shows luminous tissues present in the head (insert) and the two Aldrovandia taxa are illustrated with and without the fragile scales being easily lost
Greenland Fishes (GLF) barcoding project
All barcodes are made publicly available at www.barcodinglife.org (Ratnasingham and Hebert 2007), updated annually, with records having the prefix GLF. The Greenland fish taxa barcoded up to now are ~200 species out of ~300 currently known in this region (J.Y. Poulsen, personal observation), and can easily be identified using Cox1 barcodes with respect to known Greenland fish biodiversity. This provides for easy identification of, for example, stomach content, or similar studies that require a reference library for the Greenland fish fauna. The few taxa showing possible problematic identification using the Cox1 barcode are currently being examined, and require additional materials covering larger geographic regions.
The molecular taxonomic cladogram based on Cox1 barcodes is presented in Fig. 5, including colour-codes corresponding to geographic regions for the samples used. All notacanthiform genera included with more than one taxon were found to be monophyletic. The monotypic Lipogenys gillii (Goode and Bean 1895) was found to be a sister taxon to the remaining notacanthids, and the monotypic H. macrochir a sister taxon to the remaining halosaurs. Little intraspecific variation was found within taxa (except for N. chemnitzii) and no structure in the context of geographic sampling could be detected, although sample sizes are small. The mitogenome DNA sequence of H. macrochir is 16,655 base pairs long, and shows the two rRNAs, the 22 tRNAs, and the 13 protein coding genes in the canonical mitochondrial gene order as in other notacanthiform fishes (e.g., Inoue et al. 2004).
K2P-distance analysis of Cox1 barcodes including all notacanthiform taxa present in the Atlantic Ocean except Polyacanthonotus merretti, Aldrovandia rostrata, and A. gracilis. GLF indicates specimens sampled off Greenland. Note the five possible OTUs within the species complex N. cf. chemnitzii, including the three present off SE Greenland alone
Distributions
Notacanthiformes in Greenland waters and the subarctic Atlantic
Family Notacanthidae
Polyacanthonotus rissoanus (Figs. 3a and 4a)
Known range: Atlantic Ocean including the Mediterranean (Sulak et al. 1984; Crabtree et al. 1985; Porteiro et al. 2017). Subarctic Atlantic range: known from the Northern MAR (Porteiro et al. 2017), off Iceland (Jónsson and Pálsson 2013), off Greenland (Møller et al. 2010), and from Davis Strait Eastern Canada (ARC specimens). Greenland: the distribution of this species is extended from this study to encompass SE Greenland, in addition to already established records off SW Greenland (Table 2; Møller et al. 2010). However, the missing distribution off SE Greenland in the latter study is an error, as it was observed from multiple specimens caught annually onboard R/V Pâmiut (J.Y. Poulsen, personal observation). This is in agreement with material from 1973 (ZMH 111395) that shows this taxon present off SE Greenland (K. Sulak, personal observation). Relative to other notacanthiforms, it is second in abundance only to Notacanthus cf. chemnitzii, and is common throughout the subarctic Atlantic. Morphological variations in P. rissoanus from different geographic regions were noted by Sulak et al. (1984), although not explored further. However, personal observations by K. Sulak show that number of vertebrae, dorsal fin spines, and temperature affinity varies between North Atlantic and Mediterranean material. The limited sampling (four specimens) included in this study shows little molecular variation (Fig. 5).
Dorsal fin origin anterior to pectoral with 26–36 separated dorsal spines; pelvics 1 + 7–11; pectorals 1 + 10–16; gill rakers 23–28; pyloric caeca 3, short and whitish-light grey: benthopelagic at depths 650–1,800 m; maximum size unknown, although preanal length no more than 400 mm; sexually dimorphic, mature males with black nostrils; opercular distal flap black including parts of mouth (Sulak 1986).
Polyacanthonotus challengeri (Figs. 3b and 4b)
Known range: cosmopolitan and anti-tropical species in all major oceans (Sulak et al. 1984; Crabtree et al. 1985). Subarctic Atlantic range: known from the Northern MAR (Porteiro et al. 2017) and off Iceland (Jónsson and Pálsson 2013). Greenland: not known. This species is rarely observed, likely due to depth preferences deeper than usually surveyed. Barros-García et al. (2015) indicated at least two molecular OTUs within this taxon.
Dorsal origin posterior to pectoral with 32–46 strong separated dorsal spines; pelvic fins with fulcral spines + 8–10 spines (the last double); pectoral fins with fulcral spines + 10–14 spines; gill rakers 11–21; pyloric caeca 3 (short); body colour as P. rissoanus, although lateral line very distinct and darker; benthopelagic at depths 1,300–3,700 m; maximum size 590 mm; sexually dimorphic, mature males with darkened nostrils; females outnumber males (Sulak 1986).
Notacanthus bonaparte (Figs. 2a, 3c and 4c)
Known range: Mediterranean Ocean and the North Atlantic (Hartel et al. 2008). Subarctic Atlantic range: known from the Northern MAR (Porteiro et al. 2017), the Bill Bailey and Lousy Banks (ZMH specimens), and off Iceland (Jónsson and Pálsson 2013). New distribution (Greenland): known from one specimen off Denmark Strait SE Greenland (62.11°N, 40.11°W), deposited as ZMUB 22985 (tissue JYP#1600), sampled onboard R/V Pâmiut August 27, 2014, 3.07 °C bottom temperature with fishing depth 1,401–1,428 m using the Alfredo III trawl probing Greenland halibut abundances. Coll./ID J.Y. Poulsen. This record verifies the presence of N. bonaparte in Greenland waters, as noted by Sulak (1986), although erroneously left out by Møller et al. (2010) (personal observation, K. Sulak). The most northern record from public databases and/or Zoological collections (excluding the Greenland and Iceland specimens) is ZMH 112216 from approximately 60.16°N, 10.16°W. A personal observation by K. Sulak list ISH 3329/79 from 59.58°N, 12.07°W as N. bonaparte, verified with radiographs, with both specimens being from a Walter Herwig (WH 237/79) cruise. Observations of N. bonaparte are similar to P. challengeri, as they are probably rare due to the depth preferences of this species being below the usually fished depths in this region. Little molecular variation was found within this taxon (Fig. 5).
