Abstract
Gnathiid isopods are among the most common ectoparasitic organisms infesting marine fishes in benthic marine environments, and are best known in coral reef ecosystems. These micropredators are ecologically important, as they can impact their hosts directly through blood loss and tissue damage, and indirectly by transmitting blood parasites. Their abundance, and thus expected impact on hosts, varies greatly over multiple spatial scales. Most studies on gnathiids in coral reef systems have been conducted in the Caribbean and on the Great Barrier Reef, Australia, with few studies conducted in the heart of marine biodiversity, the “Coral Triangle”. This is the first field study of gnathiid ecology in the Philippines. The primary goals of this study were to characterize substrate associations and diel activity patterns of gnathiids in shallow reef areas. Gnathiid larvae were found to infest at least 28 species of hosts and were found in both seagrass and reef habitats. Within reef habitat, there was a significant difference in gnathiid abundance among coral reef substrates. Gnathiid larvae were most abundant in coral rubble substrates and least abundant on live hard coral. This is in spite of host abundance (fish) being greater near live coral. More and larger-sized gnathiids were collected from dusk to dawn compared to daytime, and gnathiids were able to find hosts using non-visual sensory cues. Our results are, thus, consistent with previous studies in the Caribbean.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasites make up about 40% of the earth’s biodiversity (Hatcher and Dunn 2011) and represent the most common animal lifestyle (Hudson et al. 2006). They can have high local biomass in ecological communities (Kuris et al. 2008) and, through impacts on hosts, can influence populations (e.g., Hatcher and Dunn 2011), food webs (e.g., Huxham et al. 1995; Thompson et al. 2005; Lafferty et al. 2006; Hatcher and Dunn 2011; Amundsen et al. 2009; Johnson et al. 2010), competitive interactions (e.g., Hatcher et al. 2006; Raffel et al. 2010), and, thus, community dynamics (e.g., Thomas et al. 1998; Mouritsen and Poulin 2002; Wood et al. 2007; Lafferty et al. 2008; Hatcher and Dunn 2011). Parasites generally represent a neglected element of biodiversity in ecological communities, because of their small size (“out of sight, out of mind”) and inconspicuous position on or within their hosts (Hatcher and Dunn 2011; Poulin et al. 2014). To be identified with more precision, the typically small invertebrates that comprise the majority of parasitic organisms need detailed observation and preparation compared to vertebrates and larger invertebrates (Justine 2010). However, an understanding of any ecosystem requires a comprehensive characterization of its parasite fauna.
Although parasitism is recognized as an important species interaction in marine systems (Lafferty 2013), less than 5% of publications in marine ecology journals include parasites (reviewed by Poulin et al. 2014), and most of what we know about marine parasite communities comes from studies of easily accessible, “model” systems, economically important fish species in aquaculture, and opportunistic observations, leaving high biodiversity systems such as coral reefs particularly understudied. Diversity and population abundance of parasites on hosts can vary according to the habitat and host diversity (Marcogliese 2002; Hudson et al. 2006) and the diversity of potential hosts in coral reef systems. Thus, the parasite fauna of coral reef systems is expected to be extremely high. For example, it has been estimated that over 20,000 parasite species are associated with 1000 fish species within the Capricorn section of the Great Barrier Reef (Rohde 1976), and Justine (2010) and Justine et al. (2012) estimated approximately ten parasites per fish or 17,000 parasite species for the 1700 reef fishes of New Caledonia.
Approximately 70,000 known parasite species live on the external surface of their hosts and are, thus, ectoparasites (Poulin 2007). Among arthropod ectoparasites, about 14,000 species from 400 genera feed largely, or exclusively, on vertebrate blood and body fluids (Graça-Souza et al. 2006). In marine environments, these are represented primarily by copepods and isopods (Poore and Bruce 2012). The Isopoda are one of the largest and most diverse orders of crustaceans, with 89% of members inhabiting marine environments (Kensley 1998). Like most marine invertebrates, they appear to be most diverse in coral reef systems, where it is estimated that over 5000 species have yet to be described (Kensley 1998). Out of the approximately 4000 species of identified parasitic isopods, at least 450 are known to infest fish hosts (Ravichandran et al. 2010).
Gnathiid isopods are among the most common parasitic organisms infesting marine fishes. As protelian parasites, these so-called “ticks of the sea” are unusual in that only the larvae are parasitic (Smit and Davies 2004). The small (1–3 mm) highly mobile larval phase consists of three stages that are generalist parasites on fishes (Tanaka and Aoki 1998; Smit et al. 2003). When ready to feed, they emerge from benthic substrata and use piercing mouthparts to penetrate the host skin and gills, feeding on the blood and lymph of teleost and elasmobranch fishes (Heupel and Bennett 1999; Smit and Davies 2004). After feeding, they return to the substrate, digest their meal, and molt to the next life stage (Smit et al. 2003). Following the third feeding, they metamorphose into non-feeding adult stages (Smit and Davies 2004). Feeding requires only minutes to hours, whereas non-feeding periods extend for days or weeks (Smit et al. 2003; Grutter 2003; Smit and Davies 2004). Because of the temporary association with hosts, they may also be referred to as “micropredators” (e.g., Lafferty and Kuris 2002; Raffel et al. 2008).
Gnathiids feed on multiple life history stages and species of fishes, and their impacts on hosts include lowering host blood volume (e.g., Jones and Grutter 2005; Triki et al. 2016), transmission of blood parasites (e.g., Davies et al. 2004; Smit et al. 2006; Curtis et al. 2013; Cook et al. 2015), tissue damage, and even killing the host fish (e.g., Bunkley-Williams and Williams 1998; Penfold et al. 2008; Artim et al. 2015). Gnathiid activity and infestation of hosts has been shown to be highest during nighttime and crepuscular hours (e.g., Grutter and Hendrikz 1999; Grutter et al. 2000a; Sikkel et al. 2006; Côté and Molloy 2003), influencing fish host interactions with cleaners in reef ecosystems (e.g., Grutter 2001; Sikkel et al. 2004, 2005, 2016).
While gnathiids occur in all marine environments at varying salinity levels, from the Arctic to the Antarctic, and intertidal to deep ocean (e.g., Smit and Davies 2004; Tanaka 2007; Ota et al. 2016; Quattrini and Demopoulos 2016), they are perhaps best known in coral reef environments, where they constitute the main prey item for cleaner fishes (e.g., Grutter 1999; Arnal and Côté 2000; Whiteman and Côté 2002). Most studies on coral reef gnathiids have been conducted in Australia (e.g., Grutter et al. 2000a, b, 2010; McKiernan et al. 2005; Nagel and Grutter 2007) and the Caribbean (e.g., Sikkel et al. 2000, 2006, 2009; Chambers and Sikkel 2002; Artim and Sikkel 2013).
Gnathiid abundance varies greatly over multiple spatial scales in Australia (e.g., Grutter and Poulin 1998; Grutter 1994; Jones and Grutter 2007) and the Caribbean (e.g., Cheney and Côté 2005; Sikkel et al. 2006; Welicky et al. 2013). However, the factors influencing gnathiid distribution and abundance, and, hence, the extent of host infestation are poorly understood. Most studies on gnathiid habitat associations have focused on temperate species that tend to be habitat specialists (Smit and Davies 2004; Tanaka and Nishi 2008). The few published studies that have examined gnathiid habitat associations in coral reef systems, where they are most diverse, suggested that coral reef gnathiids are more habitat generalists (e.g., Jones and Grutter 2007), but cannot persist on live coral (Artim and Sikkel 2013).
