Abstract
Marine benthic organisms can be used as indicators of the quality of environmental status and as monitoring tools to detect natural or anthropogenic perturbations. In temperate waters, metabolic and biochemical responses may be governed by physiological changes driven by seasonal factors. Gathering baseline information on the mechanisms underlying seasonal acclimation patterns is therefore a critical step towards the understanding of the physiological responses of biological indicators. In poikilothermic metazoans, the production of regulatory metabolic enzymes can be used as tools for deciphering the acclimation potential. The aim of this study was to characterize the natural seasonal variability in biometric traits and enzymatic biomarkers over a 12-month period in the sea anemone, Anemonia sulcata (Anthozoa, Cnidaria), from two areas with different environmental and anthropic impacts. Seasonality and site factors affected enzymatic kinetics at tentacle levels, while seasonality, site and biometrical characteristics modulated the same enzymes in the body column of A. sulcata. The results showed a decrease in enzymatic activity in summer and an increased synthesis of enzymes in the late fall and winter months. The interaction between biometric features and temperature appeared significant for mesolittoral sea anemones but not for infralittoral specimens. This study showed seasonal patterns of variations of biochemical responses in A. sulcata, suggesting that this species, an abundant member of shallow rocky bottom communities in temperate European seas, may represent a sensitive bio-indicator organism worth considering for further ecological studies on climate change as well as for environmental monitoring programs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In marine coastal zones, several important physiological responses are greatly influenced by abiotic and biotic factors (Odum and Barrett 2005) like food availability, the presence of predators, infection with pathogenic organisms or the interactions with conspecifics (Wiens 2011).
The concept of systemic biomonitoring asserts that the ensuring the health of aquatic ecosystems can be facilitated by integrating analytical chemical analysis with carefully selected biological endpoints measured in tissues of species of concern (Connon et al. 2011). Benthic organisms are used as indicators of oceanographic conditions, and also to monitor natural ecosystems or those affected by anthropogenic factors. A large number of studies in the literature show how benthic macroinvertebrates have been identified as excellent bioindicator species due to a number of their features (Bellante et al. 2016; Parrinello et al. 2017). Thanks to their long life cycle, they can track changes and pollutants, and they show sensitivity to various chemical or hydromorphological stress over long periods of time.
Hydroids, jellyfish, sea anemones, zoanthids, gorgonians, hard and soft corals, such as Exaiptasia pallida, Dipsastrea pallida, Cladocora caespitosa and coral reef species, are used as bioindicators of changes in water quality (Cooper et al. 2009). Furthermore, they require specific environmental conditions for survival and are sensitive to slight environmental changes. The cnidarian Hydra spp. has gained increased attention in aquatic toxicology as a sensitive and possible target species of the benthic community.
The use of new bioindicators for environmental monitoring requires previous systematic studies to establish the natural behavior of the organism (Stohler et al. 2004), identify biomarkers altered in response to environmental conditions (Prá et al. 2005; Villela et al. 2006), and establish the degree of susceptibility of the organism to specific agents (Knakievicz et al. 2008).
Physiological responses are a function of species metabolism; therefore, changes in seasonal factors act on the metabolic variables (Seebacher and Franklin 2012). In poikilothermic organisms, such as Cnidarians, a basal metazoan, environmental temperature is one of the major factors driving physiological and biochemical and enzymatic processes (Pfeifer et al. 2005) and, under extreme conditions, many of these processes shut down or compromise cellular function in other ways (Helmouth et al. 2006). It has thus become important for researchers to obtain baseline data on the physiology of marine benthic species through the seasons in order to understand the mechanism governing physiological processes.
Exploring the role of organism temperature in driving species distribution patterns has assumed a greater sense of urgency due to changes in the global climate. Furthermore, the activity and the kinetic characteristics of regulatory metabolic enzymes have been used as tools in the investigation of the adaptation potential of organisms (Ozernyuk et al. 1994; Vetter and Buchholz 1997).
As a macroinvertebrate, Anemonia sulcata may be considered a good bioindicator in systemic biomonitoring.A. sulcata is an anthozoan species living in the infralittoral zone widely distributed throughout the Mediterranean and Atlantic, from Gibraltar to the coasts of Scotland. At endodermal level, it possesses zooxanthellae of the genus Symbiodinium. The relationship with the symbiont reflects a seasonal variation; indeed, specimens change the density of algae during the year, lowering it in the summer and increasing the growth in the winter (Fitt et al. 2000).
Cnidarians are capable of acclimatizing to thermal stress by modifying various components of their cellular metabolism so that they can better perform under elevated temperature conditions (Hoegh-Guldberg 1999). Thermal stress is the major cause of current climate change-related mass bleaching events and associated mortality (Hoegh-Guldberg 1999; Donner et al. 2005), such as the loss of symbiotic dinoflagellates from the cnidarian host tissue (Douglas 2003).