Origin of first dorsal posterior to pelvic fins with 5–9 dorsal spines; anal fin with 11–14 spines + 100–140 soft rays; pelvic fins with fulcral spine + 2–4 + 7; pectorals 9–12; pyloric caeca 3–4; palatine and dentary teeth in single rows; premaxillary teeth 14–22 on each side; orbit length approximately 2/3 snout; inter-orbital distance less than eye diameter; lateral line inconspicuous; benthopelagic at depths 700–2,000 m; however, not caught above 1,400 m off Greenland; maximum size 400 mm with preanal lengths 150–200 mm; body colour pinkish or magenta, separating it clearly from N. chemnitzii, edge of gill cover and mouth darker; sexual dimorphism unknown except that females apparently grow larger than males; probably a gregarious taxon; size differences present between Mediterranean and Atlantic populations, although have not been explored further (Sulak 1986). Morphometric and meristic data presented in Table 1.
Notacanthus chemnitzii (Figs. 3d and 4d)
Known range: cosmopolitan and anti-tropical species in all major oceans (Moore et al. 2003). Subarctic Atlantic range: Known from the Northern MAR (Porteiro et al. 2017), off Iceland (Jónsson and Pálsson 2013), off Greenland (Møller et al. 2010), in Davis Strait Eastern Canada (ARC specimens), and off Norway at approximately 63°N, 6°E (ZMUB 08869), the most northern record registered in the NE Atlantic off Bjørnsund, Norway (I. Byrkjedal, personal observation). Greenland: known from multiple specimens off SW and SE Greenland, and is by far the most common notacanthid in the region. Molecular data of N. chemnitzii indicate multiple OTUs present, with three found off SE Greenland alone (Fig. 5). These results are discussed later.
Dorsal origin approximately above pelvics; premaxillary teeth 19–37 on each side; palatine and dentary teeth in multiple rows; eye approximately half snout length; inter-orbital distance approximately 2 times the eye diameter; dorsal fin origin above pelvics, with 8–12 dorsal fin spines; anal fin with 20–21 spines + more than 110 soft rays; pelvics with fulcral spine + 2–4 + 6–9; pectorals 10–17; pyloric caeca 4–8; maximum 1,200 mm TL with preanal length 440 mm; large depth distribution, from 125 to probably 2,500 m although apparently distributed in shallower waters in subarctic regions; common throughout the subarctic Atlantic; body colour brownish or greyish depending on life stage (Sulak 1986; Smith 2002). Sexual dimorphism concerning maximum size has been assumed for this taxon, although see Discussion later. Peculiarly, loss of caudal fin, plausible to have occurred in the larval or juvenile stage, with subsequent scar tissue regrowth into a truncated caudal fin is more often observed in this species than any other taxa occurring in the region (Fig. 3 insert, J.Y. Poulsen, personal observation).
Family Halosauridae
Halosauropsis macrochir (Figs. 2b, 3e and 4e)
Known range: circumglobal/cosmopolitan distribution, although records are relatively scattered (e.g., Moore et al. 2003). Subarctic Atlantic range: known from the Northern MAR only (Bergstad et al. 2012; Porteiro et al. 2017). New distribution (Greenland): known from one specimen off Denmark Strait SE Greenland (62.09°N, 40.33°W), deposited as ZMUC P3369 (tissue/field JYP#9783), sampled on board R/V Pâmiut August 5, 2011, 4.45 °C bottom temperature with fishing depth 986–1,053 m using the Alfredo III trawl probing Greenland halibut abundances. Coll./ID unknown/J.Y. Poulsen.
Dorsal origin approximately opposite pelvics; top of snout and head scaleless, opercle scaled; ventro-lateral situated lateral line distinct with 26–32 preanal enlarged tubed scales; pectorals situated dorso-posterior to the head; dorsal fin rays 11–13, the first long and segmented; pectorals 1 + 11–12; pelvics 1 + 9; pyloric caeca 10–12, relatively large, unpigmented and in double rows; body colour bluish-black with snout, top of head, isthmus, and branchiostegal membranes black; maximum TL approximately 900 mm (preanal length 320 mm) (Sulak 1986; Smith 2002). Sex ratio skewed (Bergstad et al. 2012), although no previously reported sexual dimorphism; benthopelagic in depths between 1,100 and 3,300 m (Bañón et al. 2016). Morphometric and meristic data presented in Table 1.
Aldrovandia oleosa (Figs. 2c, 3f and 4f)
Known range: circumglobal/cosmopolitan species (Moore et al. 2003). Subarctic Atlantic range: None. New distribution (Greenland): Known from one specimen off Denmark Strait SE Greenland (61.56°N, 39.53°W), deposited as ZMUB 21435 (tissue JYP#10030), sampled on board R/V Pâmiut August 2012, 3.12 °C bottom temperature with fishing depth 1366–1374 m using the Alfredo III trawl probing Greenland halibut abundances. Coll./ID J.Y. Poulsen. The newly recorded specimen from off Greenland is unambiguously identified as A. oleosa from both morphological (Table 1) and molecular data (Fig. 5). This brings the status of A. phalacra off Greenland into question, and is discussed later.
Dorsal origin posterior to pelvics; palatine and pterygoid bones widely separated (Fig. 3 insert); dorsals 1 + 9–11; pectorals 1 + 8–11: pelvics 1 + 8–9; gill rakers 17–23; pyloric caeca 5–9, unpigmented (black); lateral line situated ventro-lateral, indistinct with 16–21 pre-vent modified tubed scales; vent colour white, surrounded by a ring of pigmented skin; luminescent tissues present in ZMUB 21435, observed in the newly caught and preserved specimen as particular whitish tissue on the head, jaws, and in the oral cavity (Figs. 2c and 4f), although absent on the most posterior opercular and most ventral branchiostegal regions. The following luminous organs were observed: snout organ (SO), a premaxillary organ (PO), a mental organ (MO), a dentary organ (DO), a gular organ (GO) and luminous tissues in the oral cavity (OC). The luminous tissues are illustrated in Fig. 4f, except the GO and OC. Some of the luminous tissues can be observed in the photo of the fresh specimen (Fig. 2c), and the luminescence at time of capture was considerable (J.Y. Poulsen, personal observation). Sexual dimorphism present, with males showing a fleshy black tube associated with the anterior nostril (Sulak 1977). Benthopelagic in depths between 1,100 and 3,300 m (Bañón et al. 2016). Morphometric and meristic data presented in Table 1.
