Abstract
This study identifies the abundance and spatial distribution of siphonophores collected during spring of 2011 between the Reloncaví Fjord and the Boca del Guafo Passage, in the Chiloé Inland Sea (CIS), Chile. Nine species of siphonophore were identified (three Physonectae and six Calycophorae), of which Agalma elegans, Cordagalma ordinatum and Lensia meteori were recorded for the first time in the waters of the CIS. The dominant species were Muggiaea atlantica (88.0%) and Lensia conoidea (9.5%). The highest occurrence frequency was recorded for M. atlantica (81.2%) and L. conoidea (62.5%). Eudoxids were collected only for M. atlantica (52.1%), L. conoidea (43.7%) and Dimophyes arctica (4.2%). The highest siphonophore densities were found in the northern microbasin (Reloncaví Fjord, Reloncaví and Ancud gulfs), an area characterised by a predominance of estuarine waters (greater vertical stratification, lower temperatures and salinity, and a higher concentration of dissolved oxygen). The vertical distribution patterns showed that M. atlantica is found throughout the water column in the northern microbasin, though with preference for the depths of 0–50 m. In the southern microbasin it was distributed mainly in deeper waters (>50 m). L. conoidea tends to avoid the surface estuarine layer (0–25 m) and is mainly distributed through deeper layers (>25 m). The association with environmental variables shows that temperature and dissolved oxygen have a significantly influence over dominant species. Siphonophorae community showed unimodal responses to the environmental gradients and high interspecific variability compared with previous campaigns in the same geographical area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The study of gelatinous zooplankton, particularly planktonic cnidarians, has undergone renewed interest due to growing concern among academics, managers and the general public (Canepa et al. 2013) regarding the increases in the frequency and size of jellyfish blooms that will have a detrimental effect on several economic sectors (Purcell et al. 2007, 2013; Baxter et al. 2011; Canepa et al. 2014; De Donno et al. 2014; Mianzan et al. 2014; Tomlinson et al. 2015). Planktonic cnidarians are tactile predators that are highly efficient in terms of energy use. They are significant consumers of zooplankton and ichthyoplankton and they compete with fish larvae or juvenile fish, some of which are of commercial interest (Purcell 1985, 1991; Purcell and Arai 2001; Arai 2005; Acuña et al. 2011).
Discussions have arisen recently on the global trends of blooms of gelatinous organisms (Mills 2001; Purcell et al. 2007; Brotz et al. 2012; Condon et al. 2012). It has been shown that though variance patterns continue to follow decadal cycles (Condon et al. 2013), in some places sustained growth has been seen over time (Brotz et al. 2012; Condon et al. 2013). However, there is little information for the southeastern Pacific Ocean. Lilley et al. (2011) and Brotz et al. (2012) used a literature review to identify a lack of information and a net decrease in planktonic cnidarians associated to the Humboldt Current Large Marine Ecosystem, respectively. Of these planktonic cnidarians, siphonophores have received little attention, though they are present in all marine ecosystems and have an important role as predators and under particular circumstances are the most abundant non-crustacean predators (Purcell 1981).
Along the Chilean coast, the study of siphonophores has focused mainly on taxonomy and distribution of the most representative (or abundant) species (Palma and Apablaza 2004, ) or the most ecologically important species (high trophic level) (Palma and Apablaza 2004; Pavez et al. 2010). Most of these studies have focused on specific ecosystems, such as the Humboldt Current System (Palma 1973, 1977, 1986, 1994; Palma and Rosales 1995; Ulloa et al. 2000; Pagès et al. 2001; Palma 2008; Pavez et al. 2010), areas of blooms and minimal dissolved oxygen in northern Chile (Apablaza and Palma 2006), and in the Patagonian Fjords Ecosystem (PFE) in southern Chile (Palma and Rosales 1997; Pagès and Orejas 1999; Palma et al. 1999, 2007, 2011, 2014, 2016; Palma and Aravena 2001; Palma and Silva 2004; Villenas et al. 2009).
To date, of the total of 175 siphonophore species identified around the world (Mapstone 2014), only 55 have been recorded along the Chilean coast (Palma 2010; Oliveira et al. 2016). Along the PFE (41°20’-55°58’S), the community of siphonophores is comprised of 20 species (3 Physonectae and 17 Calycophorae). Of these, in the Chiloé Inland Sea (CIS; 41°31’-43.39°S) 15 species have been recorded (3 Physonectae and 12 Calycophorae). In the PFE, the dominant siphonophores are Muggiaea atlantica and Lensia conoidea, species of interest due to their ecological role as competitors and predators of other zooplankton species of commercial interest, such as fish larvae and eggs (Purcell 1982; Alvariño 1985; Pagès et al. 1996).