Many studies have pointed out the significance of oxidative stress in cnidarians’ bleaching (Downs et al. 2002; Wietheger et al. 2015). Reactive oxygen species production increases particularly during environmental stress (Lesser 1997; Mittler 2002) and, being highly reactive, can damage various vital bio-molecules such as DNA, proteins and lipids. Anti-oxidant enzymes normally protect aerobic organisms from ROS (Tamagno 1998).
A preliminary study of the normal fluctuations of physiological responses surveyed provides a good starting point to differentiating between normal variations due to the natural life cycle of organisms and changes due to stress situations.
Within the groups of exiting biomarkers, the variation in enzymatic kinetics is commonly used as an indicator of marine species’ biological status. In particular, esterases are enzymes used in a wide range of processes involving synthesis and hydrolysis reactions and they are involved in catabolic pathways (Stamatis et al. 1998; Lopes et al. 2011). The study of alkaline phosphatase has, in special cases, provided understanding of animal adaptation to stress conditions (Copeland 2000).
The peroxidation of membrane phospholipids is initiated by free radicals and can be used in evaluating oxidative damage. The super-family of peroxidase enzymes contains many isoforms, which partake in phagocytosis, and immune-cell-related and anti-oxidant functions (Galloway and Depledge 2001; Soares-da-Silva et al. 2002; Rodriguez et al. 2003).
The aim of the present study was to assess the basal level in a suite of enzymes belonging to the hydrolase and peroxidase categories, and to characterize the natural seasonal variability in biomarkers response over a 12-month period in A. sulcata from two areas with different environmental conditions, anthropic impacts and hydrological characteristics.
Different biomarkers have been characterized in order to derive the possible seasonal variations and the relationship to the seawater temperature, site of sampling and biometrics traits, and to represent the species as a sensitive bio-indicator organism to use during environmental monitoring programs.
Methods
Sampling
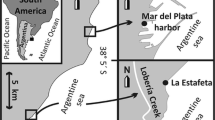
Anemonia sulcata specimens were collected from the coastal zone of Termini Imerese (Palermo, Italy) and the B zone of the Capo Gallo Marine Protected Area (Palermo, Italy), in the north of Sicily, over 12 months from January to December. Each month, two sampling and four replicates (for each sampling) were carried out both in C. Gallo and T. Imerese for a total of 192 sampled specimens. Tentacles and body were collected and stored from each specimen for a total of 384 samples (Fig. 1).
Sampling sites and experimental plan of Anemonia sulcata. Specimens were collected from the B zone of the Capo Gallo Marine Protected Area and Termini Imerese over 12 months. In both sites, two sampling sessions (S1, S2) and four replicates (R1, R2, R3, R4) were carried out per month for 96 specimens sampled at both sites and a grand total of 192 specimens and 384 samples (192 tentacles and 192 body samples)
The sampling sites showed different geomorphological features and anthropogenic impact.The T. Imerese harbor zone (37°58′41.37″N, 13°42′39.41″E) is impacted due to a discharge for the cooling water of a nearby thermoelectric power station and numerous breakwater barriers erected for the protection of the coastal line and railway which stops numerous fishing and sport boats. Animals were collected manually in the mesolittoral zone from the base of breakwaters during low tide.
Capo Gallo - Isola delle Femmine (38°21′88.52“N, 13.32′22.47“E) is a natural protected area with low anthropic pressures. Samples were collected at 2–3 m depth by scuba divers, where hydrodynamism regime and bionomic conditions were different cmpoared with T. Imerese.
Animals were sampled at the adult life stage at both sites. The size of the collected specimens ranged from a minimum of 2.5 cm to a maximum of 6.34 cm (diameter) in T. Imerese and from a minimum of 4.2 cm to a maximum of 9.2 cm (diameter) in Capo Gallo.
After each collection, specimens were transferred to aquaria and maintained in oxygenated seawater at 18 °C for 3 days.
Biometric measurement and proteic extraction
Specimens were cleaned and arranged on a glass plate, and biometric measurements were then taken at the body level (measurements of the pedal and the oral disc) and tentacles (length of the entire tentacle) using a millimeter gauge.
Tissue extractions were performed from cut tentacles and from the body, using four individual replicates for each analysis. Briefly, all tentacles were separated from the animal body with forceps and both parts suspended in 50-ml polycarbonate tubes with 4 ml of TBS buffer solution (TBS: 150 mM NaCl, 10 mM Tris HCl, pH 7.4). After homogenization by a tissue homogenator (Bioneer, Daejeon, Korea), the samples were centrifuged at 21,000g, for 20 min at 4 °C. Finally, the supernatant was recovered and stored in 2-ml Eppendorf tubes at −20 °C until using for assays.