Aldrovandia phalacra (Figs. 3g and 4g)
Known range: circumglobal/cosmopolitan species in all major oceans (Porteiro et al. 2017). Subarctic Atlantic range: known from the Northern MAR (Porteiro et al. 2017) and off SW Greenland (Okamura and Takahashi 1995; Møller et al. 2010). Greenland: known from one specimen (HUMZ 113685) off SW Greenland (Okamura and Takahashi 1995), although misspelled as A. phalacura by the latter. Confirmation of this species was not possible, however, as the specimen could not be located by HUMZ collection managers for re-identification (May 2014). It was retained as a Greenland taxon, although with some reservations. Taxonomy discussed later.
Dorsal origin posterior to pelvics; dorsals 10–12, pelvics 1 + 8, pectorals 1 + 11–13, pectorals reach pelvic origin; palatines separate; gill-rakers 19–23, lateral line situated ventro-lateral, indistinct with 24–28 pre-vent modified tubed scales; pyloric caeca 5–8, pigmented (black), in single row; body colour light grey, head bluish, with dark margins on gill cover and ventral on head; maximum length 400–500 mm (preanal length 180 mm). Sexually dimorphic, males have enlarged nostrils with anterior one tubed and darkly pigmented; females outnumber males; benthopelagic in depths between 500 and 2,300 m (Sulak 1986; Smith 2002; Bañón et al. 2016).
Key to adults of the Notacanthiformes in Atlantic subarctic waters (corresponds with Figs. 3 and 4)
-
1a. Relatively round snout, snout length 2× or less the eye length; dorsal fin spines robust and separated (Notacanthidae) ...............................................................2
-
1b. Relatively pointed snout, snout length more than 3× the eye length; one relatively short dorsal fin with 10–13 fin rays (Halosauridae) ....................................................5
-
2a. 26–40 separated dorsal fin spines ..............................3
-
2b. 6–15 separated dorsal fin spines ................................4
-
3a. Mouth not extending to posterior of anterior of eye; first dorsal fin spine in advance of pectorals .............................................Polyacanthonotus rissoanus
-
3b. Mouth extending to posterior of anterior part of eye; first dorsal fin spine posterior to pectorals ..........................................Polyacanthonotus challengeri
-
4a. Body colour pale-purplish, possibly magenta; one teeth row in lower jaw; maximum 400 mm SL ....................................................Notacanthus bonaparte
-
4b. Body colour tan to dark brown; at least two teeth rows in lower jaw; maximum 1200 mm SL ....................................................Notacanthus chemnitzii
-
5a. One row of relatively large and distinct lateral line scales ventro-laterally on body; double row of unpigmented pyloric caeca; first dorsal-fin ray as long as second and segmented; scales on opercle .................................................Halosauropsis macrochir
-
5b. One row of relatively small and indistinct lateral line scales ventro-laterally on body; pyloric caeca pigmented (black) in single row; first dorsal-fin ray much shorter than second and unsegmented; no scales on opercle ......6
-
6a. Length of palatine (variable in length) 1–4 times the distance between palatine and pterygoid bones in upper jaw; 16–21 lateral line scales before vent, relatively dark body colour; pectoral-fin rays 9–11 ...........................................................Aldrovandia oleosa
-
6b. Length of palatine less than half the distance between palatine and pterygoid bones in upper jaw; 24–28 lateral line scales before vent; relatively pale body colour; pectoral-fin rays 11–13 .......................................................Aldrovandia phalacra
Discussion
Taxonomy and distributions of subarctic Atlantic Halosauridae
With the new finding of A. oleosa, the status of A. phalacra in the NW subarctic Atlantic has become problematic, as it has only been observed by Okamura and Takahashi (1995) from one specimen caught off SW Greenland. However, this observation could not be confirmed. Aldrovandia phalacra was, peculiar, compared to only the deep-water tropical distributed A. rostrata by Okamura and Takahashi (1995), despite being more similar to the NW Atlantic distributed A. oleosa described by Sulak in 1977. Distance between the palatine and pterygoid bones and number of lateral line scales, separating the two taxa, were not included by the latter study. A smaller distance between the palatine and pterygoid bones was found by Bañón et al. (2016) in eastern Atlantic specimens caught off Spain, although allometry was noted and distances compared should be from similarly sized fishes. Barcoding Cox1 DNA sequences show unambiguous species separation of these taxa (Fig. 5), corroborated by dentition and lateral line-scale counts that separate the two species. Both A. oleosa and A. phalacra are considered circumglobal, with wide distributions (Kamikawa and Stevenson 2010). Comparison of A. oleosa (ZMUB 21435, Fig. 2) off SE Greenland, including information of A. phalacra by Okamura and Takahashi (1995), revealed no certain differences in the morphometric characters. Both Aldrovandia species have been reported from the Bear Seamount in the NW Atlantic at approximately 40°N (Moore et al. 2003).