In addition, several anthropogenic activities are also conducted in the CIS. These include the farming of marine species, reaching a total of 987 bivalve mollusc farms centres in 2010–2014, 361 centres for salmonid farming and 162 for algae (Malhué, com. pers.). Related to these activities, it is known that M. atlantica may have negative effects on salmon farming, including mortality. These effects have been documented for the northern hemisphere salmon farming (Fossa et al. 2003; Hellberg et al. 2003; Baxter et al. 2011; Hamish et al. 2011).
Thus, the main aim of this study is to contribute to the knowledge of the biodiversity, horizontal and bathymetric distribution of siphonophore species in the Chilean Inland Sea, as well as to elucidate the environmental association between the dominant species of siphonophore and oceanographic variables. The abundance of siphonophores collected during the spring of 2011, will be compared with the ones from the previous springs of 1995, 2005, 2006 and 2007.
Materials and methods
Study area
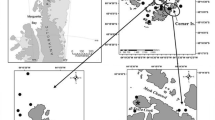
The PFE extends from Puerto Montt (41°20’S) in the north to Cape Horn (55°58’S) in the south. It comprises around 84,000 km of coastline and covers a total area of ∼240,000 km2 of complex topography with highly variable oceanographic and atmospheric conditions, constituting a structurally and functionally unique inland water ecosystem (Palma and Silva 2004). The northern part of the PFE between the Reloncaví Fjord (41°31’S) and the Boca del Guafo Passage (43°39’S) comprises the CIS, which has a surface area of 8,350 km2 (Fig. 1). For a full site description see Palma et al. (2011).
Oceanographic conditions
Topographically, the CIS is comprised of two microbasins separated by a chain of islands collectively known as Desertores Islands (Fig. 1). The northern microbasin, includes the Reloncaví Fjord, the Reloncaví and Ancud Gulfs, and the southern microbasin, the Corcovado Gulf and the Boca del Guafo Passage (Fig. 1). Both microbasins are partially filled with Subantarctic water (SAAW, salinity 33–34) flowing from the adjacent ocean, into the CIS through the Chacao Channel and the Boca del Guafo Passage. As it flows landwards, it mixes with fresh water (FW; salinity <1) flowing from the continental rivers, towards the ocean. This mixture give rise to a thin surface layer with low salinity and high dissolved oxygen, known as estuarine water (EW) (∼20-50 m thick; salinity 1–32) and a thicker and deeper layer (∼50-300 m thick), with higher salinity (∼32-33) and less dissolved oxygen, known as modified Subantarctic water (MSAAW) (Silva et al. 1997; Sievers and Silva 2008). The northern microbasin, where the EW predominates in the surface layer, has a strong surface vertical stability gradient due to the presence of a strong halocline/pycnocline centred at a depth of ∼10-30 m. The southern microbasin, has a lesser presence of EW in the surface layer, which together with the effect of turbulence due to wind and tides, leads to a quasi-homogenous vertical column (Fig. 2b). Both microbasins have a well oxygenated surface layer (6–8 mL·L−1; 80-120% saturation) but its concentration decreases with depth to 4–5 mL·L−1 (40-80% saturation) (Fig. 2c), (Silva 2008). This convergence of FW and SAAW generates a general two-layer estuarine circulation (Palma and Silva 2004; Sievers and Silva 2008).
Oceanographic and zooplankton sampling
During the CIMAR 17 Fjord Cruise, performed from 8th to 24th November 2011 with the R/V Vidal Gormaz; 13 oceanography stations distributed in a longitudinal transect from the Reloncaví Fjord to the Boca del Guafo Passage were investigated (Fig. 1).
At each station, temperature and salinity were recorded using a CTD Seabird SBE-19, from the surface to 10 m above the sea floor, or to a maximum depth of 500 m, depending on the station depth. Discreet samples were taken using a 24-bottle Niskin rosette sampler in order to measure the dissolved oxygen concentration using the Winkler method (Carpenter 1965).
The zooplankton samples were collected by trawling, using Tucker trawl nets (mouth size 1 m2 and mesh 300 μm), at three depth layers: surface (0–25 m), middle (25–50 m) and deep (50–100 m). The net system was fitted with digital flowmeters to estimate the volume filtered during each trawl. The zooplankton samples were fixed with 5% formaldehyde buffered with Borax.
From each sample, the polygastric (nectophore) and eudoxid stages were identified according to Totton (1965) and Pugh (1999). The abundances of Calycophorae were estimated considering the highest number of nectophores (anterior vs posterior) as the asexual polygastric stage, and the sum of bracts and whole eudoxids as the sexual eudoxid stage. For the Physonectae, the number of nectophores varied from species to species, and also increased with the age of the colony (Pugh 1984). The abundance of Pyrostephos vanhoeffeni was estimated considering one individual to have 20 pairs of nectophores per colony (Totton 1965). For Agalma elegans, some incomplete colonies were collected, and at stations were some nectophores were collected, it was assumed to be a single colony. Abundance levels were expressed as individuals per 1,000 m3, using the filtered water volume.