Protein content estimation
To estimate protein content, the method of Bradford (1976) was used with BSA (ranging from 0.1 to 10 mg/ml) as a standard.
Enzyme assays
Enzymatic kinetics variation was evaluated by the microplates reader RT-2100C Microplate Reader Ray after adjusting the samples at concentrations of 500 μg/ml and preparing appropriate reaction substrates in 96-well flat-bottom plate.
Alkaline phosphatase
To detect alkaline phosphatase kinetics, equal volumes (50 μl) of proteic tissue extracts (from both tentacles and body) and 4 mM p-nitrophenyl liquid phosphate (Sigma) were placed in 100 mM ammonium bicarbonate buffer containing 1 mMMgCl2 (pH 7.8, 30 °C), as described by Ross et al. (2000). The OD was continuously measured at 5-min intervals over 1 h at 405 nm in a plate reader. The initial rate of the reaction was used to calculate the activity. The enzyme unit(U) was defined as the amount of an enzyme that catalyzes the conversion of 1 μmole of substrate per minute, and in this case, the p-nitrophenyl phosphate quantity converted in p-nitrophenol in 1 min. The Abs data derived from their kinetic reactions were processed with Sigma plot software (Sigma Plot v.12.5; Systat Software, San Jose, CA, USA) in order to establish the value of Abs/min through the angular coefficient of the straight line of regression analysis applied to the graph Time versus Abs.
Esterase activity
The esterase activity was determined according to the method of Ross et al. (2000). In a ratio of 1:1, samples were incubated with 0.4 mM p-nitrophenylmyristate substrate (Sigma) in 100 mM ammonium bicarbonate containing 0.5% of Triton X-100 (pH 7.8, 30 °C). The increase of the optical density was measured at regular intervals of 5 min for 1 h at 405 nm. The activity was determined as for the alkaline phosphatase.
Peroxidase activity
To evaluate the peroxidase activity, 50 μl of each sample in 100 μL of TMB (3,3′, 5,5′-tetramethylbenzidine) was incubated for 30 min in a 96-well flat-bottom plate. The color change indicated the presence of peroxidase activity. The reaction was stopped with 2 M sulphuric acid (H2SO4) and the absorbance was read at 450 nm (modified from Quade and Roth 1997).
The Abs value of the peroxidase was multiplied for the final volume (Vf) of the well (200 μl), and then divided for the protein concentration ((Abs × Vf)/C.P. = U/mg). Standard samples without tentacles or body samples were used as blanks. One unit was defined as the amount producing an absorbance change of 1 and the activity expressed as U/mg.
Data treatment
Variables evaluated were activity of esterase, phosphatase and peroxidase enzymes, while the factors were sampling site (Capo Gallo, T. Imerese), temperature as average sea surface temperatures (SST) during the annual period from January to December, biometry of pedal and oral disc and tentacle length.
The data have been analyzed in relation to the seasonality: winter (January–March), spring (April–June), summer (July–September) and fall (October–December).
The levels of the biometrics factor were: “Big” for the tentacles with a length between 3.61 and 7 cm and oral and pedal disk with a diameter up to 4 cm; and “Small” referred to the tentacles with a length between 1 and 3.60 cm and oral and pedal disk with a diameter up to 2.40 cm.
The trend of average SST was obtained from forecasts based on satellite data; the reference catalog was Mediterranean Sea Physics Analysis And Forecast.
Statistical analysis
All measurements were performed on four replicates. The results in the figures are expressed as mean ± SE (SEM) (n = 4). Differences in activity between samples were investigated by analysis of variance (ANOVA) procedures through StatView software. Multiple comparisons of enzymes activity over time and between sites at each sampling period were made using a post-hoc comparison Tukey’s test. Factorial analyses of variance (ANOVAs) were used to detect significant differences between the sampling groups. The ANOVA assumption (homogeneity and normal distribution of the data) was verified through the Cochran Test.
Results
Morphotypes of A. sulcata are reported in Fig. 2. During the winter in Site 1 (Capo Gallo), the specimens appeared brown and the tentacle length was conspicuous (A), while at higher temperatures, the light brown morphotype was predominant (B). In Site 2 (T. Imerese) during the winter, the intertidal sampled specimens were less dark than in the infralittoral of Capo Gallo, and the oral opening appeared more developed (C). In the summer, the specimens were increasingly clear and reduced in size (D), until the discovery of specimens affected by bleaching that were then able to expel symbiotic zooxanthellae (E). High biometric measures of A. sulcata length of tentacles from Capo Gallo were collected during the fall and part of the winter, while a decrease was detected in the spring. The smaller size has been found during the summer months. Greater tentacle lengths of specimens were collected in T. Imerese during the winter and followed by a strong decrease in length at high temperatures in the summer and fall (especially in September) (Fig. 3). Oral and pedal disks maintained an almost constant size throughout the year in the samples from Capo Gallo, while these decreased in size between August and October in T. Imerese.