The finding of H. macrochir off SE Greenland in 2010, a first record for this region and the most northern specimen observed to date, is not surprising, as this species is a common component in the MAR system (Bergstad et al. 2012; Porteiro et al. 2017). It is present south and north of the Charlie Gibbs fracture zone, an important transition area related to species distribution into subarctic waters (Vecchione et al. 2010; Sutton et al. 2013). In fact, it is peculiar that it has not been observed previously, off Greenland or in Iceland waters (Jónsson and Pálsson 2013), although it is probably associated with depths that are infrequently surveyed. H. macrochir is a common species in the MAR system, previously caught in large numbers as far north as 53.27°N, 35.53°W during the MAR-ECO cruise in 2004 (Porteiro et al. 2017). Aldrovandia oleosa has never been reported from the MAR, whereas the most northern specimen of A. phalacra (ZMUB 15971) is present from 49.52°N, 29.38°W (I. Byrkjedal, personal observation). The Greenland H. macrochir specimen shows a few more lateral line scales and pyloric caeca compared to reported specimens, therefore slightly expanding the meristic ranges of these characters (Table 1). Barcoding data of North Atlantic H. macrochir specimens show OTUs with little variation, i.e., only one silent base substitution (amino acid Methionine) between the Greenland specimen and MAR materials (Fig. 5). One or two silent base substitutions in the widely used barcoding gene fragment of Cox1 (approximately 600 base pairs of a total of Cox1 1551 base pairs) are often observed between populations, or even between individuals from the same geographic region (Fig. 5 and J.Y. Poulsen, personal observation). The new record of H. macrochir off Greenland is a female, although males apparently outnumber females in this species (Bergstad et al. 2012).
It is of note that either A. oleosa or H. macrochir have been registered off SW Iceland (Jónsson and Pálsson 2013). This area is in many ways a proxy for subsequent Greenland range expansions, as the area shows highly similar abiotic environments to SE Greenland and connected by the same warmer currents, slightly higher temperatures, as well as being connected to the MAR system for a possible gateway of seamount-associated taxa (Fig. 1). Taxa extension ranges connected to bottom topological features such as the MAR and the New England seamount chain (Moore et al. 2004) are relatively poorly known. A 1 °C temperature increase has been witnessed during the last 20 years in surface layers off SW Greenland (Masson-Delmotte et al. 2012), including short temporal bursts of warmer waters affecting species distribution in surface layers (MacKenzie et al. 2014; Palsson and Astthorsson 2016), although deeper layers remain unknown if affected by these temporal variable changes. Ocean temperature change in relation to depth is vital knowledge for monitoring species distributions, as latitude is second to depth with regard to delimitation of species diversity (Sutton et al. 2008; Vecchione et al. 2010). Several notacanthiform taxa are occupying habitats inaccessible to regular bottom-trawls, as for example continental margins (Nelson 2006). The latter explanation is probably also an important factor as to why few shark species/specimens are caught in the areas surveyed (J.Y. Poulsen, personal observation). However, this must be considered in a context of habitat adaptations, as teleosts and chondrichthyans have evolved differently with regard to deep-sea trophic guilds (Musick and Cotton 2015).
Aldrovandia rostrata was depicted by Sulak (1977) to be caught in the MAR at 50°N, although this is an error (verified with K. Sulak), as it is not recorded in the species description by Günther (1878), the species account (Günther 1887), in McDowell (1973), in Sulak (1986), or in Porteiro et al. (2017). Aldrovandia affinis (Günther 1887) and Halosaurus guentheri (Goode and Bean 1896) are figured as present in subarctic Atlantic waters in online databases, although no valid records of these distributions are present (e.g., Moore et al. 2003).
Taxonomy and distributions of subarctic Atlantic Notacanthidae
Jónsson and Pálsson (2013) reported the presence of P. challengeri off Iceland, a taxon not yet confirmed off Greenland (Møller et al. 2010), as it inhabits depths not regularly surveyed. This indicates that incidental catches of halosaurs in the Irminger Sea are probably related to inaccessible fishing habitats and/or depths infrequently surveyed. Similarly to halosaurs, isolated observations of rare subarctic notacanthids below 1,000 m mostly reveal what we do not know at this time (Crabtree et al. 1985). On the contrary, P. rissoanus is a common observation off Greenland and appears to be the bathyal sister taxon to the rarely caught abyssal P. challengeri (Fig. 5). Notacanthus bonaparte was noted off Greenland by Sulak (1986), and Nielsen and Bertelsen (1992). However, it was excluded in an annotated checklist by Møller et al. (2010), as it could not be confirmed at that time. Notacanthus bonaparte is confirmed by the present study off Greenland including both meristic, morphometric, and molecular data (Tables 1 and 2). The presence of N. bonaparte in SE Greenland waters is unsurprising, as this species is also found off SE Iceland (Jónsson and Pálsson 2013), and a few specimens have been caught at the Bear Seamount in the western Atlantic (Hartel et al. 2008). Similarly to other notacanthids, N. bonaparte habitats are much deeper than N. chemnitzii, which is probably the reason of its absence in regular trawl catches in the area. The new record presented in this study of N. bonaparte was caught below 1,400 m, and considering the extensive fishing efforts carried out in the region during the last 20 years (Fig. 1), it is likely that 1,400 m is close to the upper depth limit for this taxon in this region.
Luminescent tissues in Aldrovandia oleosa
The A. oleosa specimen caught off SE Greenland has luminescent tissues present that have not been noted before in this species, nor in any other halosaurs for that matter. The observation of luminescent tissues present in A. oleosa is noteworthy and is possibly associated with a benthopelagic habitat (K. Sulak, personal observation). Several luminescent tissues are present on the head in positions known from a variety of other deep-sea fishes (e.g., Sazonov 1996), including the SO, PO, MO, DO, GO, and luminous tissues in the oral cavity (OC) (Fig. 4f). A thorough examination of luminous tissues in halosaurs is beyond the scope of this study and to be discussed elsewhere. The A. oleosa specimen was caught at 1,370 m depth, and therefore no penetrating sunlight may be expected at depth of capture. Bioluminescent tissues in deep-sea fishes are poorly known at present (e.g., Claes et al. 2013; Poulsen 2015a; Paitio et al. 2016), and morphological studies addressing such observations are required across deep-sea fish groups.