Although it lies within the heart of the Coral Triangle and, hence, the “epicenter” of marine biodiversity (Carpenter and Springer 2005), research on coral reef parasites in the Philippines lags far behind coral reef systems in Australia and the Caribbean. For example, only one new parasitic isopod species (from the family Bopyridae infecting porcellanid crabs) has been reported from the Philippines within the past two decades (e.g., Williams and Madad 2010). To our knowledge, only a single study has been conducted on gnathiid isopods in the Philippines (Sikkel et al. 2014). Therefore, the primary goals of this study were to characterize fundamental aspects of gnathiid isopod natural history in the central Philippines as a foundation for more sophisticated studies on gnathiid–host interactions and biodiversity. Specifically, we sought to characterize species diversity, host and habitat associations, and diel activity patterns of gnathiids in a shallow reef-seagrass system in this region.
Materials and methods
Study site
Research was conducted in Sibulan, a municipality of Negros Oriental, Philippines between September 2014 to May 2015 and August to October 2016. Sibulan is bordered to the south by Dumaguete City, the provincial capital of Negros Oriental, and to the north by Amlan and Bais City. Sibulan is subjected to southwest monsoon (July to October) and northeast monsoon (November to March) (Abesamis and Russ 2010). The base of operation was the Silliman University Institute of Environmental and Marine Sciences.
Sibulan has two small marine protected areas (MPAs) that prohibit fishing, swimming, and diving. Field sampling was conducted in the shallow water reef habitat (<10 m depth, water temperature of ∼27.3 °C) of Agan-an marine sanctuary (9°20′2.6″ N, 123°18′41.5″ E), located north of Dumaguete City’s airport (9°18′24.5″ N, 123°18′19.4″ E) (Fig. 1). The 6-ha reserve encloses a fringing reef and a 10–12° slope, leading to a dominant sandy and dead coral substrate. A 20-m buffer zone was established around the entire sanctuary in 2012 under Ordinance No. 8 series of 2012. The coastal current can be categorized as a strong reversing current, with flood current speeds ranging from 0.394 to 0.830 m/s and ebb current speeds ranging from 0.139 to 0.815 m/s (Calumpong et al. 1997). Approximately 30% of the hard corals in the deeper reef zone were damaged, attributed to the strong wave action during Typhoon Sendong in 2011. The breakage was evident mostly on branching and foliose type corals. Table corals were also overturned. Corals located at the intertidal zone were hardly affected. The MPA extends out reefward, enclosing a seagrass bed followed by mixed patches of hard coral, dead standing coral, limestone, algae, sponge, and soft coral. At least 59 fish species from 15 families are present, with the two most speciose families being Labridae (wrasses) and Pomacentridae (damselfish). Most of the species in these families are small- to medium-sized fishes that are not typically considered target species. More commercially important families such as Acanthuridae (surgeonfish) and Scaridae (parrotfish) are the third most speciose families.
Trap design and sampling protocol
Studies on diel activity patterns and habitat associations within reef habitat (see below) were conducted using two different trap designs: fish-baited open mesh cages (after Sikkel et al. 2006, 2014) and fish-baited PVC tube traps (after Sikkel et al. 2011) (Fig. 2). Mesh traps were constructed with a plastic hardware cloth and weighted down by sand-filled bottles, weighing approximately 2 kg. Tube traps were constructed using a 32 × 10-cm plastic tube capped at both ends with a removable cover attached to a clear plastic funnel with a 1.5-cm-diameter end spout. Four 5 × 3-cm windows at each end port covered by a 100-μm filter increased water circulation. Gnathiids entered through funnel openings and fed on the fish host. Each trap was numbered with flagging tape and placed on the reef in the afternoon. During retrieval, molding clay was used to clog openings of tube traps, preventing any gnathiids from escaping as the trap was brought to the surface. The contents of each trap, including the fish, were emptied into a plastic container. Mesh cages were retrieved with 3.8-L hermetically sealed plastic bags to minimize loss of gnathiids. After returning to the field station, the fish were allowed to remain in the containers for 2 h to allow all gnathiids, including ones that latched onto the fish host closer to retrieval time, ample time to complete feeding and dislodge from the host. The fish was then rinsed thoroughly with a wash bottle over its skin, fins, and gills with seawater into the bucket and transferred back to the housing tank. Sedation of fishes was not necessary and there was no fish mortality associated with this method. The contents of each plastic container were then filtered through a 55-μm plankton mesh and placed in Petri dishes. All gnathiids were counted using a stereoscope and kept in containers for observations. Since gnathiids can only be identified from adult males, pranizae (fed larvae) were kept in labeled containers until they fully molted to either males or females. These containers were regularly cleaned and replaced with clean seawater. Gnathiids and fish hosts were photographed on graphing paper and measured for body size and standard length (SL), respectively, using ImageJ® (Abràmoff et al. 2004).
Fish were collected by local fishermen and held in large cement tanks with running seawater and aeration. Fish were fed commercial food pellets and squid daily, and parrotfish grazed algae from the side of the tank. To minimize stress, fish were held in flow-through tanks for one day before deployment in experimental cages. After fish were retrieved from the reef and rinsed of all external parasites with seawater, they were allowed to recover in aerated seawater before being released. Gnathiid traps placed in the holding tank overnight contained no gnathiids, confirming that gnathiids were not present in the tank. For transportation to the study site, all fish hosts were placed in an aerated bucket. Once at the study site, each one was transferred from the bucket to an individual cage and placed on the substratum. Details on host species and sample sizes are reported below with each experiment.
Host finding mechanisms and trap effectiveness
To further confirm that gnathiids collected in the traps were attracted to the fish host and not simply using the trap as a refuge, the effectiveness of the fish-baited tube traps versus empty tube traps as well as the mechanisms by which gnathiids locate hosts were tested. Traps were modified to remove all visual cues but allow water circulation through the funnel. In four serial sets, eight traps were deployed: four empty traps with no fish host (NF) and four with a fish host (F), resulting in a total of 32 traps (16 of each treatment). Each trap was set in the proximity of mixed substrates that consisted of live and dead coral, algae, sponge, and coral rubble, and retrieved 24 h later. The Tripletail wrasse, Cheilinus trilobatus Lacepède, 1801 (Labridae) was used as the host, with sizes between 8 and 12.6 cm SL. The sizes of all fish hosts were similar to the size of the hosts used in the diel activity and substrate study.
Host associations
Previous studies at a nearby site (Sikkel et al. 2014) found that seven species from the families Lutjanidae, Haemulidae, Serranidae, and Scorpaenidae were all susceptible to infestation by gnathiids. To determine what other common host species were susceptible, we examined an additional 30 species from 11 families of similar size within each species using open mesh cages set from dusk to dawn as described in Sikkel et al. (2014) (Table 1). Mesh traps were placed at 3–5 m depth and anchored with two 2-kg sand-filled bottles.
Diel activity
To assess diel activity patterns in reef habitat, two sampling protocols were employed using mesh cages with Tripletail wrasse, C. trilobatus, as the host. The first sampling examined the feeding activity of gnathiids (which include seeking and feeding on a fish host) within 12-h day and 12-h nighttime blocks, while the second quantified the activity of gnathiids within multiple daytime blocks. For the two-12 h sampling periods, five collection series were conducted. For each series, a single wrasse (chosen haphazardly from the holding tank) was placed in each of the nine different traps during two time periods: day: 06:30–17:30 h and night: 18:00–05:00 h. Each mesh cage was placed in the same location on the reef during each time block for each series. Gnathiid body sizes from day and nighttime samples were also measured and compared using a Mann–Whitney U-test.
