Abstract
The fauna of three sites in the Clipperton-Clarion Fracture Zone Region of the North Pacific Ocean were evaluated as part of multiple programs supported by the US National Oceanic and Atmospheric Administration. These localities (Site A in the west and Site C and a prospective reserve area 2893–2561 km to the east) cover the range of depths and productivity observed for the region. Macrofauna densities varied with productivity, with Site A with the lowest densities and the reserve area with the highest densities. Species diversities of Polychaeta, Isopoda and Tanaidacea showed differing trends compared to export productivity, using a bootstrapped lognormal method to estimate total species. Polychaeta had the highest estimated species at the high-productivity reserve site and the lowest values at the low-productivity site A. Tanaidacea had a similar trend to that of the polychaetes. Isopoda showed an opposite species–productivity trend, with highest estimated species at the low-productivity site A and lowest values at the high-productivity reserve site. Polychaetes were most similar between sites, while isopod similarities were low. Tanaid similarities between sites A and C resembled the polychaetes value. Species turnover for isopods was high, but much less so for polychaetes and tanaids, and may be related to the dispersal potential for each taxocene. Beta diversity predicts that the average isopod species in the CCFZ has a range of approximately 2200 km2 while a polychaete species range might exceed 10,000 km2. Data from a single taxocene cannot be used as a proxy for the entire deep-sea fauna because each group has its own ecological and evolutionary responses, as well as its own history.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent interest in the deep sea of the Clipperton-Clarion Fracture Zone derives from the potential mineral wealth residing in manganese nodules (Martino and Parson 2012; Hein et al. 2013). During the period 1970–1992, the USA National Oceanic and Atmospheric Administration (NOAA) sponsored multiple research projects in the CCFZ to document the habitats and fauna, and to understand impacts on deep-sea assemblages that might be caused by mining (Hecker and Paul 1979; Jumars 1981). Bischoff and Piper (1979) report on the NOAA Deep Ocean Mining Environmental Study (DOMES) conducted during the early part of the 1970s decade. Later projects (Jumars 1980, 1981; Spiess et al. 1984; Wilson and Hessler 1987; Wilson 1990a, b, c, 1992) provided data that are presented in this paper. This paper provides a comparative overview of the macrofauna collected during these latter studies and assesses the species diversity and faunal similarity across the three sites. Sea floor images from a typical CCFZ site (14°40′N, 126°25′W, depth 4500 m) are shown in Fig. 1. Because together the three sites span much of the distance and productivity range present in the CCFZ, they provide a starting point for future research on this huge region of the abyssal sea floor. An effort has been made to find and standardize all available faunal data that have only appeared in government reports, some of which have been unavailable prior to this time. Partial data from these studies have appeared in other reports (Cunha and Wilson 2003; Glover et al. 2002; Mullineaux 1987, 1988a, b, 1989; Paterson et al. 1998; Riehl et al. 2014; Thistle and Wilson 1987, 1996; Spiess et al. 1984; Spiess, and Weydert 1987; Wilson and Hessler 1987; Wilson 1987a, b, 1990a, b, 1992).
These programs were designed to evaluate the impact of manganese nodule mining (Jumars 1981; Morgan et al. 1999; Glover and Smith 2003). For multiple reasons, our results were equivocal on how nodule mining might change fragile abyssal assemblages (Jumars 1977, 1980, 1981; Wilson and Hessler 1987). We observed that physical impacts such as miner tracks that persisted for decades (Spiess et al. 1984; Wilson 1990a; Fig. 1c). Because the nodules themselves take millennia to grow (Knoop et al. 1998), and because the fauna of the CCFZ is adapted to a low sedimentation rate, one might predict that recovery after mining will take a decades, centuries or longer (Jumars 1981; Glover and Smith 2003; Miljutin et al. 2011). Given this background, these data are presented to provide a benchmark for our knowledge of three locations and different taxocenes in this vast abyssal region. The data show that the faunal densities correlate well with surface and export productivity, but not diversities and species turnover for each group. Different taxocenes, Polychaeta and Isopoda in this case, show substantially different responses to the environment of the CCFZ.
Environmental setting
The Clipperton-Clarion Fracture Zone (CCFZ) is bounded longitudinally by 120°–160°W) and encompasses a huge area (5.1 × 106 km2) in the North Pacific Ocean, south and east of Hawaii. This area is characterized by a gradual deepening toward the west so that the eastern margin is around 4000 m deep, while south of Hawaii depths exceed 5000 m (Piper et al. 1979a, b). At 10-km scales, the seafloor varies over a 200-m depth range owing to “abyssal hills” that trend in a north–south direction, created at the Eastern Pacific Rise during the generation of new seafloor crust (Craig 1979; Mudie et al. 1972). This topography contributes to variation in the distribution of manganese nodules at all sites (Craig 1979). As sediments become deeper to the west, the topography becomes more moderated (Piper et al. 1979a, b), with different ridge and valley patterns (e.g., Piper and Blueford 1982). Because most sites are below the calcite compensation depth, sediment in this region contains little or no calcareous debris.
DOMES site A
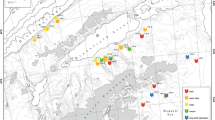
Site A is in the north Equatorial Pacific centered on 9°24′N, 151°27′W. An east–west trending valley with a depth range 4900–5200 m is the main topographic feature. The trend of the valley and rough north–south ridges of the highlands indicate that it may represent a fracture zone (Piper and Blueford 1982). The sediments contain siliceous clays with a mean clay content of 50%, the remainder being primarily siliceous ooze (Piper et al. 1979a). The highlands, covered by Quaternary sediment, are areas of deposition. Along the north margin of the valley, nodules and sediment resemble those of the highlands (Piper et al. 1979a). Early Tertiary sediment outcrops at the surface of the south margin of the valley were interpreted as ongoing erosion, perhaps by Antarctic Bottom Water flows (Piper et al. 1979a). The near-bottom daily-averaged current velocities were 2 cm/s. The average flow was to the northwest at 0.45 cm/s, with some low-frequency fluctuations having periods from 2 to 5 months (Hayes 1979). At similar sites to the east (11°N, 140°W), long-term seafloor observations for up to 3 years did not detect current velocities sufficiently strong to generate shear stresses capable of re-suspending sediments (Gardner et al. 1984). Stratographic data show that episodes of sediment erosion and redeposition took place as late as the Pleistocene (Piper et al. 1979a). The erosional events are associated with seabed current intensification during glaciation (Johnson 1972; Quinterno and Theyer 1979). The near-bottom water has salinities of 34.68 to 34.69‰ and temperatures of 1.4 ± 0.004 °C (Hayes 1979). In 1978, site A was subjected to test mining for manganese nodules (Lavelle et al. 1981), with benthic sampling before and after the event (Jumars 1980; Lavelle et al. 1981; Fig. 2). Manganese nodule cover typically varied between 10 and 25%, with some samples lacking nodules altogether (Piper and Blueford 1982). Mean nodule abundances were approximately 253 m2, ranging from 2 to 495 in a sample (estimated from data in Piper and Blueford 1982).