Biometric data of tentacles, oral and pedal disc from A. sulcata specimens collected in the infralittoral area of Capo Gallo (Site 1) and subtidal zone of Termini Imerese (Site 2). Average sea surface temperatures (SST) during the January–December period are reported in the third axis. Data represent the mean ± SE (n = 4 replicates)
In Fig. 4, the ANOVA shows statistical difference of the biometric variables between the four seasons. The tentacle lengths are higher in specimens from Capo Gallo respect to T. Imerese during whole annual period. In particular, the biometric variable of tentacles increased significantly in the fall for specimens from Capo Gallo (p < 0.01) and in winter for sample from Termini Imerese (p < 0.01). The diameter of oral and pedal disks showed conspicuous dimensions in winter and spring and a statistical difference between seasons in T. Imerese (p < 0.01) but not in Capo Gallo (p > 0.01) where, contrary to the mesolittoral site, the biometric variables of body decreased in size in winter and spring.
One-way ANOVA of the interactions of seasonality effect on tentacle length and pedal and oral disc diameter for specimens collected in Capo Gallo and Termini Imerese. Differences between means were considered significant at *p < 0.05, **p < 0.01 and ***p < 0.001. F and P values resulted significant for tentacles biometrical variable in specimens collected from Capo Gallo (p < 0.001) and the Termini Imerese subtidal area (p < 0.001). Pedal and oral disc showed significant differences in T. Imerese specimens (p < 0.001) but not in Capo Gallo samples (tentacles, p = 0.61; p = 0.76)
Enzyme activity of esterase, phosphatase and peroxidase in tentacle extracts of A. sulcata produced higher values in spring and fall (Fig. 5) both in Capo Gallo (Site 1) and in T. Imerese (Site 2) according to the whole annual scenario. A decrease was detected from May to September (at the highest temperatures), while a recovery in enzyme production followed in the fall. This state was much more evident in Site 1. Phosphatase activity, in particular, showed maximum and significant values at Site 1 in April (p < 0.05) and November, but from February to April (p < 0.005) at Site 2. The lowest production rates were observed in September in both sites extracts.
Activity of phosphatase, esterase and peroxidase enzymes in A. sulcata tentacles collected in the infralittoral area of Capo Gallo (Site 1) and subtidal zone of Termini Imerese (Site 2). Average sea surface temperatures (SST) during the January–December period are reported in the third axis. Data represent the mean ± SE (n = 4 replicates). Significant differences were detected by the Tukey post hoc test: *p < 0.05, **p < 0.01 and ***p < 0.001
High values of esterase, phosphatase and peroxidase production were detected in specimen bodies during winter and spring (January–April) at Site 1, and to a lesser degree at Site 2 (Fig. 6), with significant values (p < 0.005) from February to April. At Site 1, from May until the end of the year, all three enzymes showed the same kinetic, while at Site 2 a further decrease in September was recorded, which remained through to November.
Activity of phosphatase, esterase and peroxidase enzymes in A. sulcata body collected in the infralittoral area of Capo Gallo (Site 1) and subtidal zone of Termini Imerese (Site 2). Average sea surface temperatures (SST) during the January–December period are reported in the third axis. Data represent the mean ± SE (n = 4 replicates). Significant differences were detected by the Tukey post hoc test: *p < 0.05, **p < 0.01 and ***p < 0.001
The multifactorial analysis carried out on the overall data showed that in the tentacles, seasonality (<0.001) and site (p < 0.001) significantly modulated the enzyme activity, but not the mean of lengths of tentacles (p = 0.172) (Table 1; Fig. 7). All three enzymes production decreased significantly with increasing temperature with the exception of peroxidase at the T. Imerese site (p = 0.002) and only for the esterase enzyme, the biometrics factor showed a significant difference (p = 0.003) (Table 1). The interactions “site × biometry” (p = 0.02) confirm that the all enzyme production decrease in small organisms collected both at Capo Gallo and T. Imerese and that the minimum production is detected in summer (seasonality × biometry = 0.007).
Multifactorial ANOVA carried out on three independent enzymatic variables (phosphatase, esterase and peroxidase) detected in tentacles proteic extract relating to effects of seasonality, site and biometry factors. Interactions of first and second order about the three factors were investigated. P values resulted from differences between means and considered significant at *p < 0.05, **p < 0.01 and ***p < 0.001. Site, seasonality and interaction “site × seasonality”, “site × biometry”, “site × seasonality × biometry”, resulted significant
Interactions site*seasonality, and seasonality*biometry and site*seasonality*biometry among the factors were not significant for the totality of the enzymatic variables detected in the tentacles proteic extracts.