For the finer-scale dawn to dusk sampling, we conducted four series of samples (on 19 and 28–30 May 2015). For each series, a single wrasse was set on the reef for the full duration of the following three daytime periods: early-day: 03:30–05:45 h (n = 20); mid-day: 10:00–13:30 h (n = 20); late-day: 14:30–17:30 h (n = 20). Nine traps were also set for nighttime (18:00–05:00 h) (n = 36) after retrieval of all daytime traps. All traps were retrieved and processed after the end of each time block. Gnathiid abundances between time blocks were analyzed using GLM univariate analysis with Tukey’s post hoc test in SPSS Statistics 23. Non-parametric tests were also conducted to account for any effects of deviation from parametric assumptions. Reported means and 95% confidence intervals (CIs) were calculated using 1000 bootstrap iterations.
Broad-scale habitat associations
To determine whether gnathiids were present in both reef and seagrass habitats, 25 tube traps were deployed over 24-h sampling periods in seagrass, approximately 50 m from the reef edge. An additional 25 tube traps were placed on the reef and coral–sand interface. The marbled parrotfish, Leptoscarus vaigiensis (Quoy & Gaimard, 1824) (Labridae), was used as a host, and traps were set and retrieved during mid-day. Sampling in each habitat was conducted sequentially due to logistic constraints. Parasite prevalence for each substrate was calculated by taking the number of traps with infested fish hosts over the total number of traps.
Within-reef habitat comparison
To assess differences in gnathiid infestation relative to substrate type, two within-reef comparative studies were conducted using Tripletail wrasse, C. trilobatus, as the host in tube traps. All fish were of juvenile size, between 8 and 12.6 cm SL.
Gnathiid tube traps were deployed in 17 sampling days over a 40-day period at mid-day. One set consisted of three blocks, each with three traps (total = 9) set on chosen substrates approximately 2 m apart. Each block included traps set on dead standing hard coral (DC), live hard coral (LHC), and coral rubble (RUB). Live hard coral substrates included Montipora aequituberculata Bernard, 1897, Porites cylindrica Dana 1846, Echinopora lamellosa (Esper, 1795), Isopora brueggemanni (Brook, 1893), Acropora pulchra (Brook, 1891), Acropora hyacinthus (Dana, 1846), Goniopora sp., and Millepora sp., with percent cover on LHC ranging from 75 to 100%. To avoid damage and “chafing” of coral, traps were stabilized with 1–2-kg weights placed on sandy bottom and connected to the trap with thin nylon line. Dead coral substrate included less than 5% live cover. A buoy was set to facilitate relocation of traps.
To further examine the potential effects of substrate type on gnathiid distribution, we conducted a second experiment including live soft coral (SC) species, Sarcophyton trocheliophorum von Marenzeller, 1886 and Sinularia sp. Traps (n = 66) were placed on live SC and RUB on each of 10 days (total = 33 and 33).
A stationary point visual survey was conducted to estimate the density and biomass of fishes surrounding each trap on each substrate during dawn (05:30–06:30 h), when fish activity, associated with the night-day changeover, is high. The number and estimated size of fishes present within a 2-m radius of each trap deployed were recorded during 5-min observation periods. A Pearson correlation coefficient was calculated to examine the relationship between gnathiid abundance and fish host size (SL).
To accommodate the high number of zero values (no gnathiids in traps), the count sum over the 3 (LHC, DC, and RUB) and 2 (SC and RUB) traps set on each substrate on each sampling day was used as the dependent variable. Data were then analyzed using GLM ANCOVA, with fish density and biomass as a covariate and habitat as the fixed variable. A non-parametric Kruskal–Wallis test was also conducted due to our data not meeting normal distribution assumptions. Our data also did not meet the assumptions for a Poisson regression analysis. Reported means and 95% CIs were calculated using 1000 bootstrap iterations.
Results
Host associations
A total of 28 of the 30 fish species (from 11 families) and 73 of 85 individual fish were infested by 1 to 225 gnathiids. Gnathiids were fully and/or partially engorged (Table 1).
Diversity of gnathiids
Of the third-stage juveniles that survived and molted into adult males, four unique male morphs (Morph 1, Morph 2, Morph 3, Morph 4) were observed and do not match any previously described species (Fig. 3). Morph 1 was the most common and abundant in January to May 2015. Morph 3 was most common in August to October 2016. Morph 4 was collected only twice, during a late-day and evening set during the diel activity experiment. Morph 1 was found on Naso vlamingii (Valenciennes, 1835), Salarias fasciatus (Bloch, 1786), Dascyllus trimaculatus (Rüppell, 1829), C. trilobatus, and L. vaigiensis; Morph 2 on N. vlamingii, Abudefduf vaigiensis (Linnaeus, 1758), D. trimaculatus, and C. trilobatus; Morph 3 on A. vaigiensis, Labrichthys unilineatus (Guichenot, 1847), Halichoeres podostigma (Bleeker, 1854), Parupeneus barberinoides (Bleeker, 1852), Epinephelus merra Bloch, 1793, D. trimaculatus, C. trilobatus, and Scarus sp. (Table 1).
Host finding mechanisms and effectiveness of tube traps
Fish-baited (F) traps attracted more gnathiids than empty, no-fish (NF) traps (Fig. 4). Thirteen of the 16 (81%) F traps had gnathiids, with four traps having six gnathiids. These traps attracted a median of two gnathiids, with an interquartile range (IQR) of 4. In contrast, only one (6%) NF trap had gnathiids. Among the gnathiids recovered from NF traps, none had fed on fish blood (i.e., all were unfed).
Diel activity
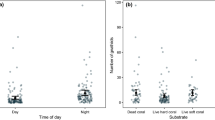
Of the 90 traps deployed, only ten traps were absent of gnathiids. Gnathiid infestation on host fish was higher at night (Fig. 5a). The daytime period (06:00–17:30 h) had a total of 255 gnathiids (median = 3, IQR = 5, bootstrap mean with 95% CI = [3.90, 8.05], range = 1–40 per fish host). In contrast, nighttime samples had a total of 439 gnathiids (median = 5, IQR = 9, bootstrap mean with 95% CI = [6.27, 14.37], range = 1–60 gnathiids per host). Although abundances appeared higher at night, a Mann–Whitney U-test showed no significance (U = 882, p = 0.29) between day and night collections, even with the removal of all outliers (U = 726, p = 0.371).
Box plots of diel activity of gnathiids. The boxes indicate gnathiid counts in the first (lower) and third (upper) quartiles, the horizontal black lines indicate medians, whiskers are minimum and maximum counts, and outliers are indicated by black circles: a gnathiid count per individual fish per time period (day and night), n = 45 fish per day and night; b gnathiid count per individual fish per time block, n = 20 fish each for the first three time blocks and 36 for the final time block
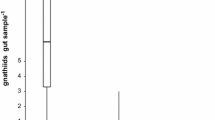
Gnathiid body sizes differed between time periods, with nighttime samples being larger than those from daytime samples (U = 363, p = 0.016) (Fig. 6). Day traps had a median body size of 1.15 mm (bootstrap mean with 95% CI = [1.15, 1.65]) and night traps 1.57 mm (bootstrap mean with 95% CI = [1.60, 1.83]). The minimum body sizes for day and night were 0.81 and 0.57 mm, respectively. The maximum body sizes were 2.07 and 2.66 mm, respectively. The interquartile ranges for day and night were both 0.98.