DOMES site A, approximate positions of samples and topography, modified from Piper and Blueford 1982, who withheld the precise locality data on request from a mining company. Figure positions were correlated with actual sample positions in Table 1, and the map axes corrected to real world positions. Sample positions are indicative and not precise. Samples from both 1977 and 1978 cruises are shown, although several samples were outside the area of the map and are indicated by a sample marker and arrow
DOMES site C
Site C is also referred to as ECHO I locality, because samples were taken on the first leg of the Scripps Institution of Oceanography (SIO) campaign ECHO (Spiess et al. 1984). The location of the Ocean Management Incorporated (OMA) test mining and the 1983 ECHO I sampling program was 14°40′N, 126°25′W at a depth of 4500 m (Figs. 1, 3). This site lies between the Clipperton and Clarion fracture zones, 2000 km to the west of the Eastern Pacific Rise and is near DOMES site C (15°N, 125°W). The temperatures, salinities, and current regime are similar to that at site A (Hayes 1979). The sediments are transitional between pelagic red clay and siliceous ooze, with generally dark yellowish-brown Quaternary sediments at the surface (Spiess et al. 1984). The bathymetry, determined from a Sea Beam survey (de Moustier 1985), shows abyssal hills trending north–south. The study site was on a relatively flat area with gentle slopes to the east, and surrounded by depressions on the east and the west (de Moustier 1985; Spiess and Weydert 1987). During 1978, OMA programs at the ECHO I locality disturbed the seafloor with various versions of a wire-towed sled or a prototype mining vehicle (Spiess and Weydert 1987). Active pumping of nodules from the seafloor and generation of a sediment plume occurred only during a few occasions and primarily during a November–December 1978 OMA/DSV cruise on the R/V Deepsea Miner II (Dick and Foell 1985). Probably owing to the low frequency of the pumping, Wilson and Hessler (1987) found no detectable effects of the test mining disturbance on the benthic fauna. Therefore, the data from this study are assumed to be more or less representative of the general benthic community in the ECHO I (site C) locality. Manganese nodules are the dominant feature of the sedimentary surface at the ECHO I site (Spiess and Weydert 1987) with coverages of 25–49% in the box corer samples (Wilson and Hessler 1987), with average long axial lengths ranging from 5.0 to 6.75 cm (Spiess and Weydert 1987). Photographic surveys using the Deep-Tow vehicle (Spiess and Weydert 1987) and acoustic reflectivities derived from Sea Beam surveys (de Moustier 1985) show that nodule coverages can range from 0 to 80% in this general area. The average nodule size (expressed as cm2) shows a significant inverse relationship with the nodules density (R = −0.96) at the sampling scale of 0.25 m2 (Wilson and Hessler 1987).
Preservational reserve area site (PRA)
The PRA Site (NOAA designated “Provisional Interim Protected Reserve Area”) is a region that was to be set aside and excluded from mining, as a stable reference area (Wilson 1990a). The site was not known to be subjected to mining impacts by the time it was sampled in 1989. The PRA site is a typical abyssal hills region of the Central Pacific (Wilson 1990a; Fig. 4) with sampling centered on 12°57′N 128°20′W, 4794 m (Fig. 5). The topography of the study area consists of a typical low relief abyssal hill complex with ridges arranged on approximately north–south axes (4700–4900 m;). Mean temperatures at the site were 1.48 °C and water movement in the region has a mean velocity of 3.23 (range 0.9–1 3.4) cm/s, with average flow toward the northeast. This current also has a well-defined tidal component that was oscillatory (D. Pillsbury, personal communication; unpublished NOAA report 1991). The bottom type has an approximately similar sedimentary facies as ECHO I samples H359 and H361, but showing higher water content in the surface light brown silicate and silt clays in all samples. A horizon at c. 10 cm was observed in one sample (PRA17) below which the sediments became coarse, semi-consolidated, and gray, similar to the discontinuity caused by the presence Tertiary sediments observed at the other sites. The higher water content of the sediments resulted in deep penetration of the sampler on several occasions. Nodule cover was fairly low, less than 10% cover in most cases, averaging 23.3 nodules/m2.. Samples this site contained 0–36 nodules, most of which had diameters less than 5 cm. Several samples had single large, granular nodules as large as 25 cm in diameter. These densities were lower than those observed at the ECHO I locality, which averaged 274.3 nodules/m2, although the PRA nodules were much larger on the average.
PRA site, geomorphology with sample positions (see Table 1). Topography derived from depth-sounding survey data taken during sampling
PRA site, topography with sample positions (see Table 1). Map scale in minutes of latitude at 12°N and 128°W
Materials and methods
Sample collection and processing
Table 1 has the locality data for all sites, which were sampled using different versions of the 0.25-m2 box corer, initially designed by R.R. Hessler, Scripps Institution of Oceanography. This paper considers only the macrofauna, those members of the fauna for which most life stages were retained on a 0.3-mm mesh screen. Megafauna (animals that might be visible in photographs) were rare in the samples, so this size class is not evaluated here. Meiofauna (taxa for which many life stages and species would pass through a 0.3 mm screen), although enumerated in the programs, are also not considered.
DOMES site A
Box corer samples were taken in a 35-km by 60-km area (Fig. 2): 24 biology samples were collected in November 1977; 26 biology samples were taken in May 1978. The sample locations were selected by picking points at random from an inner 10-km by 30-km area and from the remaining area separately. Sample locations were determined within a transponder net operated by Ocean Management Incorporated (cruise 1) or using ranges to a radar buoy at the site with an average positional accuracy of approximately ∼0.5 km (cruise 2). Piper and Blueford (1982) provide a chart of the sample locations, although not all samples were used for biological purposes. Mining was not carried out in the previously agreed impact site, so samples during the second biological cruise were not taken directly in the impacted areas (Jumars 1980). Because before (1977) and after (1978) mining samples did not appear significantly different (Jumars 1980), both datasets are treated as representative of the background benthic community. The NOAA R/V Oceanographer cruises to Site A in 1977–1978 used two versions of the USNEL 0.25-m2 box corer. One corresponded to that described by Hessler and Jumars (1974; see also Thiel 1983) except that the vent doors were on the top of the coring head rather than on the sides (design by H. Thiel). This device was used to collect box cores numbered DJ08 through DJ34. The remaining samples were collected with a box corer modified according to designs prepared by R. R. Hessler, P. A. Jumars, and J. Finger (sometimes referred to as the “Sandia” box corer). A comparison of isopod crustacean fractions collected by the two corers revealed no apparent difference in their sampling efficiency (Jumars 1980). The corer box on either sampler contained no in situ subcores. On recovery, the top water was drained through a 0.3-mm mesh sieve and the screen residues were added to the main biological sample. A subcore with an internal area of 46 cm2 was taken for geological analysis (data in Piper and Blueford 1982). The macrofaunal samples thus represent a surface area of 2454 cm2. The nodules were washed and inspected for visible macrofauna; screen residues from the nodule wash were saved as a separate faction. Nodules were then taken for geological analysis (Piper and Blueford 1982). The top 1 cm was washed gently on a 0.3-mm mesh sieve. A 9-cm-deeper layer was placed in an elutriation device (Hessler and Jumars 1974) and washed through the same size sieve. The screen residues were fixed in a sodium borate-buffered formaldehyde in filtered seawater (1:5 by volume; sodium borate is no longer recommended as a buffer because it macerates specimens left for long periods), and then later preserved in 80% ethanol after a freshwater rinse. In the laboratory of P. A. Jumars at the University of Washington, macrofaunal taxa were sorted under a dissecting microscope. Not all lower 9-cm layers were completely sorted, so only 41 samples were for density calculations.