Initiating molecular taxonomy of fish in the subarctic Atlantic Ocean
In this study, we present a publicly available barcoding initiative named the “Greenland Fishes” barcoding program. The program was initiated in 2010 by the GINR in order to facilitate molecular taxonomy of known and new fish species recorded in Greenland waters — past and future. Several studies have already used the results of this program (e.g., Orlov et al. 2015; Barros-García et al. 2016; Poulsen 2015a, b) to resolve taxonomic or extended species range issues, and we encourage the use of the results located on BOLD in molecular taxonomy projects, if useful. We also encourage contacting the authors concerning specific taxonomic groups of interest, in order to focus the sampling on taxonomic issues needing data from this region. All data is indexed to BOLD and updated annually to establish biodiversity baselines and predictions in this important region, considering ocean temperature change and the prevalent taxonomic issues, which are rampant in deeper waters, that constitute the large parts of the Greenland Exclusive Economic Zone (EEZ) water bodies. Approximately 30 new species records are present off Greenland alone that have not been published as of June 2017 (J.Y. Poulsen, personal observation). However, all records are to be found in BOLD as part of the Greenland Fishes Barcoding Project (this study, GLF records).
Concerning molecular taxonomy of the Notacanthiformes from this study, no noteworthy results are present, except N. cf. chemnitzii, which requires further validation or sampling per se (Fig. 5). Little intraspecific variation in the Cox1 barcode is observed for all taxa included, considering the geographic regions of samples. Although morphological studies are evidently necessary to validate the taxonomic status of species, molecular taxonomy is useful as an indicator of potential taxonomic issues. An example of this to follow is presented for the Mediterranean codling Lepidion lepidion (Risso 1810). It was previously considered a different species to the North Atlantic codling Lepidion eques (Günther 1887) that is now a synonym of the former, as Barros-García et al. (2016) found that no molecular structure in the Cox1 barcodes from the North Atlantic, including the Mediterranean and GLF samples of SE Greenland, was present in the Lepidion samples. This indicated one North Atlantic species only. This result consolidated a morphological study by Bañón et al. (2013) that similarly showed no structure in the morphological characters usually employed for species separation. Only in the case of N. chemnitzii is the molecular taxonomy observed (Fig. 5) noteworthy and therefore discussed below. Taxonomy of N. chemnitzii constitutes an old ichthyological conundrum which is far from resolved (McDowell 1973).
Species complex Notacanthus cf. chemnitzii
The taxon currently valid as Notacanthus chemnitzii may be a taxonomic conundrum at present, and comprises a complicated example in the grey zone of classification considering distinct populations, subspecies, or species. At present, N. chemnitzii is considered circumglobal although morphological variation is known, especially along the latitudinal cline (McDowell 1973). No studies since McDowell (1973) have examined morphometrics and meristics of N. cf. chemnitzii, whereas Cox1 barcoding data, on the contrary, have repeatedly shown several OTUs present within the taxonomic unit N. chemnitzii, both from the Pacific and from the Atlantic (Steinke et al. 2009; Ward et al. 2008; McCusker et al. 2012; Barros-García et al. 2015). The results presented in this study, including new data of specimens sampled off SE Greenland, take the issue one step further and show three OTUs present in the NW Atlantic. In fact, three haplotypes are found off SE Greenland alone (Fig. 5).
Considering the molecular delimitation of multiple OTUs, N. chemnitzii presents an interesting case of either problematic delimitation from morphological characters available, or a special case of developmental migration from southern to northern latitudes (McDowell 1973). The latter study notes that adult sizes are considerably longer at northern latitudes off New England as compared to the Gulf of Mexico. In fact, it was noted that not a single northern specimen caught showed a length approaching the southern population, consequently indicating an association between northward migration and size. An absence of large ripe females in the southern population was noted. However, gill raker counts were observed to be different, as the northern population showed 16–17 gill rakers on the first arch whereas 12–15 gill rakers were found in the southern population. The molecular results from this study support the presence of multiple Atlantic Ocean OTUs (Fig. 5) and not migration with age. Knowledge of N. chemnitzii (and notacanthiforms in general) eggs and leptocephali larvae is scarce and often non-existent on the species level (Smith 1979; Castle 1984). Although one step closer from this study, the taxonomy of the Notacanthus cf. chemnitzii species complex remains an outstanding ichthyological conundrum. We note that variations have already been established within N. cf. chemnitzii otoliths (Campana 2004; Vedishcheva et al. 2016), indicating that a thorough taxonomic study on adults could potentially resolve some of the prevalent taxonomic issues at this stage.
Abbreviations
- SE:
-
Southeast
- SW:
-
Southwest
- NW:
-
Northwest
- NE:
-
Northeast