The total enzymatic activity measured in the body was also affected significantly by temperature (p < 0.001) and site (p = 0.042) factors. Univariate results showed a significant difference in peroxidase activity (p < 0.001) in specimens from T. Imerese respect those collected in Capo Gallo (Table 2; Fig. 8). In addition, all three enzymatic components showed a significant difference in relation to biometry (p = 0.003) decreasing in small organisms especially in the impacted site (p < 0.001).
Multifactorial ANOVA carried out on three independent enzymatic variables (phosphatase, esterase and peroxidase) detected in body proteic extract relating to effects of seasonality, site and biometrical traits. Interactions of first and second order about the three variables were investigated. P values resulted from differences between means and considered significant at *p < 0.05, **p < 0.01 and ***p < 0.001. All the individual factors, site, seasonality and biometry and the interaction of the first order resulted “site*biometry “resulted significant
The interactions site*biometry resulted significant for the total activity (p = 0.007) and for the individual contribution regarding phosphatase (p < 0.001), peroxidase (p < 0.001) and esterase (p < 0.001). The site × seasonality, seasonality × biometry and site × seasonality × biometry among the factors were not significant for the totality of the enzymatic variables measured in the body samples.
Discussion
Studies of environmental signaling in animals have focused primarily on organisms with relatively constrained responses, both temporally and phenotypically. Worms and flies as existing model animals have relatively little capacity to alter their morphology in response to environmental signals (Blackstone and Bridge 2005). Hence, they exhibit little of the effect of environmental signaling on phenotypes. The nature of the interaction between environmental factors and variation can be best understood in animals that differ from conventional model organisms. The basal metazoans exhibit relatively unconstrained responses to environmental signals and may thus provide more general insight. This study analyzed the seasonal fluctuations in biometric variables and alkaline phosphatase, esterase and peroxidase enzymes in tentacles and body tissue extracts from A. sulcata during a period from January to December in two different natural populations from sites with different hydrodynamic regime, anthropogenic impact and protection levels. Specimens were sampled in the infralittoral zone of Capo Gallo, a marine protected area, and in a mesolittoral zone of the harbor area of Termini Imerese subjected to increases in turbidity and pollution.
Morphotypes and biometric characteristics have indicated differences between the two populations. The relationship between the biotic factor (as biometry) and abiotic factors (temperature, site) and the enzymatic activities involved in various biological processes including the defense system were analyzed.
Subtidal specimen phenotypes from T. Imerese showed fewer pigments than infralittoral organisms from Capo Gallo. This decrease in color is often linked to symbiosis dysfunction or reduction, which is the loss of symbionts from host tissues (Douglas, 2003). Bleaching is a stress response to environmental perturbation and it starts as a reaction to multiple stressors, changes in salinity, high visible and/or ultraviolet radiation, increased sedimentation, nutrients or pollutants (Coles and Brown 2003). However, it is now well accepted that widespread bleaching in nature is a result of elevated sea surface temperatures, associated with global climate change and with seasonally high light and/or UV radiation (Hoegh-Guldberg et al. 2007).
Seasonality and sampling site factors affected enzymatic kinetics at tentacle levels, while temperature, site and biometrical characteristics modulated the same enzymes in the body of A. sulcata. In particular, the production of enzymes increased during fall and winter, both in infralittoral (Capo Gallo) and subtidal (Termini Imerese) specimens at below 20 °C.
Phosphatase enzyme production displayed an inverse relationship with the algal bloom, being at a maximum in the fall and winter when the bloom is minimal. Algae convert the dissolved organic phosphorus (DOP) in the environment into dissolved inorganic phosphorus (DIP), which is used for organisms’ metabolic activities, thanks to the release of alkaline phosphatase into the water. When large amounts of DIP are available, alkaline phosphatase activity is repressed (Dyhrman et al. 2007). The production of phosphatase present in the body is less pronounced than in tentacles, and zooxanthellae play a crucial role in the absorption of phosphate dissolved in water (Annis and Cook 2002). Furthermore, tentacles absorb and bioaccumulate phosphates more efficiently than the body and an increase of the length of the tentacles is related to a greater presence of zooxanthellae (Pagès et al. 2016).