DOMES site C
During the Echo I cruise, the modified “Sandia” 0.25-m2 box corer with improved box and vent door seals (Wilson and Hessler 1987) was used to collect quantitative samples (Table 1; Fig. 3). For macrofaunal processing, each sample was divided into 6 separate fractions: top water, nodule washings, aspirator water (material sucked up from surface puddles of fluid and sediment), 0–1 cm layer, 1–5 cm layer, and 5–10 cm layer. This layering facilitated rapid fixation of the animals and permitted a approximate analysis of the depth distribution of the fauna. Most animals were in the fractions with the least sediment, thereby avoiding mechanical damage to the specimens and facilitating their rapid removal from the sediment during sorting. All nodules were washed and examined to remove large encrusting fauna. Some nodules were preserved for a study of the epifauna (see Mullineaux 1987, 1988a, b, 1989). The sedimentary fractions were sieved with 0.3-mm screens during the cruise. The entire top 1 cm layer and the screen residues of the lower layers were directly placed in 4% buffered formaldehyde-seawater for fixation. The larger volume fractions (1–5 cm layer, and 5–10 cm layer) were washed through a 0.3-mm screen using an elutriation device. After several days of occasional gentle agitation to aid fixation, the screen residues were washed with freshwater and placed in 80% ethanol for long-term preservation. The samples were sorted to major taxa using Wild M5 stereomicroscopes in the laboratory of R. R. Hessler at the Scripps Institution of Oceanography. Another cruise, “Quagmire II”, to site C (Wilson 1990c) the same locality as the ECHO I cruise, is not considered here.
PRA site
The box corer used at the PRA site (Table 1; Figs. 4, 5) was the same as that used at DOMES site C. All samples were divided on recovery of each sample into approximate vertical layers: top water, aspirator water, nodules and nodule wash, top 1 cm, 1–5 cm, and 5–10 cm. These layers, although not truly quantitative, provided a qualitative indication of the depth distribution of the fauna. Consequently, this layering was maintained in the laboratory and all fractions were sorted separately. Initial fixation of all fractions on the R/V Moana Wave used 4% buffered formaldehyde-seawater; after 4–6 days, the samples were washed and transferred to alcohol. Each fraction was washed with 25% ethanol in 0.3-mm mesh screens and then immersed in rose bengal for a short period (often overnight) to stain the protein-rich particles, usually specimens that were alive at sampling. In the laboratory, the major taxa were sorted into vials containing 80% ethanol and counted. The contents of selected vials were examined a second time to verify identifications. All animals were removed from the samples, including the Foraminifera. In some cases, individuals could not be counted because the group was colonial (Bryozoa, Hydrozoa, Porifera) or were highly fragmented and not possible to identify specific individuals (Foraminifera). Their presence was indicated by a nominal 1 individual in the relevant layer.
Species identification
Data for species-level identifications are available for polychaetes, isopods and tanaids at all sites (supplementary material). Morphospecies were identified for tanaids for DOMES sites A and C (ECHO I). Isopoda and Polychaeta were identified by taxonomic experts and coordinated across all sites, whereas non-isopod Peracarida and other macrofauna were identified to morphospecies at the site C. Polychaeta were identified by the late Kristian Fauchald at site A and by Kirk Fitzhugh at site C and the PRA site, who coordinated his identifications with the site A identifications. The taxonomic nomenclature in the data tables has not been updated since 1987. I identified all isopod species, with partial illustrations; most taxonomy in the data tables has been updated. Tanaids morphospecies were identified for DOMES sites A and C by Timothy Ragen and myself with consultation from the late Jurgen Sieg. Literature on most tanaid genera was collected to assist placing specimens into relevant genera; the data tables have not been updated from 1987. In a few cases, the number of taxonomically reported individuals may differ from the total for that taxon in the sorting results summaries. These numbers have not been changed to avoid ad hoc decisions on which observation was correct. All species diversity analyses omitted specimens not identified to species.
Specimen deposition
All material treated in this manuscript from all three sites, as well as meiofauna and other taxa except for the molluscs and the samples from the Quagmire II cruise, were transferred in the United States Museum of Natural History, Smithsonian Institution, collections. The Mollusca were deposited in the Malacology Collection at the Australian Museum.
Production estimates
Chih-Lin Wei (personal communication; Sweetman et al., in preparation) provided estimates for the CCFZ sites for average monthly chlorophyll-a (CHL) for years 1998–2014, net primary productivity (NPP) was estimate using the Monthly Vertical General Production Models (VGPM) from www.science.oregonstate.edu/ocean.productivity. Export particulate organic carbon (POC) flux is based on the Lutz et al. (2007) algorithm, which used a long-term monthly average of NPP (1998–2014) by multiplying an export ratio that is directly related to seasonality. The samples were collected during the decades earlier than the time range of the productivity data and therefore not representative of productivity conditions during the time of the sampling owing to decadal shifts in productivity (Smith et al. 2013). This being the case, the values are considered to be indicative of the relative productivity differences between the three sites. Thus, we expect that the most western site will have substantially lower productivity values than the two eastern sites.
Species diversity assessment
The data for each taxon (Polychaeta, Isopoda, Tanaidacea) were trimmed of specimens not identified to species. Most analyses and plots were done in LibreOffice Calc. Hypergeometric model means, i.e., Sanders rarefaction plots (Sanders 1968; Hurlbert 1971), were calculated using my Pascal program EXPSPP, based on a Fortran program written by P. A. Jumars. The rarefactions were compared to results from other biodiversity programs, BioDiversity Pro v.2 (BDPRO; McAleece et al. 1997) and EstimateS (Colwell 2013); all programs consistently reported the similar values within 0.25% (supplementary material). The bootstrapped lognormal distribution calculations were calculated using my Pascal program BOOTLOGN (current v. Bootln2016, based on a Fortran algorithm written by G. Sugihara (1980) using the doubly truncated lognormal distribution (Cohen 1950), log base 2. The lognormal distribution has been thought to be problematic because it is not based on a statistical sampling model (e.g., Colwell and Coddington 1994; Chao and Chiu 2016). This has been addressed by a nonparametric bootstrap estimator (Efron 1979; Efron and Gong 1983) that creates many pseduoreplicates (with replacement) of the original data to provide mean total species, along with variance and confidence limits. A fuller explanation of the methods can be found in Wilson (1987a; supplementary material). Bootstrap estimates were based on 1000 pseudoreplicate samples of the original species abundance data. The CHAO1 estimator (Chao 1984; Colwell and Coddington 1994) in EstimateS and BDPRO was tried on the polychaete data (supplementary material); CHAO1 found the approximate lower bound of the bootstrapped results, suggesting that nonrandom clumped distributions were impacting the frequency of singletons and doubletons, on which the index depends. The lognormal estimator uses the species abundance values directly, so should be less affected by contagious distributions. Ordinations were conducted on the species-level data but are not reported because the sparse matrices did not provide strong results.