Two indices commonly used to describe the siphonophore community (Palma et al. 2014) are dominance (DO), calculated as the percentage of each species over the total individuals collected, and frequency of occurrence (FO), which is the percentage of the presence of each species over the total number of stations sampled. For the dominant species (DO >5%), the horizontal and vertical distribution patterns were analysed.
Temperature, salinity and dissolved oxygen concentration were used to determine the oceanographic characteristics of the study zone. However, as these variables are measured at different depths than zooplankton samples, the weighted means of the environmental variables were calculated for the zooplankton samples ranges, following:
where Xij is the weighted mean of the i-th parameter at the j-th station, Zjk is the k-th depth at the j-th station, Cijk is the mean of the i-th parameter of the delta of the k-th depths at the j-th station. In addition, for the oceanographic variables (temperature, salinity and dissolved oxygen), vertical cross-sections were generated using the kriging function (with linear interpolation; Surfer 8.0).
Data analysis
Environmental association between the dominant species and the environmental variables were assessed through generalised additive models (GAM). The use of a negative binomial distribution of errors and a log-link function was implemented to avoid over-dispersion and prediction of negative abundances, respectively (Wood 2006). Due to the high variability of the filtered water volume through the nets (data not shown), the models were fitted using the volume of filtered water (log-transformed) as an offset inside the model. The optimal model was achieved using a backward selection procedure until all explanatory variables were significant (Zuur et al. 2009).
From a community perspective, the environmental restriction over the siphonophore community was evaluated using canonical correspondence analysis (CCA) (Ter Braak 1986). CCA considers specific unimodal responses in the composition of the community against the environmental variables. To determine the model with the best fit (the highest inertia explained by only the significant environmental variables), a smart variable selection protocol was used (using a forward stepwise model selection), by applying a permutation procedure. The functions cca and ordistep from the “vegan” library were used for the CCA and the permutation test, respectively (Oksanen et al. 2015). The software R, version 3.3.1 (R Core Team 2016) was used for all statistical tests.
Inter-year preliminary comparisons were done using only stations located in the same geographical position along the longitudinal transect from the spring cruises of the CIMAR campaigns in 1995 (C1F), 2005 (C10F), 2006 (C11F), 2007 (C12F) and 2011 (C17F, this study). All cruises were conducted during the same period of the year (October-November). As the nets used differ among cruises (Bongo nets for C1F and Tucker Trawl nets for all the rest) the comparison was made using the standardised values of abundance of polygastric phase (i.e. nectophores).
Results
Hydrography
The surface layer form the Reloncaví Fjord to the Boca del Guafo Passage (∼50 m) showed high values for temperature (10–12.6 °C) and dissolved oxygen (6–8 mL·L−1), and a high range of salinity variation (1–33) (Fig. 2). The highest values for temperature and dissolved oxygen and the lowest salinity were found at the head of the Reloncaví Fjord, while the lowest values for temperature and dissolved oxygen and the highest salinity were found at the Boca del Guafo Passage (Fig. 2).
A strong halocline (≈1.3 m−1) was present at the Reloncaví Fjord and the Reloncaví Gulf and centred at a depth of ∼10 m (Stations B7 and 8; Fig. 2b). This halocline decreased in intensity towards the ocean, reaching ∼0.04 m−1 at the Boca del Guafo Passage. The deep layer (>50 m) was less stratified, colder (8-10 °C), more saline (32–34) and less oxygenated (4–6 mL·L−1), than the surface layer (Fig. 2a-c).
Specific composition
A total of nine species of Siphonophore were identified: six Calycophorae and three Physonectae. The presence of Agalma elegans, Cordagalma ordinatum and Lensia meteori represent new records for the Chiloé Inland Sea, and the presence of C. ordinatum represents a new record for all the Patagonian Fjord Ecosystem. The Siphonophorae community was strongly dominated by Muggiaea atlantica (DO = 88.0%) and Lensia conoidea (DO = 9.5%), which accounted for 97.5% of all the organisms collected. The highest occurrence frequencies recorded were 81.2% for M. atlantica and 62.5% for L. conoidea. The eudoxid stage was found only for M. atlantica (52.1%), L. conoidea (43.7%) and Dimophyes arctica (4.2%). For D. arctica, the polygastric stage was not collected (Table 1).