For both Capo Gallo and T. Imerese organisms, esterase production in the tentacles and body reflected a similar trend to that proposed earlier for phosphatase activity. Therefore, with activity lower in the summer and greater enzyme production in the late fall and winter months, there was also a correlation here with seasonality and sampling site. In subtidal specimens’ biometry and seasonality, interactions also appeared significant. In Capo Gallo, the ANOVA showed statistically significant differences in the months of April and November for tentacles and in February, March and April for the body. In addition to the defense system, the esterase enzyme in A. sulcata could be involved in processing of metabolism of fatty acids (Helisto and Korpela 1998; Kulkarni and Gadre 2002), and its production could be linked to the activity of photosynthetic symbionts according to a relationship of inverse proportionality. The molecules needed for the organisms are produced by symbionts and there is no esterase involved in the degradation of complex molecules from the environment. The hydrolytic activity associated with the anthozoan tentacles was even one order of magnitude higher than activity in the digestive tracts of other macroinvertebrates. Tentacles can be responsible for extracorporeal digestion, which depends on the presence of hydrolase in the ectoderm. Reduction in body size, tentacle length and enzymatic expression for T. Imerese specimens were significantly affected by increased temperature and, moreover, low enzymatic values were registered in the fall period. Organisms sampled in T. Imerese were subjected to strong environmental stress as rapid changes in temperature due to possible exposure to the air during low tide in the summer rapid warming days, pollution from boats and the dredging carried out with the purpose of gathering bottom sediments, resulting in increased death and reduction in size.
An increase in peroxidase activity in spring months could also be attributed to greater UV ray intensity to prevent damage to the cytoplasmic membranes and to cellular functionality generally (Lobo et al. 2010). A decrease during the period of maximum UV intensity implies that Anemonia. sulcata contains ectodermal levels in some protein structures—Green Fluorescent Protein (GFP)—that shield the animal from the rays and make the light available for the photosynthetic activity of zooxanthellae (Wiedenmann, 2002). The March–April period, during which the UV ray intensity increases, can be conceived as one of “acclimatization”; the animal produces the GFP to protect itself from UV rays and the production of ROS (Wiedenmann et al. 2004). Increased activity was expressed in the body of organisms sampled in T. Imerese, presumably because there is greater exposure to light and little shelter at this site. Furthermore, there is also a larger amount of ectodermal tissue (containing GFP) in the tentacles than in the body.
At the Capo Gallo site, we found a majority, albeit borderline, of activity expressed in the tentacles compared with the body. We attributed this to the fact that organisms protect themselves by hiding in rock crevices where the tentacles are more exposed to light than the body.
The performance of enzymatic fluctuations does not seem to depend on body size. Peroxidase was only slightly lower at the T. Imerese site, where the animals were reduced in size. The lipid peroxidation (LPO) levels and seasonal trends from this study are in the same range as the study by Nahrgang et al. (2012) in which the authors reported seasonal baseline in three species of scallop of complementary parameters commonly used stress biomarkers, as they provided valuable information on the physiological status of the studied organisms.
Giarratano et al. (2011) also identified a lower LPO expression in mussels from different sites in summer compared with winter. Food availability influences the biochemistry of animals and might generally explain the higher lipid peroxidation during low food availability in winter months.
The data collected and physiological responses investigated can be considered reliable biomarkers, as they are reproducible, displayed, controlled and comparable. Thus, they constitute a valid time series that reflects the possible changes due to the natural system.
Environmental factors including temperature, oxygen, food availability and light irradiation can affect physiological parameters of benthic macroinvertebrates. In particular, seasonal changes and temperature are the most studied abiotic factors, being able to affect many physiological processes in ectothermic animals and the structure of ecosystems. From a biological perspective, changes in temperature drive consequences on the dynamics of marine benthic organisms and brought about changes to their physiology and phenology. Local patterns of acclimatization, phenotypic plasticity and genetic adaptation can cause differing responses to changing environmental conditions among individuals within a population, among different populations and among closely related species (Bradshaw and Holzapfel 2006; Helmuth et al. 2006a, b; Pearson et al. 2009; Stillman 2003). Understanding how current and future range boundaries are likely to be set by aspects of weather and climate thus demands that we understand how large-scale environmental ‘signals’ are distilled down to the level of the organism, namely to factors that define the organism’s fundamental (physiological) niche (Kearney 2006). Equally important to such an approach is exploring how physiological responses to these environmental drivers vary in space and time.
The measure of a complete set of enzymatic and morphological parameters provided useful information to determine the physiological status of the anemone and its biological processes used as environmental biomarkers. Thus, the present study showed seasonal variation in biological responses in addition to the knowledge of biology of the species, suggesting that this species—a widely distributed and abundant member of shallow rocky bottom communities in temperate European seas—may represent a sensitive bio-indicator organism, worth considering for further ecological studies on climate change as well as for environmental monitoring programs.