When sampled on a finer time scale, gnathiid activity varied greatly (Fig. 5b). Early-day traps had a maximum and minimum of 0 and 4 gnathiids, respectively (median = 1, IQR = 2.8, bootstrap mean with 95% CI = [0.72, 2.63]). Mid-day had 0 and 6 gnathiids, respectively (median = 1, IQR = 2.8, bootstrap mean with 95% CI = [0.91, 2.56]). Late-day had 0 and 9 gnathiids, respectively (median = 2, IQR = 4.5, bootstrap mean with 95% CI = [2.14, 7.79]). Evening had 0 and 20 gnathiids, respectively (median = 3, IQR = 8.5, bootstrap mean with 95% CI = [3.32, 7.16]). With the removal of an outlier from late-day (gnathiid count 31), time of day had a significant effect on gnathiid activity (F = 4.920, df = 3, p = 0.003). Early-day (p = 0.010) and mid-day (p = 0.012) exhibited lower gnathiid activity compared to nighttime blocks. However, late-day was not significantly different to the other periods (early-day p = 0.588, mid-day p = 0.612, evening p = 0.327). The non-parametric Kruskal–Wallis test yielded similar results (χ2 = 11.910, df = 3, p = 0.008). Overall, gnathiid counts appeared to remain low during early-day and mid-day hours, gradually increasing in late-day, and peaking at night.
Broad-scale habitat associations
Gnathiids were found in all habitats, with a total of 23 individuals from 10 out of the 25 traps in seagrass bed (40% prevalence) and 23 from 12 out of the 25 traps in coral–sand interface (48% prevalence). Coral reef traps had the lowest, with only a total of 12 gnathiids from 5 out of the 25 traps (20% prevalence).
Within-reef habitat: substrate study
Fish densities differed statistically between substrates (χ2 = 15.294, df = 2, p < 0.001); however, biomass did not (χ2 = 1.74, df = 2, p = 0.419). Live coral substrates had higher fish density and biomass (bootstrap mean with 95% CI = [12.21, 26.89] fish/m2 and bootstrap mean with 95% CI = [0.16, 0.31] kg/m2, respectively) compared to dead standing coral (bootstrap mean with 95% CI = [6.15, 10.93] fish/m2 and bootstrap mean with 95% CI = [0.12, 0.26] kg/m2, respectively) and coral rubble (bootstrap mean with 95% CI = [5.11, 9.65] fish/m2 and bootstrap mean with 95% CI = [0.11, 0.25] kg/m2, respectively).
Among the 150 total traps deployed within the 17 sets, gnathiids were found in 28 out of 50 traps on both DC and RUB (56%) compared with 20 of the 50 traps set on LHC (40%). Due to the narrow size range of fish hosts within a given species, no significant relationship between host size and gnathiid abundance was found (r = 0.049, p = 0.56). ANCOVA showed no significant effect of fish density and biomass on gnathiid abundance (F = 1.31, df = 2, p = 0.282); however, gnathiid abundances were significantly different among substrate types (F = 6.016, df = 2, p = 0.005) (Fig. 7a). The Kruskal–Wallis test also showed a significant difference between substrates (χ2 = 7.076, df = 2, p = 0.029), consistent with the parametric tests. RUB traps had the highest total gnathiid count, with 73 gnathiids with minimum and maximum gnathiid counts of 0 and 11, respectively (median = 3, IQR = 7, bootstrap mean with 95% CI = [2.69, 6.08]) and ranging from 1 to 11 per trap. DC had a total of 51, with minimum and maximum counts of 0 and 5, respectively (median = 3, IQR = 3, bootstrap mean with 95% CI = [2.11, 4.12]), with a range of 1–9 per trap. LHC had the lowest total number of 28 and minimum and maximum counts of 0 and 3, respectively (median = 1, IQR = 2.8, bootstrap mean with 95% CI = [1.11, 2.21]), ranging from 0 to 4 per trap.
Box and whisker plots of gnathiid loads (a and b) and body size (c) in relation to substrate type. The horizontal black lines indicate medians, boxes indicate counts and body size within the first and third quartiles, and whiskers indicate minimum and maximum gnathiid counts or body size. Outliers are shown as black dots. Numbers for a and b represent gnathiid abundances per substrate per sampling set. LHC = live hard coral; DC = dead standing hard coral; RUB = coral rubble; SC = soft coral
Comparing RUB with SC, RUB had 147 total gnathiids, with a minimum and maximum of 0 and 14, respectively (median = 3, IQR = 5, bootstrap mean with 95% CI = [3.027, 5.971]) and ranging from 1 to 15 per trap. SC had 92 total gnathiids with a minimum and maximum of 0 and 8, respectively (median = 2, IQR = 4.5, bootstrap mean with 95% CI = [1.517, 4.156]), with a range of 1–17 gnathiids. While this difference was marginally non-significant with all data included (t = 1.723, df = 1, p = 0.09), with the removal of an extreme outlier from RUB and SC (gnathiid counts 15 and 17, respectively), RUB had significantly higher gnathiid loads compared to SC (t = 2.176, df = 1, p = 0.033) (Fig. 7b). A Mann–Whitney U-test also showed significance between both substrates (U = 347.5, p = 0.011) with the inclusion of all outliers.
Gnathiid body sizes differed among substrates (F = 5.350, p = 0.002) (Fig. 7c). RUB traps had the largest sized gnathiids (median = 1.6, IQR = 0.821, bootstrap mean with 95% CI = [1.50, 1.90]), ranging from 0.68 to 2.79 mm, followed by LHC (median = 1.34, IQR = 1.08, bootstrap mean with 95% CI = [1.08, 1.62]), ranging from 0.60 to 2.34 mm, then DC (median = 1.31, IQR = 0.403, bootstrap mean with 95% CI = [1.18, 1.47]), ranging from 0.60 to 2.07 mm, and, finally, SC as the smallest (median = 1.01, IQR = 0.201, bootstrap mean with 95% CI = [0.90, 1.18]), ranging from 0.834 to 1.46 mm. Gnathiid sizes differed between RUB and DC (p = 0.037), as well as with SC (p = 0.005); however, gnathiid sizes of LHC did not differ among the other substrates (p = 0.958, p = 0.077, p = 0.478). Non-parametric tests also showed a significant difference in body sizes between substrates (χ2 = 13.771, df = 3, p = 0.003).
Discussion
Combining previous studies (Sikkel et al. 2014; Cruz-Lacierda and Nagasawa 2017) and this one, 34 of 37 investigated fish species from 14 families in the central Philippines are infested by gnathiid isopods. While the previous studies made no attempt to identify gnathiid species, in this study, five fish species were infested by more than one gnathiid morph and the most common gnathiids were found to infest ten species. We found that fish from the families Acanthuridae, Labridae, Pomacanthidae, and Pomacentridae were particularly heavily infested. Although gnathiids are regarded as host generalists, certain host species appear more susceptible than others. For example, in the Caribbean, among 16 host species experimentally compared by Coile and Sikkel (2013), members of the families Haemulidae (grunts) and Lutjanidae (snappers) were found to be most susceptible to Gnathia marleyi Farquharson, Smit & Sikkel, 2012. In the Indo-Pacific, laboratory host- choice experiments of Gnathia aureumaculosa Ferreiera, Smit, Grutter & Davies, 2009 in the Great Barrier Reef (GBR) showed an apparent preference for wrasses (Labridae), despite infesting all other tested hosts (Nagel and Grutter 2007). In the only quantitative comparison in the Philippines, Sikkel et al. (2014) reported that lionfish [Pterois volitans (Linnaeus, 1758)] were significantly less susceptible to gnathiids than members of three other families. However, this comparison was limited only to families that included species ecologically similar to lionfish.