Faunal similarity
Normalized expected species shared (NESS; Grassle and Smith 1976) was used to assess faunal similarity for each of the taxa. Original implementations of this method gave anomalously high values (>1.0) in matrices like the isopod and tanaid datasets where many species are present as only single individuals. A revised version of the NESS algorithm (NEWNESS, E. Gallagher, personal communication) was used as a program compiled in Fortran77. Similarities are reported either at a shared species of m = 50 for comparability or using mmax, the maximum number of shared species for the set of samples so that the index is sensitive to rare species.
Data repository
Faunal data and an extended discussion of the bootstrapped lognormal estimator are available in the supplementary material. Additional data and detailed analyses, as well as Linux binaries of Bootlogn and ExpSpp, are available from me on request.
Results
Faunal assessment, DOMES site A
The faunal data for DOMES site A study is from Jumars (1980), although other reports covered portions of the fauna (Glover et al. 2002; Paterson et al. 1998; Thistle and Wilson 1987, 1996; Wilson 1987a, b). Jumars (1980), however, only discussed the statistical outcome relative to the before and after OMA test mining impact, so the data in that report are the main source of information (Tables 2, 3). Only 41 of the 52 macrofauna samples were completely sorted, so only the complete data were used for the summary values. All samples, however, contributed to the species data (see “Species diversity and turnover between CCFZ sites”). The most abundant group in the fauna was Polychaeta (38.57%) followed by Tanaidacea (12.83%) and Isopoda (11.91%). The sessile groups associated with nodules (Porifera, Tunicata, Entoprocta and Bryozoa) together made a substantial fraction (14.69%) of the total fauna. The samples were not fractioned to the level used in latter programs, although data from nodules and top 1 cm were kept separated in many samples. Where the nodule and top fractions were kept separate, they accounted for an average of 68.1% of the fauna in each sample, with 15.6% from the nodules and 56.4% from the top layer. Macrofauna abundances were poorly correlated with either nodule abundances (R 2 = 0.19) or depth (R 2 = 0.03) at this site. Jumars (1980) found substantial spatial autocorrelation in the DOMES site A samples, so that adjacent samples closely resembled one another.
Faunal assessment, DOMES site C – ECHO I locality
Samples at this site were grouped as potential mining impact samples being near mining tracks (“tests”) or those taken away from the mining tracks (“controls”). All samples except one (H361) had manganese nodules. Macrofauna numbers varied considerably (43 individuals, H352 to 155 individuals, H349; Table 4) in the 15 samples (Fig. 3). The average density of macrofauna was 370.7 ind/m2 [standard deviation (SD) 123.92]. The test samples had a higher mean abundance than the controls, 96 versus 82.6 individuals, but the tests were less variable, test (SD = 16.6) compared to the controls (SD = 32.8), because the test sample area was clustered around miner tracks and therefore were from a smaller area. Similar to DOMES site A, most of the fauna lives at the surface. In all, the top 1 cm layer, nodule wash and other filtered water from the surface (aspirator water, top water) accounted for 71.12% of the fauna the samples. The lower two layers (1–5 cm, 5–10 cm) accounted for 21.41 and 7.47% of the fauna, respectively. A substantial number of individuals appeared in the nodule wash (15.80% of the total), demonstrating that many taxa were living on the nodules. These include Brachiopods, some Ascidacea, some Cnidaria, limpet-like Gastropoda, Monoplacophora, Polyplacophora, Porifera, and some Polychaeta. These groups either fell off the nodules during sampling or they were removed during the washing procedure. A few specimens proved to be highly tenacious, and remained on the nodules that were preserved for later study (L. Mullineaux, personal communication); because those specimens were so few, their omission from the results reported here should not greatly affect our conclusions. As expected, the Polychaeta were the most abundant group, with the greatest numbers in control sample H349, and “no nodule” sample H361 (Table 4). The arthropods were the second most abundant group in these samples, being dominated by the Tanaidacea and the Isopoda. The Mollusca were the third most abundant group, within which the gastropods and the bivalves were most abundant.
Faunal assessment, PRA site
The summary macrofaunal data are in Table 5. The average density of macrofauna is 774.50 ind/m2 (SD 254.8) over a range of 444 (PRA18) to 1324 (PRA17) individuals. These densities are 2 times higher than those seen at the DOMES site C (ECHO I locality). Macrofaunal density was inversely correlated with depth; slope = −0.268), although although weakly (R 2 = 0.055), mostly due to the influence of outlier PRA17. This inverse relationship is improved if PRA17 is removed from the data set (slope = −0.623, R 2 = 0.429). Relationships with nodule densities or maximum sizes were not apparent probably because nodules have much less coverage at PRA than at other sites. Unlike the other sites, the PRA site shows a slightly deeper penetration into the sediment by the fauna. While the top layers (nodules, washes and top 1 cm) account for 62.51% of the fauna, 37.5% of the fauna were found below 1 cm (1–5 cm 27.97%; 5–10 cm, 9.52%). This might be attributed to the higher water content of the sediments.
Comparative faunal abundances
Of the three sites, PRA showed the highest abundances in all categories (Table 6) and DOMES site A had the least. Correlated with the east to west decrease in surface and sea floor export production (Wei, personal communication), the mean faunal density (macrofaunal ind/m2) also decreased (Table 6). The PRA site had the highest values for CHL, NPP and POC as well as values for macrofaunal density. Although the CHL values for sites A and C (showing the best linear correlation with macrofauna density: R 2 = 0.9896) are minimally lower than the PRA site (70.25 and 80.89%, respectively), the macrofaunal abundances and all components of the fauna are substantially much higher at PRA than at sites A and C (29.04 and 47.86% of PRA abundances, respectively). Data for peracarids and polychaetes are provided in Table 6 but these differences are parallel across all members of the fauna. This great disparity between the mean productivity and the macrofaunal abundances (Table 6) may be the result of the persistence of deep-sea populations, thus integrating POC flux at the sea floor over a longer time frame than the available measurements (1998–2014). Another possibility might be the PRA site experienced especially high productivity during the decade 1980–1989 than was observed during the 1998 to 2014 time frame of the productivity estimates. Subjective observations in 1989 indicated that region around the PRA sites had high fish populations; fishing ships were seen all around the R/V Moana Wave while on station.
Species diversity and turnover between CCFZ sites
Summary data are presented in Tables 7, 8 and 9, and the full data can be found the supplementary material archived with this journal. The taxocenes Polychaeta, Isopoda and Tanaidacea are treated separately owing to differences in presumptive dispersal characteristics and population dynamics.