Horizontal distribution
The abundance of siphonophores fluctuated between 10 and 7,980 in. 1,000 m−3 in the Ancud Gulf (St. 21) and Reloncaví Fjord (St. 6), respectively, averaging 1,904 in. 1,000 m−3. The highest densities were found in the northern microbasin, with significant decline in abundance seen south of Desertores Islands, in the Corcovado Gulf and in the Boca del Guafo passage. Muggiaea atlantica, with a mean of 1,680.8 in. 1,000 m−3, decreased from north to south along the transect and its highest densities were found in the Reloncaví Fjord, with concentrations >5,400 in. 1,000 m−3. South of the Desertores Islands and towards the Boca del Guafo Passage, a strong drop in abundance and occurrence was recorded (Fig 3a). Lensia conoidea showed a more skewed distribution, with a mean of 181.7 in. 1,000 m−3, concentrated in the far north of the transect, with a maximum of 5,429 in. 1,000 m−3 in the Reloncaví Gulf (St. 7; Fig 3b). Elsewhere on the transect, it was found at low numbers and infrequently. The other seven species comprised only 2.5% of the siphonophores and were collected occasionally in the CIS.
Vertical distribution
The vertical distribution patterns of the dominant species showed marked differences throughout the study area. Muggiaea atlantica was found through the entire water column in the northern microbasin (Fig. 4a), with a preference for the layer at 0–50 m in the northern area (Reloncaví Fjord and Gulf), while southwards of Ancud Gulf, it was found deeper (>50 m). On the other hand, Lensia conoidea tended to avoid the surface layer with estuarine waters (0–25 m), being found mainly below 25 or 50 m at most stations (Fig. 4b).
Vertical distribution of the abundance (in percentage) of dominant species. a Muggiaea atlantica, and b Lensia conoidea in a north–south transect between Reloncaví Fjord and Guafo Passage (Chiloé Interior Sea), spring 2011. Scale bar represents 100% of the abundance per station. Grey boxes diurnal tows, black boxes nocturnal tows
Association with environmental variables
In the case of Muggiaea atlantica, the environmental variables that best explain its variability (15.26%) were sampling depth and temperature. The highest abundances were positively correlated with depth (Fig. 5a left panel) and temperature showed a unimodal response where the highest densities were found at ∼10.4 °C (Fig. 5a right panel). For Lensia conoidea, dissolved oxygen and salinity were significant in accounting for its variability (60.8%). Dissolved oxygen values in the range of 4.5-5.5 mL·L−1 had a positive effect on abundance values, while higher values showed a negative effect (Fig. 5b left panel). An almost linear correlation was observed for salinity, where high values of salinity favoured abundance of this species (Fig. 5b right panel).
Functional responses of dominant species of siphonophores. a Muggiaea atlantica and b Lensia conoidea to the oceanographic variables [from left to right: total depth (m), temperature (°C), dissolved oxygen (mL·L−1) and salinity] in spring 2011. The continuous black line indicates the GAM model fit and in grey the areas of confidence intervals of the model
Siphonophore community structure was mainly driven by temperature and salinity (64.3% of explained deviance). Most of the community showed a positive correlation with salinity and a central position on the axes for temperature and dissolved oxygen (Fig. 6). Muggiaea atlantica showed unimodal responses with temperature, dissolved oxygen and salinity, while Lensia conoidea was associated with stations with lower temperature and higher salinity and dissolved oxygen (Fig. 6).
Canonical correspondence analysis triplot. Sampling stations shown as grey dots, greyscale according to depth. Green triangles correspond to the centroids of the analysis. Species present in the community follows Ae Agalma elegans, Co Cordagalma ordinatum, Lc Lensia conoidea,Lm Lensia meteori, Ma Muggiaea atlantica, Pv Pyrostephos vanhoeffeni, Sf Sphaeronectes fragilis, Sk Sphaeronectes koellikeri. Oceanographic (explanatory) variables are indicated in blue [Temp temperature (°C), Sal salinity, Dissox dissolved oxygen (mL·L−1)]. The percentage of variability (inertia) explained is indicated on each axis
Inter-year comparisons
Summary statistics for all the cruises are shown in Table 2. Results for C17F vary from those reported in Table 1, since the calculations in Table 2 cover only those stations that coincide in space. The Calycophora Lensia meteori and the physonectids Agalma elegans and Cordagalma ordinatum represent new records for the CIS with low abundance. Two Calycophorae species (Abylopsis tetragona and Vogtia serrata) and one Physonectae (Physophora hydrostatica) were recorded only once during the first CIMAR Campaign (C1F) and never found again.
Along the cruises, two species of Calycophorae (Dimophyes arctica and Sphaeronectes koellikeri) present a clear pattern of diminishing abundance over the time. In both cases, their abundances were high during the first campaign (C1F for of D. arctica and C10F for S. koellikeri) and decreased in number until C17F, where D. arctica polygastric stages were not recorded. In the cases of Lensia conoidea and Muggiaea atlantica, their abundance followed opposite patterns. The first (C1F) and last (C17F) campaign were good for L. conoidea which was considered as a dominant species, but during the intermediate campaigns (C10F, C11F, C12F), its abundance was low and was not considered as a dominant species. On the other hand, Muggiaea atlantica showed its maximum values of abundance during the intermediate CIMAR campaigns, even reaching a maximum of more than 14.000 individuals per cubic metre. Beside this, this species have dominated the temporal records.