A major challenge of gnathiid biodiversity studies is that larval (feeding) stages of gnathiids often appear morphologically similar among species and species identification usually requires adult males. Although molecular techniques are improving our ability to match life history stages (Grutter et al. 2000b; Ota et al. 2016), doing so also requires identifying species morphologically. To date, some 200 species of gnathiids have been described, mostly from tropical and subtropical latitudes. However, this represents a small fraction of potential species. Based on male morphology, all four species collected in our study are, as yet, undescribed (proper species descriptions with associated genetic data will be presented in a future manuscript). This is in spite of the fact that our study area included only 600 m2. In one highly studied, larger, area of the GBR, Lizard Island (9.90 km2), 11 newly described species were discovered within one year (Farquharson et al. 2012; Svavarsson and Bruce 2012). By comparison, in the entire Caribbean, only 11 species have been described. The central Philippines, a known biodiversity hot spot located within the Coral Triangle, has a higher concentration of species per unit area—more than anywhere in Indonesia, including Wallacea (Carpenter and Springer 2005). Our findings underscore the fact that there are still major gaps in our knowledge on gnathiid isopod biodiversity in the Philippines.
Our findings on diel activity of gnathiids are consistent with findings from other coral reef studies; gnathiids overall, and especially larger ones, are more active at night (Grutter and Hendrikz 1999; Grutter et al. 2000a; Chambers and Sikkel 2002; Côté and Molloy 2003; Sikkel et al. 2006, 2009). However, we do not know whether our observations reflect differences in activity within versus among species. For example, in the eastern Caribbean, Gnathia marleyi appears to undergo an ontogenetic niche shift, whereby first-stage larvae are more active during dawn and daytime, with second- and third-stage larvae becoming more nocturnal. Moreover, the majority of larvae collected at midnight develop into females, whereas third-stage larvae collected at first light develop mainly into males (Sikkel et al. 2009). In the GBR, Grutter et al. (2000a) found a higher percentage of larger (≥1 mm) gnathiid juveniles at night compared with more smaller sized juveniles (≤1 mm) during the day. However, the extent to which this is due to within- versus among-species differences is unclear. Given the larger species diversity on the GBR, it is possible that smaller species are more diurnal and larger species more nocturnal (Nagel et al. 2008). Regardless of the underlying mechanism, possible explanations for this size-related shift in activity could be due to predation pressure by cleaners that might preferentially target larger gnathiids (Grutter et al. 2000a), and/or predation pressure by nocturnally active corals that would more likely consume smaller gnathiids (Artim and Sikkel 2013). Gnathiids in general may be more successful in seeking a host meal during night when more fish are sedentary. Increased gnathiid activity at night would result in high exposure to gnathiids for diurnally active fishes that seek refuge in reef habitat at night and may thus select for physiological or behavioral responses that enable fish to minimize exposure (Grutter et al. 2011; Sikkel et al. 2016).
In order to seek a blood meal at night, gnathiid parasites appear to use olfactory cues and behavioral responses to ultimately detect, locate, and exploit a suitable host. Our findings are consistent with those of Sikkel et al. (2011) in that gnathiids in our study were shown to be capable of finding hosts using only olfactory cues. While gnathiids have large, compound eyes that may enable them to navigate at night for host detection (Smit and Davies 2004), immobile hosts may still be difficult to locate when hidden. Thus, visual cues provided by resting or slow-moving fish may not alone be sufficient to attract gnathiids, while olfactory cues alone are (Sikkel et al. 2011). Gnathiids have long antennae that may enable them to locate hosts using chemical cues (Nagel et al. 2008), and Nagel et al. (2008) found that nocturnal gnathiids had significantly longer antennules and larger eyes than diurnal ones. These differences were attributable to the different environmental levels of light. Marine crustaceans use multiple sensory mechanisms when seeking prey or evading a predator (Nagel et al. 2008; Garm and Watling 2013). However, in this study, we focused on only olfactory and visual cues and found that gnathiid parasites feed mainly at night and can rely on detecting chemical cues from a a host (Sikkel et al. 2011). Nevertheless, nothing is known about the molecules gnathiids use to locate hosts.
Gnathiids in our study were found in all habitats but were least likely to be found on live hard coral, in spite of the fact that live hard coral was associated with higher host availability. The most likely explanation for this is that corals are consumers of free-living gnathiids and gnathiids try to avoid contact with them (Artim and Sikkel 2013). However, a wider range of gnathiid sizes was observed in both live coral and coral rubble, possibly indicating a range of small to large gnathiids on the substrate. There can be two possible explanations for this pattern, as suggested by Artim and Sikkel (2013): (1) a large number of newly hatched larvae are distributed more in substrates with less predators (coral rubble) and (2) younger larvae are less maneuverable and less likely to be able to avoid live coral, while the older, larger larvae are stronger swimmers and better at avoiding or escaping live coral. In the Caribbean, Artim and Sikkel (2013) found stage 1 larvae to be the least capable of avoiding contact with live coral, while larger gnathiids were more successful and contacted live polyps less frequently.
Little is known about gnathiid habitat associations in coral reef systems and few studies have explored the effects of habitat on gnathiid distribution and abundance within the reef over small spatial scales. Jones and Grutter (2007) observed higher abundance of larger gnathiids on coral reef margins of Lizard Island, GBR, where fish hosts were also the most abundant, suggesting that greater feeding success in those areas may be driving habitat associations. Sikkel et al. (2016) reported that fish placed in cages and deployed on the reef at night harbored significantly more gnathiids than those placed in the adjacent seagrass habitat. In contrast, gnathiid infestations were comparable during the day when gnathiid activity was low. The reciprocal effects of fish hosts on gnathiid distribution and gnathiid distribution on fish hosts remain virtually unexplored.
While much attention has been devoted to the effects of growth and survival of cultured fisheries, few studies in the Philippines have investigated parasite–host relationships in natural marine systems. With the extensive network of MPAs established to improve and sustain fishery production in the Philippines, it is essential to to conserve and restore reefs and their associated biodiversity (e.g., Alcala 2004; Green et al. 2015; Abesamis et al. 2016). MPAs and no-take zones have proven to promote the increase of fish densities and fish sizes that could lead to a spillover effect, density-dependent movements of fish to outside the protected area due to space limitation and territorial interactions (Roberts and Polunin 1991; Rowley 1994; Kramer and Chapman 1999; Abesamis and Russ 2005). Large-bodied fish, which tend to harbor more parasites as they age and grow, are more attractive and targeted by fishers; therefore, the removal of fish may also result in “fishing out” marine parasites (Guégan and Hugueny 1994; Arneberg 2002; Wood et al. 2010; Wood and Lafferty 2015).
New knowledge of parasite ecology in the Philippine marine ecosystem could aid in the implementation and management of MPAs. Habitat degradation and unregulated fishing of large, higher trophic level, fish and any fish host preferentially infested by specific parasite groups may not only reduce parasite transmission, but also reduce food web complexity and parasite biodiversity (Wood et al. 2010; Lafferty 2013; Wood and Lafferty 2015). Therefore, more studies must be conducted to further understand the relationships between gnathiid parasites and their hosts, gnathiid distribution across or within habitats, and general parasite biology and biodiversity in the Philippines.