Polychaeta
Being the most abundant category of the fauna, both in individuals and in species (Table 7), places the polychaetes as the dominant member of the abyssal soft bottom assemblage. They might function as a proxy for rest of the fauna although other taxa should be considered independently. The reproductive mode of polychaetes possibly allows for long-range dispersal, although available evidence for abyssal regions indicates brooding behavior is frequent (Young 2003). Other reproductive modes seen in shallow water taxa, including asexual budding and parthenogensis, may also occur (e.g., spioniform families; Blake and Arnofsky 1999; Franke 1999). No consistent trends appear in the polychaete species diversity values. Although the PRA site had the greatest number of individuals and highest density, this site had the fewest species in the pooled samples. DOMES site C had the most species, but at an intermediate density. All rarefaction graphs have not reached an asymptote indicating that the total species count has not been recovered in the samples. These plots (Fig. 6) also do not cross, and are showing the same trends but at differing levels. The lognormal estimates of total polychaete species (observed species and unobserved species, those below Preston’s (1962a, b) veil line show a pattern that trends with density at each site (Table 7). Site A has the fewest estimated total species, while the PRA site is estimated to be the most species rich. The match of the lognormal distribution mode to the data was best for site A, but poorest for site C although substantially better that those found for the lower density crustacean taxa. The mode of the estimated lognormal distribution (supplementary data) matched the sample distribution for site A, as indicated by the fit probability, but the sample mode was separated from the estimated mode by one standard deviation for site C and the PRA site. At all stations, the sample mode, the rarest species that were present as only singletons or doubletons, was the most common category, which leaves open the possibility that the distribution may change with additional sampling. The PRA site has the lowest evenness; this assemblage has a higher dominance (some species are much more common than others) and the tail of the lognormal distribution, the rare species, is longer, thus resulting a higher estimated total species. The bootstrapped lognormal estimates are preferred for site C and the PRA site because the fit of the original distribution was poorer, and because they are more conservative.
Considering the three sites in aggregate, a species accumulation plot (Figs. 7 and 8), similar to that done by Grassle and Maciolek (1992) based on individuals sampled and sample area sorted by longitude east to west, shows an almost perfect fit (R 2 = 0.995) to a logarithmic equation. A species accumulation plot based on individuals sampled is also modeled well by a logarithmic equation although not as closely (R 2 = 0.968). If the samples are sorted from west to east, however, linear equations fit the data better, probably because the abundances were substantially lower in the site A samples. These regression models imply that polychaetes diversity will continue to increase as one takes more samples from the sites in aggregate. These equations do not, however, take account for unsampled areas between the sites, so assessing the similarity of the intermediate sites is needed for understanding beta diversity in the CCFZ (McClain and Rex 2015).
Isopoda
The peracarid crustaceans, and especially the Isopoda, are good representatives for that part of the fauna that has low dispersal potential. Asellota, which have no swimming larval dispersal phase in their life cycle (Wilson 1991), are the dominant isopod group in abyssal samples. Although some taxa may have presumptively higher dispersal potential as adults, such as the Munnopsidae (Wilson 1989; Hessler 1993; Osborn 2009), most species are primarily non-natatory epifaunal or infaunal (Thistle and Wilson 1987, 1996). Descendant individuals of any isopod taxon, or any benthic animal in general, could potentially walk from one end of the CCFZ to the other, but then the question becomes at what distance can the taxon maintain gene flow. A isopod species probably cannot maintain genetic connectivity over this distance, given the patchy nature of the habitat and clumped dispersion pattern observed in deep-sea species (Jumars 1976).
The Isopoda have a distinctly different pattern of species diversity than found in the Polychaeta (Table 8) as well as much lower abundances. While polychaete species diversity showed a positive relationship with density and number of individuals, the isopods showed a negative relationship. Even though the PRA site has approximately the same number of specimens as DOMES site A, it has 27 fewer species, a pattern that is also found in the total species estimates. Site C (ECHO I site) has the same number of species as the PRA site but many fewer individuals; the PRA isopod density (mean ind/m2) is twice that of site C. Additionally, site C has a rarefaction plot (Fig. 9) that rises quickly but crosses the site A graph to lower values at around 110 individuals. As in the polychaetes, the isopod collector’s curves by individuals (Fig. 10) or by area (Fig. 11) are rising, so more species remain to be found, which is also supported by the lognormal fit to the isopod species abundance distributions (Table 8). Because the number of specimens included in the isopod data set were many fewer than the polychaetes, the fit of the data to lognormal distributions was poorer, to the extent that the site C data generated several “data set too small” warnings during bootstrap iterations. Half of the singletons are not included in sample distributions so this most abundant category is especially reduced in the already small site C isopod dataset. In low abundance datasets such as these, poor lognormal estimates may result. The site A dataset, however, had an acceptable fit probability. The mode of the sample distributions were all within 1 standard deviation of the estimated distribution, although the patterns were substantially attenuated. The theoretical total species estimates follow the observed species in the samples, with site A having 143.7 species, PRA next with 90.6 and then site C with 79. The site C result is low owing to the small size of the data set.
Tanaidacea
The tanaids were identified to morphospecies for sites A and C; the PRA site specimens are currently unidentified. The tanaids represent a different reproductive mode (Johnson et al. 2001) from that of the isopods: males are exceedingly rare in samples, probably owing to the short lifetime of non-feeding terminal males. The tanaids show a level of species diversity and abundance (Table 9) that is similar to that of the isopods, but with a different pattern. While isopods had the highest species diversity at site A, tanaids had approximately similar species diversity at both sites A and C (Fig. 12), which is similar to the isopod species diversity at the PRA site. Tanaid species diversity is substantially less than isopods at site A and site C (compare Tables 8 and 9). Because these data are sparse, the total species estimates show a poorer fit to the lognormal distribution. Nevertheless, the bootstrap estimator for total species suggests that site C is more speciose than site A (112 species vs. 86 species, respectively). This result is supported by an ad hoc extension of the rarefaction curves (Fig. 12), for which the site C curve would cross the site A curve if more individuals were in the data.
Faunal similarity
The Polychaeta and Isopoda datasets were each pooled by locality and NESS was calculated using the same sample size, m = 50, for all taxocenes so the similarities would be comparable (Table 10). The values for the polychaetes and for the isopods are strikingly different, with the former showing similarities of 0.66 to 0.73 over the entire span of distances from 2893 to 357 km, whereas the latter are less that 0.5 even for the relatively nearby pair, Site C to the PRA site (Fig. 13). The single value for the tanaids, Site A – Site C, is shows approximately the same value as the same pair of sites for the polychaetes. This result shows that although data for the isopods and tanaids are smaller compared to the polychaete data, they can nevertheless have distinctly different similarities. These differences have important consequences for the total species richness of the CCFZ. Because isopod species composition changes with distance at a much higher rate than the polychaetes, i.e. has a higher beta diversity, if integrated across the entire CCFZ, the isopods have a greater numbers of species. The NESS estimator is sensitive to sample size, m, so that as m is higher, the measure becomes more sensitive to the rarer species (Grassle and Smith 1976). If NESS is calculated for the maximum m for each data set (Table 11), the mean polychaete NESS estimate, which had a much higher mmax than 50 (317), the mean similarity decreased by 7.72%. The Isopods (mmax = 76), which had many rare species, actually became somewhat more similar (1.91%) on the average. The single tanaid value (mmax = 86), which had approximately similar abundances to the isopods, decreased by 3.33%.