Discussion
The species Agalma elegans, Cordagalma ordinatum and Lensia meteori were recorded for the first time in the CIS, and the presence of C. ordinatum constitutes its first record in the PFE. The other members of the siphonophore community had already been recorded in the CIS (Palma and Silva 2004; Villenas et al. 2009; Palma et al. 2011). Therefore, the richness of the Siphonophorae Order in the CIS has increased to 12 species, i.e. 57% of the species recorded through the entire PFE (21 spp.) and 22% of the 55 species recorded in the Humboldt Current System between Arica (18°S) and Puerto Montt (45°S) (Palma, 2010; Oliveira et al. 2016). The high dominance of Muggiaea atlantica and Lensia conoidea in the CIS is consistent with the results obtained for the entire PFE, where these two species are also dominant (Palma and Rosales 1997; Pagès and Orejas 1999; Palma and Silva 2004; Palma et al. 2007, 2011, 2014, 2016). It is important to note that L. conoidea is also a dominant siphonophore in the Norwegian fjords in the northern hemisphere (Hosia and Bamstedt 2007; 2008), which demonstrates its capacity for adaptation to estuarine ecosystems.
Abundance of dominant siphonophores showed high variability along the CIS (Pagès and Orejas 1999; Palma and Silva 2004; Palma et al. 2007, 2011, 2014, 2016). Horizontal distribution showed that Muggiaea atlantica is associated with EW and Lensia conoidea with MSAAW. This behaviour explains their coexistence and high dominance in the CIS (Palma 2008; Villenas et al. 2009; Palma et al. 2011, 2014). The higher abundance of organisms in the northern microbasin is likely due to the higher stability of the water column, favoured by a strong halocline/pycnocline centred at a depth of ∼10-30 m in comparison to the southern microbasin, where no halocline/pycnocline was found (Fig. 2) (Silva et al. 1997). In different studies carried out in the CIS, it has been stated that the environmental differences between the two microbasins affect the biodiversity and abundance of siphonophores (Palma and Rosales 1997; Villenas et al. 2009; Palma et al. 2011). The results obtained in spring of 2011 agree with other results for jellyfish and siphonophores found in the CIS in the springs of 1995, 2004, 2005 and 2006 (Palma and Rosales 1997; Villenas et al. 2009; Palma et al. 2011). It is also important to note the high biological productivity of the northern microbasin, as reflected by the high values of zooplanktonic biomass and the abundance of copepods, and larval stages of fish and crustaceans (Balbontín and Bernal 1997; Mujica and Medina 1997; Villenas et al. 2009). This condition offers higher trophic availability for siphonophores (Alvariño 1985).
The dominant species showed different patterns in their vertical distribution (Fig. 4). In the far north of the study area, M. atlantica was distributed mainly towards the surface (0–50 m) and associated with EW with a higher temperature (>11 °C), lower salinity (<32) and high dissolved oxygen content (>6 mL·L−1) (Fig. 2). However, south of the Ancud Gulf, it was deeper, where it was found below 50 m and therefore associated with MSAAW, with lower temperature and dissolved oxygen and higher salinity. This displacement of M. atlantica to greater depths along the north–south gradient was also found by Palma et al. (2011). A possible explanation for this phenomenon is the presence of a more stable water column in the northern area, allowing for a shallow distribution of M. atlantica that tolerates higher ranges of environmental variables (Fig. 6) and a more mixed water column to the south due to the entrance of oceanic waters from the west where L. conoidea can occur. In this way, L. conoidea was generally found below depths of 50 m (Fig. 4) and associated with MSAAW with lower temperatures (10.0-10.5 °C), dissolved oxygen (<6 mL·L−1) and higher salinity (>32) (Fig. 2). The more surface distribution of M. atlantica (0–50 m) and the deeper distribution of L. conoidea (50–100 m) has also been reported for the central area of the PFE (Penas Gulf to Concepción Channel; 47°-50°10’S; Palma et al. 2014).
The Siphonophorae community of the CIS has been surveyed at irregular intervals since 1995 (Palma and Rosales 1997; Villenas et al. 2009; Palma et al. 2011). Comparisons showed that two Calycophorae species (Abylopsis tetragona and Vogtia serrata) and one Physonectae (Physophora hydrostatica) were recorded only once during the first CIMAR Campaign (C1F) and never found again. The Calycophorae Dimophyes arctica was dominant during the first cruise (C1F) and since then it has shown a constant decrease in abundance. During the latest cruise, no nectophores and only eudoxid stages for this species were found (Table 1). The fact that only eudoxid stages were recorded reflects the presence of this species, but the decline in abundance can be attributed to a sustained decrease. This decrease in abundance is in agreement with Brotz et al. (2012), who suggested a net decrease in gelatinous zooplankton abundance along the Humboldt Current Large Marine Ecosystem. However, two species of Lensia have shown inter-annual variability with increases during the last three campaigns, especially for L. meteori, which had not been recorded previously in this area. Another species showing high values and more importantly a clear dominance through the years is Muggiaea atlantica. This species shared the dominance with two or three other species, but since 2006 (C11F) its high abundance values meant it was the only dominant species with very high abundance. Therefore, we suggest that long-term changes in the Siphonophorae community in Patagonian fjords have to be addressed considering not only total numbers but rather species-specific variability.