- OTU:
-
Operational Taxonomic Unit
- MAR:
-
Mid-Atlantic Ridge
- SPF:
-
Subpolar Front
References
Bañón R, Arronte JC, Vázquez-Dorado S, Río JLD, Carlos AD (2013) DNA barcoding of the genus Lepidion (Gadiformes: Moridae) with recognition of Lepidion eques as a junior synonym of Lepidion lepidion. Mol Ecol Res 13:189–199. doi:10.1111/1755-0998.12045
Bañón R, Arronte JC, Armesto Á, Barros-García D, Carlos AD (2016) Halosaur fishes (Notacanthiformes: Halosauridae) from Atlantic Spanish waters according to integrative taxonomy. Zootaxa 4184(3):471–490. doi:10.11646/zootaxa.4184.3.3
Barros-García D, Arronte JC, Fernández-Peralta L, García R, De Carlos A (2015) DNA barcoding of deep-water notacanthiform fishes (Teleostei, Elopomorpha). Zool Scr 45(3):263–272. doi:10.1111/zsc.12154
Barros-García D, Bañón R, Arronte JC, De Carlos A (2016) New data reinforcing the taxonomic status of Lepidion eques as synonym of Lepidion lepidion (Teleostei, Gadiformes). Biochem Syst Ecol 68:6–10
Bergstad OA, Clark L, Hansen HØ, Cousins N (2012) Distribution, population biology, and trophic ecology of the deepwater Demersal fish Halosauropsis macrochir (Pisces: Halosauridae) on the mid-Atlantic ridge. PLoS One 7(2):e31493. doi:10.1371/journal.pone.0031493
Bloch ME (1788) Ueber zwey merkwürdige Fischarten. Abh Böhmischen Ges Wiss 3:278–282
Byrkjedal I, Godø OR, Heino M (2004) Northward range extensions of some mesopelagic fishes in the northeastern Atlantic. Sarsia 89:484–489. doi:10.1080/00364820410009265
Campana SE (2004) Photographic atlas of fish otoliths of the Northwest Atlantic Ocean. NRC Research Press, Ottawa, pp 1–284
Castle PHJ (1984) Notacanthiformes and Anguilliformes: Development. In: Moser HG, Richards WJ, Cohen DM, Fahay MP, Kendall AW Jr, Richardson SL (eds) Ontogeny and systematics of fishes. American Society of Ichthyology and Herpetology Special Publication 1. Allen Press, Lawrence Kansas, pp 62–93
Chen J-N, López JA, Lavoué S, Miya M, Chen W-J (2014) Phylogeny of the Elopomorpha (Teleostei): evidence from six nuclear and mitochondrial markers. Mol Phylogenet Evol 70:152–161
Chernova NV (2014) New species of the genus Careproctus (Liparidae) from the Kara Sea and identification key for congeners of the North Atlantic and Arctic. J Ichthyol 54(10):757–780
Claes JM, Dean MN, Nilsson D-E, Hart NS, Mallefet J (2013) A deepwater fish with ‘lightsabers’ — dorsal spine-associated luminescence in a counterilluminating lanternshark. Sci Rep 3:1308. doi:10.1038/srep01308
Crabtree RE, Sulak KJ, Musick JA (1985) Biology and distribution of species of Polyacanthonotus (Pisces: Notacanthiformes) in the western North Atlantic. Bull Mar Sci 36:235–248
De Filippi F, Verany GB (1857) Sopra alcuni pesci nuovi o poco noti del Mediterraneo. Nota. Mem Accad Sci Torino (Ser 2) 18:187–199
Eschmeyer WN, Fricke R, van der Laan R (eds) (2017) Catalog of fishes: genera, species, References. (http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp). Electronic version Accessed 10 Jan 2017
Figueroa DE, Brunetti NE, Sakai M (2008) The southernmost record of notacanthiform Tiluropsis leptocephali, with notes on possible species identity. Mar Biodiv Rec 1(e53):1–4. doi:10.1017/S1755267207005805
Fossheim M, Primicerio R, Johannesen E, Ingvaldsen RB, Aschan MM, Dolgov AV (2015) Recent warming leads to a rapid borealization of fish communities in the Arctic. Nat Clim Chang 5:673–678. doi:10.1038/nclimate2647
Gilchrist JDF, von Bonde C (1924) Deep-sea fishes procured by the S.S. “Pickle” (part II). Rep Fish Mar Biol Surv Union S Afr 3(7):1–24
Goode GB, Bean TH (1895) A revision of the order Heteromi, deep-sea fishes, with a description of the new generic types Macdonaldia and Lipogenys. In: Scientific results of explorations by the U. S. Fish Commission steamer Albatross. Proc US Nat Mus 17(1013):455–470
Goode GB, Bean TH (1896) Oceanic ichthyology, a treatise on the deep-sea and pelagic fishes of the world, based chiefly upon the collections made by the steamers Blake, Albatross, and Fish Hawk in the northwestern Atlantic, with an atlas containing 417 figures. Spec Bull US Nat Mus 2:1–553
Günther A (1878) Preliminary notices of deep-sea fishes collected during the voyage of H.M.S. Challenger. Ann Mag Nat Hist 2:248–251
Günther A (1887) Report on the deep-sea fishes collected by H. M. S. Challenger during the years 1873–76. Zoology 22:241
Hartel KE, Kenaley CP, Galbraith JK, Sutton TT (2008) Additional records of deep-sea fishes from off greater New England. Northeast Nat 3:317–334
Inoue JG, Miya M, Tsukamoto K, Nishida M (2004) Mitogenomic evidence for the monophyly of elopomorph fishes (Teleostei) and the evolutionary origin of the leptocephalus larva. Mol Phylogen Evol 32:274–286. doi:10.1016/j.ympev.2003.11.009
Izzo C, Doubleday ZA, Grammer GL, Gilmore KL, Alleway HK, Barnes TC, Disspain MC, Giraldo AJ, Mazloumi N, Gillanders BM (2016) Fish as proxies of ecological and environmental change. Rev Fish Biol Fish 26(3):265–286. doi:10.1007/s11160-016-9424-3
Jónsson G, Pálsson J (2013) Islenskir Fiskar (Icelandic fishes), 2th edn. Mál og Menning, Reykjavík, pp 1–493
Kamikawa DJ, Stevenson DE (2010) New records of Aldrovandia oleosa (Notacanthiformes: Halosauridae) from the eastern North Pacific Ocean. Calif Fish Game 96(3):216–220
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Knudsen SW, Møller PR (2008) Careproctus kidoi, a new Arctic species of snailfish (Teleostei: Liparidae) from Baffin Bay. Ichthyol Res 55:175–182. doi:10.1007/s10228-007-0034-x