References
Annis ER, Cook CB (2002) Alkaline phosphatase activity in symbiotic dinoflagellates (zooxanthellae) as a biological indicator of environmental phosphate exposure. Mar Ecol Prog Ser 245:11–20
Bellante A, Piazzese D, Cataldo S, Parisi MG, Cammarata M (2016) Evaluation and comparison of trace metal accumulation in different tissues of potential bioindicator organisms: macrobenthic filter feeders Styela plicata,Sabella spallanzanii, and Mytilus galloprovincialis. Environ Toxicol Chem 35:3062–3070
Blackstone N, Bridge DM (2005) Model systems for environmental signaling. Integr Comp Biol 45(4):605–614
Bradford M (1976) A rapid and sensitive method for quantification of microgram quantities of protein using the principle of protein dye binding. Anal Biochem 72:248–254
Bradshaw WE, Holzapfel CM (2006) Evolutionary response to rapid climate change. Science 312:1477–1478
Coles S L, Brown BE (2003) Coral bleaching-capacity for acclimatization and adaptation. Adv Mar Biol 46:183–223
Connon RE, Beggel S, D'Abronzo LS, Giest JP, Pfieff J, Longuinov AV, Vulpe CD, Werner I (2011) Linking molecular biomarkers with higher level condition indicators to identify effects of copper exposures on the endangered delta smelt (Hypomesus transpacificus). Environ Toxicol Chem 30:290–300
Copeland R (2000) Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis. Wiley-VCH, New York
Donner SD, Skirving WJ, Little CM, Oppenheimer M, Hoegh-Guldberg O (2005) Global assessment of coral bleaching and required rates of adaptation under climate change. Glob Change Biol 11(12):2251–2265
Douglas AE (2003) Coral bleaching––how and why? Mar Pollut Bull 46(4):385–392
Downs CA, Fauth JE, Halas JC, Dustan P, Bemiss J, Woodley CM (2002) Oxidative stress and seasonal coral bleaching. Free Radic Biol Med 33(4):533–543
Dyhrman ST, Ammerman JW, Van Mooy BA (2007) Microbes and the marine phosphorus cycle. Oceanography 20:110–11610
Fitt WK, McFarland FK, Warner ME, Chilcoat GC (2000) Seasonal patterns of tissue biomass and densities of sym biotic dinoflagellates in reef corals and relation to symbioses. Mar Bleaching Limnol Oceanogr 45:677–685
Galloway TS, Depledge MH (2001) Immunotoxicology in invertebrates: measurement and ecotoxicological review. Ecotoxicology 10:5–23
Giarratano E, Gil M, Mlanga (2011) Seasonal and pollution induced variation in biomarkers of transplanted mussels within the beagle channel. Mar Pollut Bull 62:1337–1344
Helistö P, Korpela T (1998) Effects of detergents on activity of microbial lipases as measured by the paranitrophenylalkanoate esters method. Enzym Microb Technol 23:113–117
Helmuth B, Broitman BR, Blanchette CA et al (2006a) Thermal stress in the rocky intertidal zone: implications for climate change. Ecol Monogr 76(4):461–479
Helmuth B, Broitman BR, Blanchette CA, Gilman S, Halpin P, Harley CD, O'Donnell M, Hofmann GE, Menge B, Strickland D (2006b) Mosaic patterns of thermal stress in the rocky intertidal zone: implications for climate change. Ecol Monogr 76:461–479
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50(8):839–866
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K et al (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Kearney M (2006) Habitat, environment and niche: what are we modelling? Oikos 115:186–191
Knakievicz T, Ferreira H (2008) Evaluation of copper effects upon Girardia tigrina freshwater planarians based on a set of biomarkers. Chemosphere 71:419–428
Kulkarni N, Gadre RV (2002) Production and properties of alkaline, thermophilic lipase from Pseudomonas fluorenscens NS2W. J Ind Microbiol Biotechnol 28:344–348
Lesser MP (1997) Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs 16(3):187–192
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev 4(8):118–126
Lopes D, Fraga L, Fleuri L, Macedo G (2011) Lipase and esterase - to what extent can this classification be applied accurately? lipases e esterases: como definir e classificar? Tecnol Aliment 31(3):608–613
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nahrgang J, Brooks SJ, Evenset A, Camus L, Jonsson M, Smith TJ, Lukina J, Frantzen M, Giarratano E, Renaud PE (2013). Seasonal variation in biomarkers in blue mussel (Mytilus edulis), Icelandic scallop (Chlamys islandica) and Atlantic cod (Gadus morhua): implications for environmental monitoring in the Barents Sea. Aquat Toxicol. 127:21–35
Odum EP, Barrett GW (2005) Fundamentals of ecology. Thomson Brooks/Cole, Belmont