References
Acuña JL, López-Urrutia Á, Colin S (2011) Faking giants: the evolution of high prey clearance rates in jellyfishes. Science 333:1627–1629
Alvariño A (1985) Predation in the plankton realm; mainly with reference to fish larvae. Invest Mar Center Interdisc Cienc Mar 2:1–122
Arai MN (2005) Predation on pelagic coelenterates: a review. J Mar Biol Assoc U K 85:523–536
Balbontín F, Bernal R (1997) Distribución y abundancia del ictioplancton en la zona austral de Chile. Cienc Tecnol Mar 20:155–163
Baxter EJ, Rodger HD, McAllen R, Doyle TK (2011) Gill disorders in marine-farmed salmon: investigating the role of hydrozoan jellyfish. Aquac Environ Interact 1:245–257
Brotz L, Cheung WWL, Kleisner K, Pakhomov E, Pauly D (2012) Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia 690(1):3–20
Canepa A, Sastre S, Piñol L, Fuentes V (2013) Thirty years of jellyfish blooms in Catalonia: narratives and empirical evidences. In: Fourth International Jellyfish Bloom Symposium, Hiroshima, 5-7 June 2013
Canepa A, Fuentes V, Sabatés A, Piraino S, Boero F, Gili J-M (2014) Pelagia noctiluca in the Mediterranean Sea. In: Lucas CH, Pitt KA (eds) Jellyfish blooms. Springer, Dordrecht, pp 237–266
Carpenter J (1965) The Chesapeake Bay Institute technique for the Winkler dissolved oxygen method. Limnol Oceanogr 10:141–143
Condon RH, Graham WM, Pitt KA, Cathy H (2012) Questioning the rise of gelatinous zooplankton in the world’s oceans. Bioscience 62:160–169
Condon RH, Duarte CM, Pitt KA, Robinson KL, Lucas CH, Sutherland KH, Mianzan HW, Bogeberg M, Purcell JE, Decker MB, Uye S, Madin LP, Brodeur RD, Haddock SHD, Malej A, Parry GD, Eriksen E, Quiñones J, Acha M, Harvey M, Arthur JM, Graham WM (2013) Recurrent jellyfish blooms are a consequence of global oscillations. Proc Natl Acad Sci U S A 110:1000–1005
De Donno A, Idolo A, Bagordo F, Grassi T, Leomanni A, Serio F, Guido M, Canitano M, Zampardi S, Boero F, Piraino S (2014) Impact of stinging jellyfish proliferations along south Italian coasts: human health hazards, treatment and social costs. Int J Environ Res Public Health 11:2488–2503
Fosså JH, Flood PR, Olsen AB, Jensen F (2003) Sma og usynlige, men plagsomme maneter av arten Muggiaea atlantica. Fisken Havet 2:99–103
Hamish DR, Henry L, Mitchell SO (2011) Non-infectious gill disorders of marine salmonid fish. Rev Fish Biol Fish 21:423–440. doi:10.1007/s11160-010-9182-6
Hellberg H, Olsen AB, Jensen F (2003) Clinical signs and histopathology in farmed Atlantic salmon (Salmo salar) associated with large numbers of the jellyfish Muggiaea atlantica (Siphonophore). Book of Abstracts, 11th EAFP International Conference, St. Julians, , September 2003
Hosia A, Båmstedt U (2007) Seasonal changes in the gelatinous zooplankton community and hydromedusa abundances in Korsfjord and Fanafjord, western Norway. Mar Ecol Prog Ser 351:113–127
Hosia A, Båmstedt U (2008) Seasonal abundance and vertical distribution of siphonophores in western Norwegian fjords. J Plankton Res 30(8):951–962
Lilley MKS, Beggs SE, Doyle TK, Hobson VJ, Stromberg KHP, Hays GC (2011) Global patterns of epipelagic gelatinous zooplankton biomass. Mar Biol 158:2429–2436
Mapstone GM (2014) Global diversity and review of Siphonophorae (Cnidaria: Hydrozoa). PLoS ONE 9(2):e87737. doi:10.1371/journal.pone.0087737
Mianzan HW, Quiñones J, Palma S, Schiariti A, Acha EM, Robinson KL, Graham WM (2014) Chrysaora plocamia: a poorly understood jellyfish from South American waters. In: Pitt KA, Lucas CH (eds) Jellyfish blooms. Springer, Dordrecht, pp 219–236
Mills C (2001) Jellyfish blooms: are populations increasing globally in response to changing ocean conditions? Hydrobiologia 451:55–68
Mujica A, Medina M (1997) Larvas de crustáceos decápodos de los canales australes de Chile (41°30’S-46°40’S). Cienc Tecnol Mar 20:147–154
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H (2015) vegan: Community Ecology Package. R package version 2.3-0. http://CRAN.R-project.org/package=vegan. Accessed 10 July 2015