Normalized Expected Species Shared (m = 50) for Polychaeta and Isopoda plotted against distances between those sites. Filled diamonds, Polychaeta; filled circles, Isopoda; filled square, Tanaidacea, single value. Linear regression, great circle distances separating sites and NESS: Polychaeta, y intercept 0.7410103, slope −0.0000212; Isopoda, y intercept: 0.5051448121, slope −0.0001145
Discussion
Sampling taxocenes
These results suggest that, although the data were nearly adequate for the abundant polychaetes, more quantitative samples (more than 15–16 box corer samples) are needed for recovering robust estimates of diversity in the isopods or tanaids. This is especially true for the isopods, which had extremely high level of beta diversities relative to the polychaetes. For example, if individuals in the theoretical species abundance distribution (7397 ind) are totaled and divided by the isopod density for Site A (27.02 ind/m2,), the approximate minimum area needed to collect the estimated 129 species isopods at Site A is 274 m2, which amounts to 1095 box corer samples. For a less diverse habitat like the ECHO I locality at Site A, the same calculation yields 19 m2 and a somewhat more achievable number of 76 box corers, which is approximately the same number of samples taken at Site A during the two R/V Oceanographer cruises. Increasing the box corer size to 1.0 m2 is one approach that has been discussed, but the operation and processing of such large samples becomes impractical, and probably would not result in saved ship time. Clearly, capturing all species is impractical, so working with smaller numbers of samples and using estimation techniques will have to suffice. If the isopods were distributed randomly at Site A, 50 samples should have given a good fit to the lognormal distribution but, because they have a contagious dispersion pattern, more samples might have given a better estimate.
Another implication of these results is that combining data for all taxa into single diversity estimates may obscure the patterns that dominate benthic assemblages. In highly species-rich assemblages, the probability of sampling a particular species is low, so many samples are required capture a useful sample of that single species. The other aspect of this problem is that establishing the distribution of any particular species over an arbitrary distance multiplies this problem by however many distinct sites are desired. Although non-quantitative samplers, such as the epibenthic sled, capture large swath of an assemblage, these samplers are notoriously biased in which taxa they capture, and will often miss strongly infaunal taxa; for example, compare Thambematidae abundances between box corers and epibenthic sleds in Harrison (1987). Because of this bias, non-quantitative samplers will also strongly distort species-abundance relationships. On manganese nodule dominated sea floors, sleds are also poor samplers of the nodule epifauna, which often require special handling to assess accurately (Mullineaux 1987).
Diversity and productivity
The polychaete data have been used to assess the influence of surface and export productivity (Paterson et al. 1998; Glover et al. 2002). The isopod data, however, show a different trend from the polychaete data. The faunal density estimates show the strongest correlation with CHL, although estimated export POC shows the same trend. The bootstrap lognormal estimated total species, on the other hand, varies with regard to POC. The polychaetes show a positive relationship with POC, ranging from 203 total species at site A to 310 species at the PRA site. The isopods have a inconsistently negative relationship to POC. The isopods are especially speciose (143 bootstrap lognormal estimated total species) at the low productivity site A but have many fewer species at the higher productive sites C and PRA (79 and 91 species, respectively). Although habitat heterogeneity might explain the differences (such as sedimentary heterogeneity; Etter and Grassle 1992), sediments, nodule composition and geomorphology are similar across the entire CCFZ (Piper et al. 1979a, b), with the strongest variables being sedimentary rate and productivity. The tanaids, for which only data from sites A and C are available, show a trend similar to that of the polychaetes, with the bootstrap lognormal estimated total species highest at the high POC PRA site (112 species) and lowest at the low POC site A (87 species). Here, diversity is not necessarily related to POC and might even have a negative relationship in the isopods. Despite only having data from three sites, these results are at least indicative and worthy of additional study. These findings are in stark contrast to a recent multi-investigator study (Woolley et al. 2016) which found that export POC and proximity to slope habitats drive deep-sea diversity. One might attribute their results to the target of their research, the megafaunal taxocene Ophiuroidea, although they argued that this was a general result for deep-sea assemblages. These results point to the pitfalls of using one taxocene as a proxy for the entire fauna as each group has its own unique ecological and evolutionary response to the deep-sea environment.
Beta diversity and reproductive mode
As proposed by McClain and Rex (2015), beta diversity can be assessed using distance decay methods, Here, this is provided by the slope of a linear regression of the distances and NESS between pairs of sites (Fig. 13; Table 10) and multiplying it by the mean total species richness at all three sites for isopods or polychaetes (Tables 7, 8). Admittedly, these regressions provide a poor match to the data, but they provide an approximate comparison of the beta diversity in polychaetes and isopods. The calculation suggests that isopods change at a rate of 0.012 species per km, and the inverse of which gives an approximate linear species range of 84 km. The much more species rich and abundant polychaetes, on the other hand, change little over greater distances, 0.0056 species per km, so the average species range is 180 km. If this estimate is expanded to an areal estimate, assuming an approximately circular distribution, an isopod species range could be 2,228 km2 while a polychaete species range could be 10,289 km2. Although the level of species nestedness and species replacement (cf. McClain and Rex 2015) cannot be addressed directly, the low similarity in the isopods between widely separated sites suggests that the main process is species replacement, while the similarity between sites in the polychaetes argues for a higher degree of nestedness, although certainly replacement is also taking place.
The reproductive modes of the two taxocenes may provide an understanding of these differences. Because some polychaetes may have larval dispersal combined with slow development at deep sea temperatures that extends the larval period, each species could maintain connectivity over great distances in the deep sea. Furthermore, modes of reproduction in polychaetes include both sexual and asexual reproduction, which contribute to the reproductive success of each individual. The polychaete result may also be explained as a negative bias owing to our inability to distinguish closely related species (Nygren 2014). If so, polychaete species turnover could be much higher in the CCFZ. The direct developing isopods have no swimming larval dispersal, and can only disperse as adults or juveniles. In addition, isopod species are much more readily identified to species, owing to their abundance of external morphological features, which allows one to separate members of species flocks such as the northern hemisphere Eurycope complanata species complex (Wilson 1983). The rarity of each isopod species might seem to militate against the persistence of the rare species because the probability of encounter for reproductive adults would be extremely low. The reproductive ability of females to mate at an early age and retain sperm until fertilization (Wilson 1991) increases the likelihood of successful reproduction. Additionally, deep-sea species in aggregate show a clumped distribution (Jumars 1976), which also improves the probability of reproductive encounters. Consequently, the range of an isopod species can be quite small, with low connectivity between regions.
The single NESS value for the Tanaidacea at sites A and C implies that this group might have a beta diversity that is similar to the Polychaeta, which runs counter to the idea that Peracarida, in general, should have low connectivity and high beta diversities. Isopods and tanaids, however, differ considerably in their reproductive mode (Johnson et al. 2001). While the isopods have males and females that are fully functional as adults, tanaid terminal males are non-feeding and are highly transformed for locomotion. While sex ratios of isopods are more or less equal, the observed sex ratios of tanaids are highly skewed toward females; males are extremely rare in the DOMES samples (unpublished data). The males are rare in the samples because they are both highly motile and less likely to be sampled, but also because their residence time in the assemblage is short compared to the adult females. These observations imply that the tanaid males might be the agent of connectivity in this otherwise sedentary, largely tubicolous infaunal group (Wilson 1987a, b).