Ozernyuk ND, Klyachko OS, Polosukhina ES (1994) Acclimation temperature affects the functional and structural properties of lactate dehydrogenase from fish (Misgurnus fossilis) skeletal muscles. Comp Biochem Physiol 107B:141–145
Pagès C, Godinot C, D'Angelo C, Wiedenmann J, Grover R (2016) Phosphorus metabolism of reef organisms with algal symbionts. Ecol Monogr 86(3):262–272
Parrinello D, Bellante A, Parisi MG, Sanfratello MA, Indelicato S, Piazzese D, Cammarata M (2017) The ascidian Styela plicata hemocytes as a potential biomarker of marine pollution: in vitro effects of seawater and organic mercury. Ecotoxicol Environ Saf 136:126–134
Pearson GA, Lago-Leston A, Mota C (2009) Frayed at the edges: selective pressure and adaptive response to abiotic stressors are mismatched in low diversity edge populations. J Ecol 97:450–462
Pfeifer S, Schiedek D, Dippner JW (2005) Effect of temperature and salinity on acetyl- 825 cholinesterase activity, a common pollution biomarker, in Mytilus sp. from the south-westernBaltic sea. J Exp Mar Biol Ecol 320:93–103
Prá D, Lau AH, Knakievicz T, Carneiro FR, Erdtmann B (2005) Environmental genotoxicity assessment of an urban stream using freshwater planarians. Mutat Res 585:79–85
Quade MJ, Roth JA (1997) A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet Immunol Immunopathol 58:239–248
Rodriguez A, Angeles Esteban M, Meseguer J (2003) Phagocytosis and peroxidase release by seabream (Sparus aurata L.) leucocytes in response to yeast cells. Anat Rec 272A:415–423
Ross NW, Firth KJ, Wang A, Burka JF, Johnson SC (2000) Changes in hydrolytic enzyme activities of naïve Atlantic salmon Salmo salar skin mucus due to infection with the salmon louse Lepeophtheirus salmonis and cortisol implantation. Dis Aquat Org 41:43–51
Seebacher F, Franklin C (2012) Determining environmental causes of biological effects: the need for a mechanistic physiological dimension in conservation biology. Philos Trans R Soc Lond B Biol Sci. 19, 367(1596): 1607–1614
Soares da Silva IM, Ribeiro J, Valongo C, Pinto R, Vilanova M, Bleher R, Machado J (2002) Cytometric, morphologic and enzymatic characterisation of haemocytes in Anodontacygnea. Comp Biochem Physiol A 132:541–553
Stamatis H, Christakopoulos P, Kekos D et al (1998) Studies on the synthesis of short-chain geranyl esters catalyzed by Fusarium oxysporum esterase in organic solvents. J Mol Catal B 4:229–236
Stillman J (2003) Acclimation capacity underlies susceptibility to climate change. Science 301(5629):65
Stohler RA, Curtis J, Minchella DJ (2004) A comparison of microsatellite polymorphism and heterozygosity among field and laboratory populations of Schistosoma mansoni. Int J Parasitol 34(5):595–601
Tamagno E, Aragno M, Boccuzzi G, Gallo M, Parola S, Fubini B, Poli G, Danni O (1998) Oxygen free radical scavenger properties of dehydroepiandrosterone. Cell Biochem Funct 16:57–63
Vetter RAH, Buchholz F (1997) Catalytic properties of two pyruvate kinase isoforms in Nordic krill, Meganyctiphanes norvegica, with respect to seasonal temperature adaptation. Comp Biochem Physiol A 116:1–10
Villela IV, de Oliveira IM, da Silva J, Henriques JA (2006) DNA damage and repair in haemolymph cells of golden mussel (Limnoperna fortunei) exposed to environmental contaminants. Mutat Res 605:78–86
Wiedenmann J, Schenk A, Rocker C, Girod A, Spindler K, Nienhaus GU (2002) A far-red fluorescent protein with fast maturation and reduced oligomerization tendency from Entacmaea quadricolour (Anthozoa, Actinaria). Proc Natl Acad Sci U S A 99:11646–11651
Wiedenmann J, Ivanchenko S, Oswald F, Nienhaus GU (2004) Identification of GFP-like proteins in nonbioluminescent, azooxanthellate Anthozoa opens new perspectives for bioprospecting. Mar Biotechnol 6:270–277
Wiens (2011). The niche, biogeography and species interactions. Philos Trans R Soc Lond B Biol Sci 366(1576): 2336–2350
Wietheger A, Fisher P, Gould K, Davy S (2015) Sensitivity to oxidative stress is not a definite predictor of thermal sensitivity in symbiotic dinoflagellates. Mar Biol 162:2067–2077
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by FFR-Cammarata from Scientific Research University of Palermo (2014) and MC RITMARE Project (CNR and CONISMA). .
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by S. Piraino
Rights and permissions
About this article
Cite this article
Parisi, M.G., Lentini, A. & Cammarata, M. Seasonal changes in morpho-functional aspects of two Anemonia sulcata (Pennant, 1777) wild populations. Mar Biodiv 47, 561–573 (2017). https://doi.org/10.1007/s12526-017-0695-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-017-0695-2