MacKenzie BR, Payne MR, Boje J, Høyer JL, Siegstad H (2014) A cascade of warming impacts brings bluefin tuna to Greenland waters. Glob Chang Biol 20:2484–2491. doi:10.1111/gcb.12597
Masson-Delmotte VM, Swingedouw D, Landais A, Seidenkrantz MS, Gauthier E, Bichet V, Massa C, Perren B, Jomelli V, Adalgeirsdottir G, Christensen JH, Arneborg J, Bhatt U, Walker DA, Elberling B, Chaulet FG, Ritz C, Gallée H, Van Den Broeke M, Fettweis X, de Vernal A, Vinther B (2012) Greenland climate change: from the past to the future. WIREs Clim Change 3:427–449. doi:10.1002/wcc.186
McCusker MR, Denti D, Van Guelpen L, Kenchington E, Bentzen P (2012) Barcoding Atlantic Canada’s commonly encountered marine fishes. Mol Ecol Res 1755:1–12. doi:10.1111/1755-0998.12043
McDowell SB (1973) Halosauridae. In: Woods LP, Sonoda PM (eds) Fishes of the Western North Atlantic. Suborder Halosauroidei. Memoirs of the Sears Foundation of Marine Research 1, p 132–228
Miller MJ, Tsukamoto K (2004) An introduction to leptocephali biology and identification. Ocean Research Institute, The University of Tokyo, Tokyo, pp 1–96
Moore JA, Hartel KE, Craddock JE, Galbraith JK (2003) An annotated list of deep-water fishes from off the New England region, with new area records. Northeast Nat 10:159–248
Moore JA, Vecchione M, Collette BB, Gibbons R, Hartel KE (2004) Selected fauna of bear seamount (New England seamount chain), and the presence of “natural invader” species. Arch Fish Mar Res 51:241–250
Musick JA, Cotton CF (2015) Bathymetric limits of chondrichthyans in the deep sea: a re-evaluation. Deep Sea Res II Top Stud Oceanogr 115:73–80. doi:10.1016/j.dsr2.2014.10.010
Møller PR, Nielsen JG, Knudsen SW, Poulsen JY, Sünksen K, Jørgensen OA (2010) A checklist of the fish fauna of Greenland waters. Zootaxa 2378:1–84
Nelson JS (2006) Fishes of the world, 4th edn. John Wiley & Sons, Hoboken
Nielsen JG, Bertelsen E (1992) Fisk i grønlandske farvande. Atuakkiorfik, Nuuk, Greenland, 68 pp
Okamura O, Takahashi N (1995) Halosauridae. In: Okamura O, Amaoka K, Takeda M, Yano K, Okada K, Chikuni S (eds) Fishes collected by the R/V Shinkai Maru around Greenland. Japan Marine Fishery Resources Research Center, Tokyo, pp 47–52
Olivar MP, Hulley PA, Castellón A, Emelianov M, López C, Tuset VM, Contreras T, Molí B (2017) Mesopelagic fishes across the tropical and equatorial Atlantic: biogeographical and vertical patterns. Progr Oceanogr 151:116–137. doi:10.1016/j.pocean.2016.12.001
Orlov AM, Orlova SY, Volkov AA, Pelenev DV (2015) First record of humpback anglerfish (Melanocetus johnsonii) (Melanocetidae) in Antarctic waters. Polar Res 34:25356. doi:10.3402/polar.v34.25356
Paitio J, Oba Y, Meyer-Rochow VB (2016) Bioluminescent fishes and their eyes. In: Thirumalai J (ed) Luminescence — an outlook on the phenomena and applications. InTech, Rijeka, pp 297–332. doi:10.5772/62517
Palsson J, Astthorsson OS (2016) New and historical records of the ocean sunfish Mola mola in Icelandic waters. J Fish Biol 90(3):1126–1132. doi:10.1111/jfb.13237
Porteiro F, Sutton T, Byrkjedal I, Orlov A, Heino M, Menezes G, Bergstad OA (2017) Fishes of the northern mid-Atlantic ridge collected during the MAR-ECO cruise in June-July 2004. Arquipelago Life Mar Sci Suppl 10:1–125
Poulsen JY (2015a) A new species of pencil smelt Nansenia boreacrassicauda (Microstomatidae, Argentiniformes) from the North Atlantic Ocean. Zootaxa 4020(3):517–532. doi:10.11646/xootaxa.4020.3.6
Poulsen JY (2015b) Fifth confirmed record and North Atlantic range expansion of the rare pelagic bobtail snipe eel genus Neocyema (Cyematidae, Elopomorpha). Mar Biodiv Rec 8(e53):1–5. doi:10.1017/S175526721500024X
Poulsen JY, Sado T, Hahn C, Byrkjedal I, Moku X, Miya M (2016) Preservation obscures pelagic deep-sea fish diversity: doubling the number of sole-bearing opisthoproctids and resurrection of the genus Monacoa (Opisthoproctidae, Argentiniformes). PLoS One 11(8):e0159762. doi:10.1371/journal.pone.0159762
Ratnasingham S, Hebert PDN (2007) BOLD: the barcode of life data system (www.barcodinglife.org). Mol Ecol Notes 7:355–364. doi:10.1111/j.1471-8286.2006.01678.x
Risso A (1810) Ichthyologie de Nice, ou histoire naturelle des poissons du Département des Alpes Maritimes. F. Schoell, Paris, 388 pp
Risso A (1840) Observations sur quelques poissons de la mer de Nice. Archiv für Naturgeschichte 6:376–393
Rose GA (2005) On distributional responses of North Atlantic fish to climate change. ICES J Mar Sci: J Conseil 62(7):1360–1374
Ryan WBF, Carbotte SM, Coplan J, O’Hara S, Melkonian A, Arko R, Weissel RA, Ferrini V, Goodwillie A, Nitsche F, Bonczkowski J, Zemsky R (2009) Global multi-resolution topography (GMRT) synthesis data set. Geochem Geophys Geosyst 10:Q03014. doi:10.1029/2008GC002332
Sazonov YI (1996) Morphology and significance of the luminous organs in alepocephaloid fishes. Bioinformat Ecol Series 11:151–163
Smith DG (1970) Notacanthiform Leptocephali in the Western North Atlantic. Copeia 1:1–9
Smith DG (1979) Guide to the Leptocephali (Elopiformes, Anguilliformes, and Notacanthiformes). NOAA Technical Report NMFS Circular 424, U.S. Department of Commerce, 1–39
Smith DG (2002) Halosauridae and Notacanthidae. In: Carpenter KE (ed) FAO species identification guide for fishery purposes. The living marine resources of the Western Central Atlantic 2. Bony fishes part 1 (Acipenseridae to Grammatidae). FAO, Rome, pp 685–689
Srivathsan A, Meier R (2012) On the inappropriate use of Kimura-2-parameter (K2P) divergences in the DNA-barcoding literature. Cladistics 28:190–194. doi:10.1111/j.1096-0031.2011.00370.x