What these beta diversity results mean between the sites where we don’t have samples is unknown. We do not have fine-scaled sampling that connects assemblages that are statistically different (using NESS or any other estimator). Unlike shallow water that has a coarse grained physical structure with distance, the deep-sea in the CCFZ is continuous but environmentally varying with distance. Do we have deep-sea connectivity for populations that are genetically linked over long distances by way of continuous replacement of one form with another? Or do we have zones of introgression or even abrupt breaks and well-defined species distributions? Samples at a fine scale over a large distance are needed to answer this question. So, although we might say that one species at site A is different from one at site C, the possibility exists that they are connected but the transition between forms is unobserved. We can discuss connectivity as long as we are clear that we do not know the fine details.
References
Bischoff JL, Piper DZ (eds) (1979) Marine geology and oceanography of the Pacific manganese nodule province vol 9. Marine Science. Plenum (Springer US), New York
Blake JA, Arnofsky PL (1999) Reproduction and larval development of the spioniform Polychaeta with application to systematics and phylogeny. Hydrobiologia 402:57–106. doi:10.1023/a:1003784324125
Chao A (1984) Nonparametric estimation of the number of classes in a population. Scand J Stat 11:265–270
Chao A, Chiu C-H (2016) Nonparametric estimation and comparison of species richness. Els, pp. 1–11. John Wiley & Sons, Ltd, Chichester
Cohen AC Jr (1950) Estimating the mean and variance of normal populations from singly truncated and doubly truncated samples. Ann Math Stat 21:557–569
Colwell RK (2013) EstimateS: Statistical estimation of species richness and shared species from samples. Version 9. User’s Guide and application published at: http://purl.oclc.org/estimates. Accessed 3 Nov 2016
Colwell RK, Coddington JA (1994) Estimating terrestrial biodiversity through extrapolation. Philos Trans R Soc Lond B 345:101–118
Craig JD (1979) The relationship between bathymetry and ferromanganese deposits in the north equatorial Pacific. Mar Geol 29:165–186. doi:10.1016/0025-3227(79)90107-5
Cunha MR, Wilson GDF (2003) Haplomunnidae (Crustacea: Isopoda) reviewed, with a description of an intact specimen of Thylakogaster Wilson & Hessler, 1974. Zootaxa 326:1–16
de Moustier C (1985) Inference of manganese nodule coverage from Sea Beam acoustic backscattering data. Geophysics 50:989–1001. doi:10.1190/1.1441976
Dick RE, Foell EJ (1985) Analysis of SIO deep tow photographs of mining device tracks, “ECHO-1” cruise, mining test site. Deepsea Ventures, Inc., Gloucester Point, Virginia
Efron B (1979) Bootstrap methods: another look at the jackknife. Ann Stat 7:1–26
Efron B, Gong G (1983) A leisurely look at the bootstrap, the jackknife, and cross-validation. Am Stat 37:36–48. doi:10.1080/00031305.1983.10483087
Etter RJ, Grassle JF (1992) Patterns of species diversity in the deep sea as a function of sediment particle size diversity. Nature 360:576–578
Franke H-D (1999) Reproduction of the Syllidae (Annelida: Polychaeta). Hydrobiologia 402:39–55. doi:10.1023/a:1003732307286
Gardner WD, Sullivan LG, Thorndike EM (1984) Long-term photographic, current, and nephelometer observations of manganese nodule environments in the Pacific. Earth Planet Sci Lett 70:95–109. doi:10.1016/0012-821X(84)90212-7
Glover AG, Smith CR (2003) The deep-sea floor ecosystem: current status and prospects of anthropogenic change by the year 2025. Environ Conserv 30:219–241
Glover AG, Smith CR, Paterson GLJ, Wilson GDF, Hawkins L, Sheader M (2002) Polychaete species diversity in the central Pacific abyss: local and regional patterns, and relationships with productivity. Mar Ecol Prog Ser 240:157–170
Grassle JF, Maciolek NJ (1992) Deep-sea species richness: regional and local diversity estimates from quantitative bottom samples. Am Nat 139:313–341
Grassle JF, Smith W (1976) A similarity measure sensitive to the contribution of rare species and its use in investigation of variation of marine benthic communities. Oecologia 25:13–22
Harrison K (1987) Deep-sea asellote isopods of the north-east Atlantic: the family Thambematidae. Zool Scr 16:51–72
Hayes SP (1979) Benthic current observations at DOMES sites A, B, and C in the tropical central north pacific ocean. In: Bischoff JL, Piper DZ (eds) Marine geology and oceanography of the Pacific manganese nodule province. PlenuPress (Springer US), New York, pp 83–112
Hecker B, Paul AZ (1979) Abyssal community structure of the benthic infauna of the Eastern equatorial pacific: DOMES sites A, B, and C. In: Bischoff JL, Piper DZ (eds) Marine geology and oceanography of the Pacific manganese nodule province. Plenum (Springer US), New York, pp 287–308
Hein JR, Mizell K, Koschinsky A, Conrad TA (2013) Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: comparison with land-based deposits. Ore Geol Rev 51:1–14
Hessler RR (1993) Swimming morphology in Eurycope cornuta (Isopoda: Asellota). J Crust Biol 13:667–674
Hessler RR, Jumars PA (1974) Abyssal community analysis from replicate box cores in the central North pacific. Deep-Sea Res 21:185–209
Hurlbert SN (1971) The nonconcept of species diversity: a critique and alternative parameters. Ecology 52:577–586
Johnson DA (1972) Ocean-floor erosion in the equatorial pacific. Geol Soc Am Bull 83:3121–3144. doi:10.1130/0016-7606(1972)83[3121:oeitep]2.0.co;2
Johnson WS, Stevens M, Watling L (2001) Reproduction and development of marine peracaridans. Adv Mar Biol 39:107–220
Jumars PA (1976) Deep-sea species diversity: does it have a characteristic scale? J Mar Res 34:217–246
Jumars PA (1977) Potential environmental impact of deep-sea manganese nodule mining: community analysis and prediction vol unpublished manuscript no.13. US National oceanic and atmospheric administration. Pacific Marine Environmental Laboratory, Seattle
Jumars PA (1980) Impact of a pilot-scale manganese nodule mining test on the benthic community Contract No. 03-78-BOl-17. Department of Oceanography, University of Washington, Seattle
Jumars PA (1981) Limits in predicting and detecting benthic community responses to manganese nodule mining. Mar Min 3:213–229
Knoop PA, Owen RM, Morgan CL (1998) Regional variability in ferromanganese nodule composition: northeastern tropical. Pac Ocean Mar Geol 147:1–12. doi:10.1016/S0025-3227(97)00077-7