Oliveira OMP, Araujo EM, Ayón P, Cedeño-Posso CM, Cepeda AA, Córdova P, Galea HR, Genzano GN, Haddad MA, Mianzan HW, Migotto AE, Miranda LS, Miranda TP, Morandini AC, Nagata RM, Nascimento KB, Nogueira M Jr, Palma S, Quiñones J, Rodriguez CS, Scarabino F, Schiariti A, Tronolone VB, Marques AC (2016) Census of Cnidaria (Medusozoa) and Ctenophora from South American marine waters. Zootaxa 4194(1):1–256
Pagès F, Orejas C (1999) Medusae, siphonophores and ctenophores of the Magellan region. Sci Mar 63(Suppl):51–57
Pagès F, González HE, González SR (1996) Diet of the gelatinous zooplankton in Hardangerfjord (Norway) and potential predatory impact by Aglantha digitale (Trachymedusae). Mar Ecol Prog Ser 139:69–77
Pagès F, González H, Ramón M, Sobarzo M, Gili J-M (2001) Gelatinous zooplankton assemblages associated with water masses in the Humboldt Current System, and potential predatory impact by Bassia bassensis (Siphonophora: Calycophorae). Mar Ecol Prog Ser 210:13–24
Palma S (1973) Contribución al estudio de los sifonóforos encontrados frente a la costa de Valparaíso. I. Taxonomía. Invest Mar Valparaíso 4:17–88
Palma S (2008) Zooplankton distribution and abundance in the austral Chilean channels and fjords. In: Silva N, Palma S (eds) Progress in the oceanographic knowledge of Chilean interior waters, from Puerto Montt to Cap Horn. Comité Oceanográfico Nacional-Pontificia Universidad Católica de Valparaíso, Valparaíso, 161 pp
Palma S (2010) Cnidarios Planctónicos (Cnidaria). In: Palma S, Báez P, Pequeño G (eds) Bibliografía sobre Biodiversidad Acuática de Chile. Comité Oceanográfico Nacional, Valparaíso, pp 51–58
Palma S, Apablaza P (2004) Abundancia estacional y distribución vertical del zooplancton gelatinoso carnívoro en un área de surgencia en el norte del Sistema de la Corriente de Humboldt. Invest Mar Valparaíso 32(1):49–70
Palma S, Aravena G (2001) Distribución de sifonóforos, quetognatos y eufáusidos en la región magallánica. Cienc Tecnol Mar 24:47–59
Palma S, Rosales S (1995) Composición, abundancia y distribución estacional del macroplancton de la bahía de Valparaíso. Invest Mar Valparaíso 23:49–66
Palma S, Rosales S (1997) Sifonóforos epipelágicos de los canales australes chilenos (41°30’- 46°40’S). Cienc Tecnol Mar 20:125–146
Palma S, Silva N (2004) Distribution of siphonophores, chaetognaths and euphausiids and oceanographic conditions in the fjords and channels of southern Chile. Deep-Sea Res II 51(6–9):513–535
Palma S, Ulloa R, Linacre L (1999) Sifonóforos, quetognatos y eufáusidos de los canales australes entre el Golfo de Penas y Estrecho de Magallanes. Cienc Tecnol Mar 22:111–142
Palma S, Apablaza P, Soto D (2007) Diversity and aggregation areas of planktonic cnidarians of the southern channels of Chile (Boca del Guafo to Pulluche Channel). Invest Mar Valparaíso 35(2):71–82
Palma S, Silva N, Retamal MC, Castro LE (2011) Seasonal and vertical distributional patterns of siphonophores and medusae in the Chiloé Interior Sea, Chile. Cont Shelf Res 31(3–4):260–271
Palma S, Retamal MC, Silva N, Silva C (2014) Siphonophores spatial and vertical distribution and its relation with oceanographic conditions in Patagonian fjords, southern Chile. Sci Mar 78(3):339–351
Palma S, Retamal MC, Silva N, Canepa A (2016) Siphonophores in fjords and channels in southern Patagonia: biodiversity, spatial distribution and environmental association. J Mar Biol Assoc U K. doi:10.1017/50025315416001302
Pavez MA, Landaeta ME, Castro LR, Schneider W (2010) Distribution of carnivorous gelatinous zooplankton in the upwelling zone of central Chile (austral spring 2001). J Plankton Res 32(7):1051–1065
Pugh PR (1984) The diel migrations and distribution within a mesopelagic community in the North East Atlantic. 7 Siphonophores. Prog Oceanogr 13:461–48
Pugh P (1999) Siphonophorae. In: Boltovskoy D. (ed) South Atlantic zooplankton, vol 1. Backhuys, Leiden, pp 467–511
Purcell JE (1981) Dietary composition and diel feeding patterns of epipelagic siphonophores. Mar Biol 65:83–90