References
Abesamis RA, Russ GR (2005) Density-dependent spillover from a marine reserve: long-term evidence. Ecol Appl 15:1798–1812. doi:10.1890/05-0174
Abesamis RA, Russ GR (2010) Patterns of recruitment of coral reef fishes in a monsoonal environment. Coral Reefs 29:911–921. doi:10.1007/s00338-010-0653-y
Abesamis RA, Stockwell BL, Bernardo LP, Villanoy CL, Russ GR (2016) Predicting reef fish connectivity from biogeographic patterns and larval dispersal modelling to inform the development of marine reserve networks. Ecol Indic 66:534–544. doi:10.1016/j.ecolind.2016.02.032
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophoton Int 11:36–42
Alcala AC (2004) Marine reserves as tool for fishery management and biodiversity conservation: natural experiments in the central Philippines, 1974–2000. Silliman University-Angelo King Center for Research and Environmental Management, Dumaguete City
Amundsen PA, Lafferty KD, Knudsen R, Primicerio R, Klemetsen A, Kuris AM (2009) Food web topology and parasites in the pelagic zone of a subarctic lake. J Anim Ecol 78:563–572. doi:10.1111/j.1365-2656.2008.01518.x
Arnal C, Côté IM (2000) Diet of broadstripe cleaning gobies on a Barbadian reef. J Fish Biol 57:1075–1082. doi:10.1111/j.1095-8649.2000.tb02213.x
Arneberg P (2002) Host population density and body mass as determinants of species richness in parasite communities: comparative analyses of directly transmitted nematodes of mammals. Ecography 25:88–94. doi:10.1034/j.1600-0587.2002.250110.x
Artim JM, Sellers JC, Sikkel PC (2015) Micropredation by gnathiid isopods on settlement-stage reef fish in the eastern Caribbean Sea. Bull Mar Sci 91:479–487. doi:10.5343/bms.2015.1023
Artim JM, Sikkel PC (2013) Live coral repels a common reef fish ectoparasite. Coral Reefs 32:487–494. doi:10.1007/s00338-012-0995-8
Bunkley-Williams L, Williams EH (1998) Isopods associated with fishes: a synopsis and corrections. J Parasitol 84:893–889. doi:10.2307/3284615
Calumpong HP, Estacion JS, Lepiten MV, Acedo CE (eds) (1997) Status of the coastal resources of the Negros learning site (Manjuyod to Dauin). Silliman University Marine Laboratory, Dumaguete City. COE-CRM document no. 1/97
Carpenter KE, Springer VG (2005) The center of the center of marine shore fish biodiversity: the Philippine Islands. Environ Biol Fish 72:467–480. doi:10.1007/s10641-004-3154-4
Chambers SD, Sikkel PC (2002) Diel emergence patterns of ecologically important, fish-parasitic, gnathiid isopod larvae on Caribbean coral reefs. Caribb J Sci 38:37–43
Cheney KL, Côté IM (2005) Mutualism or parasitism? The variable outcome of cleaning symbioses. Biol Lett 1:162–165. doi:10.1098/rsbl.2004.0288
Coile AM, Sikkel PC (2013) An experimental field test of susceptibility to ectoparasitic gnathiid isopods among Caribbean reef fishes. Parasitology 140:888–896. doi:10.1017/S0031182013000097
Cook CA, Sikkel PC, Renoux LP, Smit NJ (2015) Blood parasite biodiversity of reef-associated fishes of the eastern Caribbean. Mar Ecol Prog Ser 533:1–13. doi:10.3354/meps11430
Côté IM, Molloy PP (2003) Temporal variation in cleanerfish and client behaviour: does it reflect ectoparasite availability? Ethology 109:487–499. doi:10.1046/j.1439-0310.2003.00883.x
Cruz-Lacierda ER, Nagasawa K (2017) Occurrence of praniza larvae of a gnathiid (Isopoda, Gnathiidae) on wild-caught orange-spotted grouper, Epinephelus coioides (Hamilton, 1822) (Perciformes, Serranidae), in Lingayen Gulf, Philippines. Crustaceana 90:101–110. doi:10.1163/15685403-00003626
Curtis LM, Grutter AS, Smit NJ, Davies AJ (2013) Gnathia aureamaculosa, a likely definitive host of Haemogregarina balistapi and potential vector for Haemogregarina bigemina between fishes of the Great Barrier Reef, Australia. Int J Parasitol 43:361–370. doi:10.1016/j.ijpara.2012.11.012
Davies AJ, Smit NJ, Hayes PM, Seddon AM, Wertheim DF (2004) Haemogregarina bigemina (Protozoa: Apicomplexa: Adeleorina)—past, present and future. Folia Parasitol 51:99–108. doi:10.14411/fp.2004.015
Farquharson C, Smit NJ, Sikkel PC (2012) Gnathia marleyi sp. nov. (Crustacea, Isopoda, Gnathiidae) from the eastern Caribbean. Zootaxa 3381:47–61. doi:10.5281/zenodo.212837
Garm A, Watling L (2013) The crustacean integument: setae, setules, and other ornamentation. Funct Morphol Divers 1:167–198
Graça-Souza AV, Maya-Monteiro C, Paiva-Silva GO, Braz GR, Paes MC, Sorgine MH, Oliveira MF, Oliveira PL (2006) Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem Molec 36:322–335. doi:10.1016/j.ibmb.2006.01.009
Green AL, Maypa AP, Almany GR, Rhodes KL, Weeks R, Abesamis RA, Gleason MG, Mumby PJ, White AT (2015) Larval dispersal and movement patterns of coral reef fishes, and implications for marine reserve network design. Biol Rev 90:1215–1247. doi:10.1111/brv.12155
Grutter AS (1994) Spatial and temporal variations of the ectoparasites of seven reef fish species from Lizard Island and Heron Island, Australia. Mar Ecol Prog Ser 115:21–30. doi:10.3354/meps115021
Grutter AS (1999) Fish cleaning behaviour in Noumea, New Caledonia. Mar Freshw Res 50:209–212. doi:10.1071/MF97078
Grutter AS (2001) Parasite infection rather than tactile stimulation is the proximate cause of cleaning behaviour in reef fish. Proc R Soc Lond B Biol Sci 268:1361–1365. doi:10.1098/rspb.2001.1658
Grutter AS (2003) Feeding ecology of the fish ectoparasite Gnathia sp. (Crustacea: Isopoda) from the Great Barrier Reef, and its implications for fish cleaning behaviour. Mar Ecol Prog Ser 259:295–302. doi:10.3354/meps259295
Grutter AS, Cribb TH, McCallum H, Pickering JL, McCormick MI (2010) Effects of parasites on larval and juvenile stages of the coral reef fish Pomacentrus moluccensis. Coral Reefs 29:31–40. doi:10.1007/s00338-009-0561-1
Grutter AS, Hendrikz J (1999) Diurnal variation in the abundance of juvenile parasitic gnathiid isopods on coral reef fish: implications for parasite–cleaner fish interactions. Coral Reefs 18:187–191. doi:10.1007/s003380050178
Grutter AS, Lester RJ, Greenwood J (2000a) Emergence rates from the benthos of the parasitic juveniles of gnathiid isopods. Mar Ecol Prog Ser 207:123–127. doi:10.3354/meps207123
Grutter AS, Morgan JAT, Adlard RD (2000b) Characterising parasitic gnathiid isopod species and matching life stages with ribosomal DNA ITS2 sequences. Mar Biol 136:201–205. doi:10.1007/s002270050677
Grutter AS, Poulin R (1998) Intraspecific and interspecific relationships between host size and the abundance of parasitic larval gnathiid isopods on coral reef fishes. Mar Ecol Prog Ser 164:263–271. doi:10.3354/meps164263