Steinke D, Zemlak TS, Boutillier JA, Hebert PDN (2009) DNA barcoding of Pacific Canada’s fishes. Mar Biol 156:2641–2647. doi:10.1007/s00227-009-1284-0
Sulak KJ (1977) Aldrovandia oleosa, a new species of the Halosauridae, with observations on several other species of the family. Copeia 1:11–20
Sulak KJ (1986) Halosauridae, Notacanthidae. In: Whithead PJP, Bauchot ML, Hureau JC, Nielsen J, Tortonese E (eds) Fishes of the North-Eastern Atlantic and the Mediterranean, volume 2. UNESCO, Paris, pp 593–603
Sulak KJ, Crabtree RE, Hureau J-C (1984) Provisional review of the genus Polyacanthonotus (Pisces, Notacanthidae) with description of a new Atlantic species, Polyacanthonotus merretti. Cybium 8(4):57–68
Sutton TT, Porteiro FM, Heino M, Byrkjedal I, Langhelle G, Anderson CI, Horne J, Søiland H, Falkenhaug T, Godø OR, Bergstad OA (2008) Vertical structure, biomass and topographic association of deep-pelagic fishes in relation to a mid-ocean ridge system. Deep Sea Res II Top Stud Oceanogr 55(1):161–184. doi:10.1016/j.dsr2.2007.09.013
Sutton TT, Letessier TB, Bardarson B (2013) Midwater fishes collected in the vicinity of the sub-polar front, mid-North Atlantic Ocean, during ECOMAR pelagic sampling. Deep-Sea Res II 98:292–300. doi:10.1016/j.dsr2.2013.08.001
Vaillant LL (1888) Expéditions scientifiques du “Travailleur” et du “Talisman” pendant les années 1880, 1881, 1882, 1883. Poissons. Paris, 406 pp
Vecchione M, Bergstad OA, Byrkjedal I, Falkenhaug T, Gebruk AV, Godø OR, Gislason A, Heino M, Høines ÅS, Menezes GMM, Piatkowski U, Priede IG, Skov H, Søiland H, Sutton T, Wenneck TL (2010) Biodiversity patterns and processes on the mid-Atlantic ridge. Chapter 6. In: McIntyre AD (ed) Life in the world’s oceans: diversity, distribution, and abundance. Blackwell, Oxford. doi:10.1002/9781444325508.ch6
Vecchione M, Falkenhaug T, Sutton T, Cook A, Gislason A, Hansen HØ, Heino M, Miller PI, Piatkowski U, Porteiro F, Søiland H, Bergstad OA (2015) The effect of the North Atlantic Subpolar Front as a boundary in pelagic biogeography decreases with increasing depth and organism size. Prog Oceanogr 138:105–115. doi:10.1016/j.pocean.2015.08.006
Vedishcheva EV, Orlov AM, Orlova SY, Trofimova AO (2016) First data on the age, growth processes, and otoliths of snub-nosed spiny eel Notacanthus chemnitzii (Notacanthidae). J Ichthyol 56(6):890–898. doi:10.1134/S0032945216060102
Ward RD, Zemlak TS, Bronwyn HI, Last PR, Hebert PDN (2005) DNA barcoding Australia’s fish species. Phil Trans R Soc B 360:1462. doi:10.1098/rstb.2005.1716
Ward RD, Costa FO, Holmes BH, Steinke D (2008) DNA barcoding of shared fish species from the North Atlantic and Australasia: minimal divergence for most taxa, but Zeus faber and Lepidopus caudatus each probably constitute two species. Aquat Biol 3:71–78. doi:10.3354/ab00068
Wisz MS, Broennimann O, Grønkjær P, Møller PR, Olsen SM, Swingedouw D, Hedeholm RB, Nielsen EE, Guisan PL (2015) Arctic warming will promote Atlantic—Pacific fish interchange. Nat Clim Chang 5:261–265. doi:10.1038/nclimate2500
Acknowledgements
T. Kawai (Hokkaido University Museum, Japan) for specimen search, A. Bentley (Kansas University, USA) and other identifiers for access to North Atlantic fish barcoding data, I. Eidus & R. Thiel (Zoological Museum Hamburg, Germany) and R. Milne (Atlantic Reference Centre, Canada) for specimen data, E. Johannesen & R. Wienerroither (Institute for Marine Research, Norway) for information, T. Wenneck (Institute of Marine Research, Norway) and A. Orlov (Russian Research Institute of Fisheries and Oceanography, Russia) for MAR-ECO photographs, J. Nielsen and R/V Pâmiut crew (Greenland Institute of Natural Resources, Greenland) for photos and sampling during surveys, M. Krag (Zoological Museum University of Copenhagen, Denmark) for shipping of specimens and information, J. Hlidberg (private, www.fauna.is, Island) for help with illustrations, K. Graham, A. Hay, J. King, M. Lockett, M. McGrouther, K. Parkinson, J.R. Paxton, and S. Reader (Australian Museum, Australia) for help with X-rays, photography, and logistics, D. Barros-García (University of Vigo, Spain) for specimen and sequence information, and K. Sulak (U.S. Geological Survey, USA) and anonymous reviewer for valuable suggestions and comments. We owe a special thanks to I. Byrkjedal and G. Langhelle (Natural History Collections Bergen, Norway) for continuous help with specimens, tissue samples, curating, and providing morphometric/meristic data whenever needed, L. Lindblom (University of Bergen, Norway) for help with molecular work, I. Chemshirova (Zoological Society London, U.K.) for correcting manuscript, and to H. Siegstad (Greenland Institute of Natural Resources) for logistics. This research received no specific grant from any funding agency, commercial, or not-for-profit sectors (part of a larger effort to register biodiversity and facilitate onboard fish identification in Greenland waters).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by O. A. Bergstad
Rights and permissions
About this article
Cite this article
Poulsen, J.Y., Thorkildsen, S. & Arboe, N.H. Identification keys to halosaurs and notacanthids (Notacanthiformes, Elopomorpha) in the subarctic Atlantic Ocean including three new distributional records and multiple molecular OTUs of Notacanthus cf. chemnitzii . Mar Biodiv 48, 1009–1025 (2018). https://doi.org/10.1007/s12526-017-0762-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-017-0762-8