Purcell JE (1982) Feeding and growth of the siphonophore Muggiaea atlantica (Cunningham 1893). J Exp Mar Biol Ecol 62:39–54
Purcell JE (1985) Predation on fish eggs and larvae by pelagic cnidarians and ctenophores. Bull Mar Sci 37:739–755
Purcell JE (1991) A review of cnidarians and ctenophores feeding on competitors in the plankton. Hydrobiologia 216(217):335–342
Purcell JE, Arai MN (2001) Interactions of pelagic cnidarians and ctenophores with fish: a review. Hydrobiologia 451:27–44
Purcell JE, Uye S-I, Lo WT (2007) Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Mar Ecol Prog Ser 350:153–174
Purcell JE, Baxter EJ, Fuentes VL (2013) Jellyfish as products and problems of aquaculture. In: Geoff A, Gavin B (eds). Advances in aquaculture hatchery technology. Woodhead Publishing, Sawston, pp 404-430
R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/.
Sievers HA, Silva N. (2008) Water masses and circulation in austral Chilean channels and fjords. In: Silva N, Palma S (eds) Progress in the oceanographic knowledge of Chilean interior waters, from Puerto Montt to Cap Horn. Comité Oceanográfico Nacional-Pontificia Universidad Católica de Valparaíso, Valparaíso, pp 53–58
Silva N (2008) Dissolved oxygen, pH and nutrients in the austral Chilean channels and fjord. In: Silva N, Palma S (eds) Progress in the oceanographic knowledge of Chilean interior waters, from Puerto Montt to Cap Horn. Comité Oceanográfico Nacional-Pontificia Universidad Católica de Valparaíso, Valparaíso, pp 37–43
Silva N, Calvete C, Sievers H (1997) Características oceanográficas físicas y químicas de canales australes chilenos entre Puerto Montt y Laguna San Rafael (Crucero Cimar-Fiordo 1). Cienc Tecnol Mar 20:23–106
Ter Braak CJF (1986) Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167–1179
Tomlinson B, Maynou F, Sabatés A, Fuentes V, Canepa A, Sastre S (2015) Systems approach modelling of the interactive effects of fisheries, jellyfish and tourism in the Catalan coast. Estuar Coast Shelf Sci. doi:10.1016/j.ecss.2015.11.012
Totton A (1965) A synopsis of the Siphonophora. British Museum (Natural History), London, 230 pp
Ulloa R, Palma S, Linacre L, Silva N (2000). Seasonal changes in the bathymetric distribution of siphonophores, chaetognaths and euphausiids associated to water masses off Valparaiso, Chile (Southeast Pacific). In: Farber J (ed) Oceanography of the Eastern Pacific I, Centro de Investigacion Cientifica y de Education Superior de Ensenada (CICESE), Ensenada, pp 72–83.
Villenas F, Soto D, Palma S (2009) Cambios interanuales en la biomasa y biodiversidad de zooplancton gelatinoso en el sur de Chile (Primaveras 2004 y 2005). Rev Biol Mar Oceanogr 44(2):309–324
Wood SN (2006) Generalized additive models: an introduction with R. Chapman and Hall/CRC, Baton Rouge, 384 pp
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
The authors gratefully acknowledge the National Oceanography Committee (CONA) for the partial financing of the projects CONA-C17F 11–06 by S. Palma, and CONA- C17F 11–08 by N. Silva, as well as the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT) for support of A. Canepa through project CONICYT-PAI: 82140034. We also gratefully acknowledge the crew of the R/V Abate Molina from the Instituto de Fomento Pesquero, Dr. Leonardo Castro for help with zooplankton samples, and María Inés Muñoz, head of sample collection, both of the University of Concepcion, and also Paola Reinoso and Gresel Arancibia for the sampling work and chemical analysis of the samples. Finally, we thank to Elena Guerrero and two other anonymous reviewers for their comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Piraino
Rights and permissions
About this article
Cite this article
Palma, S., Cabello, F., Silva, N. et al. Siphonophores of the Chiloé Inland Sea: biodiversity, spatial distribution and environmental association. Mar Biodiv 48, 1731–1742 (2018). https://doi.org/10.1007/s12526-017-0662-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-017-0662-y