Grutter AS, Rumney JG, Sinclair-Taylor T, Waldie P, Franklin CE (2011) Fish mucous cocoons: the ‘mosquito nets’ of the sea. Biol Lett 7:292–294. doi:10.1098/rsbl.2010.0916
Guégan JF, Hugueny B (1994) A nested parasite species subset pattern in tropical fish: host as major determinant of parasite infracommunity structure. Oecologia 100:184–189. doi:10.1007/BF00317145
Hatcher MJ, Dick JT, Dunn AM (2006) How parasites affect interactions between competitors and predators. Ecol Lett 9:1253–1271. doi:10.1111/j.1461-0248.2006.00964.x
Hatcher MJ, Dunn AM (2011) Parasites in ecological communities: from interactions to ecosystems. Cambridge University Press, Cambridge
Heupel MR, Bennett MB (1999) The occurrence, distribution and pathology associated with gnathiid isopod larvae infecting the epaulette shark, Hemiscyllium ocellatum. Int J Parasitol 29:321–330. doi:10.1016/S0020-7519(98)00218-5
Hudson PJ, Dobson AP, Lafferty KD (2006) Is a healthy ecosystem one that is rich in parasites? Trends Ecol Evol 21:381–385. doi:10.1016/j.tree.2006.04.007
Huxham M, Raffaelli D, Pike A (1995) Parasites and food web patterns. J Anim Ecol 64:168–176. doi:10.2307/5752
Johnson PT, Dobson A, Lafferty KD, Marcogliese DJ, Memmott J, Orlofske SA, Poulin R, Thieltges DW (2010) When parasites become prey: ecological and epidemiological significance of eating parasites. Trends Ecol Evol 25:362–371. doi:10.1016/j.tree.2010.01.005
Jones CM, Grutter AS (2005) Parasitic isopods (Gnathia sp.) reduce haematocrit in captive blackeye thicklip (Labridae) on the Great Barrier Reef. J Fish Biol 66:860–864. doi:10.1111/j.0022-1112.2005.00640.x
Jones CM, Grutter AS (2007) Variation in emergence of parasitic and predatory isopods among habitats at Lizard Island, Great Barrier Reef. Mar Biol 150:919–927. doi:10.1007/s00227-006-0416-z
Justine JL (2010) Parasites of coral reef fish: how much do we know? With a bibliography of fish parasites in New Caledonia. Belgian J Zool 140:155–190
Justine JL, Beveridge I, Boxshall GA, Bray RA, Miller TL, Moravec F, Trilles JP, Whittington ID (2012) An annotated list of fish parasites (Isopoda, Copepoda, Monogenea, Digenea, Cestoda, Nematoda) collected from snappers and bream (Lutjanidae, Nemipteridae, Caesionidae) in New Caledonia confirms high parasite biodiversity on coral reef fish. Aquat Biosyst 8:22. doi:10.1186/2046-9063-8-22
Kensley B (1998) Estimates of species diversity of free-living marine isopod crustaceans on coral reefs. Coral Reefs 17:83–88. doi:10.1007/s003380050100
Kramer DL, Chapman MR (1999) Implications of fish home range size and relocation for marine reserve function. Environ Biol Fish 55:65–79. doi:10.1023/A:1007481206399
Kuris AM, Hechinger RF, Shaw JC, Whitney KL, Aguirre-Macedo L, Boch CA, Dobson AP, Dunham EJ, Fredensborg BL, Huspeni TC, Lorda J, Mababa L, Mancini FT, Mora AB, Pickering M, Talhouk NL, Torchin ME, Lafferty KD (2008) Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature 454:515–518. doi:10.1038/nature06970
Lafferty KD (2013) Parasites in marine food webs. Bull Mar Sci 89:123–134. doi:10.5343/bms.2011.1124
Lafferty KD, Allesina S, Arim M, Briggs CJ, De Leo G, Dobson AP, Dunne JA, Johnson PT, Kuris AM, Marcogliese DJ, Martinez ND, Memmott J, Marquet PA, McLaughlin JP, Mordecai EA, Pascual M, Poulin R, Thieltges DW (2008) Parasites in food webs: the ultimate missing links. Ecol Lett 11:533–546. doi:10.1111/j.1461-0248.2008.01174.x
Lafferty KD, Dobson AP, Kuris AM (2006) Parasites dominate food web links. Proc Natl Acad Sci 103:11211–11216. doi:10.1073/pnas.0604755103
Lafferty KD, Kuris AM (2002) Trophic strategies, animal diversity and body size. Trends Ecol Evol 17:507–513. doi:10.1016/S0169-5347(02)02615-0
Marcogliese DJ (2002) Food webs and the transmission of parasites to marine fish. Parasitology 124:83–99. doi:10.1017/S003118200200149X
McKiernan JP, Grutter AS, Davies AJ (2005) Reproductive and feeding ecology of parasitic gnathiid isopods of epaulette sharks (Hemiscyllium ocellatum) with consideration of their role in the transmission of a haemogregarine. Int J Parasitol 35:19–27. doi:10.1016/j.ijpara.2004.10.016
Mouritsen KN, Poulin R (2002) Parasitism, community structure and biodiversity in intertidal ecosystems. Parasitology 124:101–117. doi:10.1017/S0031182002001476
Nagel L, Grutter AS (2007) Host preference and specialization in Gnathia sp., a common parasitic isopod of coral reef fishes. J Fish Biol 70:497–508. doi:10.1111/j.1095-8649.2007.01320.x
Nagel L, Montgomerie R, Lougheed SC (2008) Evolutionary divergence in common marine ectoparasites Gnathia spp. (Isopoda: Gnathiidae) on the Great Barrier Reef: phylogeography, morphology, and behaviour. Biol J Linn Soc 94:569–587. doi:10.1111/j.1095-8312.2008.00997.x
Ota Y, Fujita Y, Hirose M (2016) Occurrence of temporary fish ectoparasites (Isopoda; Gnathiidae) in low-salinity subterranean habitats of Miyako-jima Island, Ryukyu Islands, southwestern Japan. Plankton Benthos Res 11:87–95. doi:10.3800/pbr.11.87
Penfold R, Grutter AS, Kuris AM, McCormick MI, Jones CM (2008) Interactions between juvenile marine fish and gnathiid isopods: predation versus micropredation. Mar Ecol Prog Ser 357:111–119. doi:10.3354/meps07312
Poore GC, Bruce NL (2012) Global diversity of marine isopods (except Asellota and crustacean symbionts). PLoS One 7:e43529. doi:10.1371/journal.pone.0043529
Poulin R (2007) Evolutionary ecology of parasites. Princeton University Press, Princeton
Poulin R, Blasco-Costa I, Randhawa HS (2014) Integrating parasitology and marine ecology: seven challenges towards greater synergy. J Sea Res 113:3–10. doi:10.1016/j.seares.2014.10.019
Quattrini AM, Demopoulos AW (2016) Ectoparasitism on deep-sea fishes in the western North Atlantic: in situ observations from ROV surveys. Int J Parasitol Parasites Wildl 5:217–228. doi:10.1016/j.ijppaw.2016.07.004
Raffel TR, Hoverman JT, Halstead NT, Michel PJ, Rohr JR (2010) Parasitism in a community context: trait-mediated interactions with competition and predation. Ecol 91:1900–1907. doi:10.1890/09-1697
Raffel TR, Martin LB, Rohr JR (2008) Parasites as predators: unifying natural enemy ecology. Trends Ecol Evol 23:610–618. doi:10.1016/j.tree.2008.06.015
Ravichandran S, Rameshkumar G, Balasubramanian T (2010) Infestation of isopod parasites in commercial marine fishes. J Parasit Dis 34:97–98. doi:10.1007/s12639-010-0014-3
Roberts CM, Polunin NV (1991) Are marine reserves effective in management of reef fisheries? Rev Fish Biol Fish 1:65–91. doi:10.1007/BF00042662
Rohde K (1976) Species diversity of parasites on the Great Barrier Reef. Parasitol Res 50:93–94
Rowley RJ (1994) Marine reserves in fisheries management. Aquat Conserv 4:233–254. doi:10.1002/aqc.3270040305
Sikkel PC, Cheney KL, Côté IM (2004) In situ evidence for ectoparasites as a proximate cause of cleaning interactions in reef fish. Anim Behav 68:241–247. doi:10.1016/j.anbehav.2003.10.023
Sikkel PC, Fuller CA, Hunte W (2000) Habitat/sex differences in time at cleaning stations and ectoparasite loads in a Caribbean reef fish. Mar Ecol Prog Ser 193:191–199. doi:10.3354/meps193191
Sikkel PC, Herzlieb SE, Kramer DL (2005) Compensatory cleaner-seeking behavior following spawning in female yellowtail damselfish. Mar Ecol Prog Ser 296:1–11. doi: 10.3354/meps296001
Sikkel PC, Schaumburg CS, Mathenia JK (2006) Diel infestation dynamics of gnathiid isopod larvae parasitic on Caribbean reef fish. Coral Reefs 25:683–689. doi:10.1007/s00338-006-0154-1
Sikkel PC, Sears WT, Weldon B, Tuttle BC (2011) An experimental field test of host-finding mechanisms in a Caribbean gnathiid isopod. Mar Biol 158:1075–1083. doi:10.1007/s00227-011-1631-9
Sikkel PC, Tuttle LJ, Cure K, Coile AM, Hixon MA (2014) Low susceptibility of invasive red lionfish (Pterois volitans) to a generalist ectoparasite in both its introduced and native ranges. PLoS One 9:e95854. doi:10.1371/journal.pone.0095854
Sikkel PC, Welicky RL, Artim JM, McCammon AM, Sellers JC, Coile AM, Jenkins WG (2016) Nocturnal migration reduces exposure to micropredation in a coral reef fish. Bull Mar Sci 93:475–489. doi:10.5343/bms.2016.1021
Sikkel PC, Ziemba RE, Sears WT, Wheeler JC (2009) Diel ontogenetic shift in parasitic activity in a gnathiid isopod on Caribbean coral reefs. Coral Reefs 28:489–495. doi:10.1007/s00338-009-0474-z
Smit NJ, Basson L, Van As JG (2003) Life cycle of the temporary fish parasite, Gnathia africana (Crustacea: Isopoda: Gnathiidae). Folia Parasitol 50:135–142. doi:10.14411/fp.2003.024
Smit NJ, Davies AJ (2004) The curious life-style of the parasitic stages of gnathiid isopods. Adv Parasitol 58:289–391. doi:10.1016/S0065-308X(04)58005-3
Smit NJ, Grutter AS, Adlard RD, Davies AJ (2006) Hematozoa of teleosts from Lizard Island, Australia, with some comments on their possible mode of transmission and the description of a new hemogregarine species. J Parasitol 92:778–788. doi:10.1645/GE-756R.1
Svavarsson J, Bruce NL (2012) New and little-known gnathiid isopod crustaceans (Cymothoida) from the northern Great Barrier Reef and the Coral Sea. Zootaxa 3380:1–33. doi:10.5281/zenodo.212640
Tanaka K (2007) Life history of gnathiid isopods—current knowledge and future directions. Plankton and Benthos Research 2:1–11. doi:10.3800/pbr.2.1
Tanaka K, Aoki M (1998) Crustacean infauna of the demosponge Halichondria okadai (Kadota) with reference to the life cycle of Gnathia sp. (Isopoda: Gnathiidea). In: Watanabe Y, Fusetani N (eds) Sponge science: multidisciplinary perspectives. Springer-Verlag, Tokyo, pp 259–267
Tanaka K, Nishi E (2008) Habitat use by the gnathiid isopod Elaphognathia discolor living in terebellid polychaete tubes. J Mar Biol Assoc UK 88:57–63. doi:10.1017/S0025315408000039
Thomas F, Renaud F, de Meeûs T, Poulin R (1998) Manipulation of host behaviour by parasites: ecosystem engineering in the intertidal zone? Proc R Soc Lond B Biol Sci 265:1091–1096. doi:10.1098/rspb.1998.0403
Thompson RM, Mouritsen KN, Poulin R (2005) Importance of parasites and their life cycle characteristics in determining the structure of a large marine food web. J Anim Ecol 74:77–85. doi:10.1111/j.1365-2656.2004.00899.x
Triki Z, Grutter AS, Bshary R, Ros AF (2016) Effects of short-term exposure to ectoparasites on fish cortisol and hematocrit levels. Mar Biol 163:187. doi:10.1007/s00227-016-2959-y
Welicky RL, Cheney KL, Coile AM, McCammon A, Sikkel PC (2013) The relationship between lunar periodicity and activity of fish-parasitic gnathiid isopods in the Caribbean. Mar Biol 160:1607–1617. doi:10.1007/s00227-013-2213-9
Whiteman EA, Côté IM (2002) Cleaning activity of two Caribbean cleaning gobies: intra- and interspecific comparisons. J Fish Biol 60:1443–1458. doi:10.1111/j.1095-8649.2002.tb02439.x
Williams JD, Madad AZ (2010) A new species and record of branchial parasitic isopods (Crustacea: Isopoda: Bopyridae: Pseudioninae) of porcellanid crabs from the Philippines. Exp Parasitol 125:23–29. doi:10.1016/j.exppara.2009.09.012
Wood CL, Byers JE, Cottingham KL, Altman I, Donahue MJ, Blakeslee AM (2007) Parasites alter community structure. Proc Natl Acad Sci 104:9335–9339. doi:10.1073/pnas.0700062104
Wood CL, Lafferty KD (2015) How have fisheries affected parasite communities? Parasitology 142:134–144. doi:10.1017/S003118201400002X
Wood CL, Lafferty KD, Micheli F (2010) Fishing out marine parasites? Impacts of fishing on rates of parasitism in the ocean. Ecol Lett 13:761–775. doi:10.1111/j.1461-0248.2010.01467.x
Acknowledgments
The authors thank the late Mayor Emmanuel Diputado, the Department of Agriculture and the Bantay Dagat of the Sibulan Municipality for logistic support and permitting access to the marine sanctuary of Agan-an. We also thank the director, Dr. Hilconida P. Calumpong, Dr. Janet S. Estacion, Dr. Rene A. Abesamis, and the staff of Silliman University Institute for Environmental and Marine Sciences for assistance and use of lab space. We thank Dianne Mira Yap, Danielle Mark Fukuda, Jean Asuncion Utzurrum, Floramae Joyce Neri, Denzyl Divinagracia, Persie Mark Sienes, and Mary Shodipo for the field assistance. Finally, we thank Dioscoro Inocencio, Rafael Ceriales, Andres B. Tubat, Paseo Tubat, and Jose G. Somoza Sr. for fish collections and field support, and Nico J. Smit for assistance with gnathiid species identification. This work was funded in part by the US National Science Foundation (OCE-1536794, PC Sikkel, PI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The use and care of animals used in this study was approved by the Arkansas State University Institutional Animal Care and Use Committee (IACUC) protocol number 778227-1. All other applicable international, national, and institutional guidelines for the care and use of animals were followed.
Additional information
Communicated by H. Hillebrand
Rights and permissions
About this article
Cite this article
Santos, T.R.N., Sikkel, P.C. Habitat associations of fish-parasitic gnathiid isopods in a shallow reef system in the central Philippines. Mar Biodiv 49, 83–96 (2019). https://doi.org/10.1007/s12526-017-0756-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-017-0756-6