Lavelle JW, Ozturgut E, Swift SA, Erickson BH (1981) Dispersal and resedimentation of the benthic plume from deep-sea mining operations: a model with calibration. Mar Min 3:59–93
Lutz MJ, Caldeira K, Dunbar RB, Behrenfeld MJ (2007) Seasonal rhythms of net primary production and particulate organic carbon flux to depth describe the efficiency of biological pump in the global ocean. J Geophys Res Oceans 112:26. doi:10.1029/2006jc003706
Martino S, Parson LM (2012) A comparison between manganese nodules and cobalt crust economics in a scenario of mutual exclusivity. Mar Policy 36:790–800. doi:10.1016/j.marpol.2011.11.008
McAleece N, Gage JD, Lambshead PJD, Paterson GLJ (1997) BioDiversity professional statistics analysis software. Scottish Association for Marine Science and the Natural History Museum, London, http://www.sams.ac.uk/peter-lamont/biodiversity-pro. Accessed Oct 2016
McClain CR, Rex MA (2015) Toward a conceptual understanding of beta-diversity. In: Futuyma DJ (ed) Annual review of ecology, evolution, and systematics, vol 46, pp. 623–642. doi:10.1146/annurev-ecolsys-120213-091640
Miljutin DM, Miljutina MA, Arbizu PM, Galéron J (2011) Deep-sea nematode assemblage has not recovered 26 years after experimental mining of polymetallic nodules (Clarion-Clipperton Fracture Zone, Tropical Eastern Pacific). Deep Sea Res I Oceanogr Res Pap 58:885–897. doi:10.1016/j.dsr.2011.06.003
Morel A, Berthon J-F (1989) Surface pigments, algal biomass profiles, and potential production of the euphotic layer: Relationships reinvestigated in view of remote-sensing applications. Limnol Oceanogr 34:1545–1562. doi:10.4319/lo.1989.34.8.1545
Morgan CL, Odunton NA, Jones AT (1999) Synthesis of environmental impacts of deep seabed mining. Mar Georesour Geotechnol 17:307–356. doi:10.1080/106411999273666
Mudie JD, Grow JA, Bessey JS (1972) A near-bottom survey of lineated abyssal hills in the equatorial pacific. Mar Geophys Res 1:397–411. doi:10.1007/bf00286741
Mullineaux LS (1987) Organisms living on manganese nodules and crusts: distribution and abundance at three North Pacific sites. Deep Sea Res A Oceanogr Res Pap 34:165–184
Mullineaux LS (1988a) The role of settlement in structuring a hard-substratum community in the deep sea. J Exp Mar Biol Ecol 120:247–261
Mullineaux LS (1988b) Taxonomic notes on large agglutinated foraminifers encrusting manganese nodules, including the description of a new genus, Chondrodapis (Komokiacea). J For Res 18:46–53. doi:10.2113/gsjfr.18.1.46
Mullineaux LS (1989) Vertical distributions of the epifauna on manganese nodules: Implications for settlement and feeding. Limnol Oceanogr 34:1247–1262
Nygren A (2014) Cryptic polychaete diversity: a review. Zool Scr 43:172–183. doi:10.1111/zsc.12044
Osborn KJ (2009) Relationships within the Munnopsidae (Crustacea, Isopoda, Asellota) based on three genes. Zool Scr 38:617–635
Paterson GLJ, Wilson GDF, Cosson N, Lamont PA (1998) Hessler and Jumars (1974) revisited: abyssal polychaete assemblages from the Atlantic and Pacific. Deep-Sea Res II Top Stud Oceanogr 45:225–252
Piper DZ, Blueford JR (1982) Distribution, mineralogy, and texture of manganese nodules and their relation to sedimentation at DOMES Site A in the equatorial North Pacific. Deep Sea Res A Oceanogr Res Pap 29:927–951. doi:10.1016/0198-0149(82)90019-X
Piper DZ, Cook HE, Gardner JV (1979a) Lithic and acoustic stratigraphy of the equatorial north Pacific: DOMES sites A, B, and C. In: Bischoff JL, Piper DZ (eds) Marine geology and oceanography of the Pacific manganese nodule province. Plenum (Springer US), New York, pp 309–348
Piper DZ, Leong K, Cannon WF (1979b) Manganese nodule and surface sediment compositions: DOMES sites A, B, and C. In: Bischoff JL, Piper DZ (eds) Marine geology and oceanography of the Pacific manganese nodule province. Plenum (Springer US), New York, pp 437–474
Preston FW (1962a) The canonical distribution of commonness and rarity: part I. Ecology 43:185–215. doi:10.2307/1931976
Preston FW (1962b) The canonical distribution of commonness and rarity: Part II. Ecology 43:410–432. doi:10.2307/1933371
Quinterno P, Theyer F (1979) Biostratigraphy of the equatorial north Pacific: DOMES sites A, B, and C. In: Bischoff JL, Piper DZ (eds) Marine Geology and oceanography of the Pacific manganese nodule province. Plenum (Springer US), New York, pp 349–364
Riehl T, Wilson GDF, Malyutina MV (2014) Urstylidae – a new family of abyssal isopods (Crustacea: Asellota) and its phylogenetic implications. Zool J Linn Soc 170:245–296. doi:10.1111/zoj.12104
Sanders HL (1968) Marine benthic diversity: a comparative study. Am Nat 102:243–282
Smith KL, Ruhl HA, Kahru M, Huffard CL, Sherman AD (2013) Deep ocean communities impacted by changing climate over 24 y in the abyssal northeast. Pac Ocean Proc Natl Acad Sci 110:19838–19841. doi:10.1073/pnas.1315447110
Spiess FN, Weydert M (1987) Operational aspects, geology and physical effects of dredging. In: Spiess FN, Hessler RR, Wilson GDF, Weydert M (eds) Environmental effects of deep-sea dredging (final report to the national oceanic and atmospheric administration on contract NA83-SAC-00659), vol SIO Reference 87-5. Scripps Institution of Oceanography, La Jolla, pp 1–23
Spiess FN, Hessler R, Wilson G, Weydert M, Rude P (1984) ECHO I cruise report vol 84-3. Scripps Institution of Oceanography, La Jolla. doi:10.13140/RG.2.1.4131.0242
Sugihara G (1980) Minimal community structure: an explanation of species abundance patterns. Am Nat 116:770–787
Thiel H (1983) Meiobenthos and nanobenthos of the deep sea. In: Rowe GT (ed) The sea, vol 8. Wiley, New York, pp 167–230
Thistle D, Wilson GDF (1987) A hydrodynamically modified, abyssal isopod fauna. Deep-Sea Res 34:73–87. doi:10.1016/0198-0149(87)90123-3
Thistle D, Wilson GDF (1996) Is the HEBBLE isopod fauna hydrodynamically modified? A second test. Deep-Sea Res Part A Oceanogr Res Pap 43:545–554
Wilson GDF (1983) Systematics of a species complex in the deep-sea genus Eurycope, with a revision of six previously described species (Crustacea, Isopoda, Eurycopidae). Bull Scripps Inst Oceanogr 25:1–64
Wilson GDF (1987a) Crustacean communities of the manganese nodule province. Scripps Institution of Oceanography, La Jolla. doi:10.13140/RG.2.1.4645.0729
Wilson GDF (1987b) Differences in dispersal and speciation between deep-sea tanaids and isopods (Crustacea). Am Zool 23:140A
Wilson GDF (1989) A systematic revision of the deep-sea subfamily Lipomerinae of the isopod crustacean family Munnopsidae. Bull Scripps Inst Oceanogr 27:1–138
Wilson GDF (1990a) Manganese nodule mining impact studies: RUM 3 -- R/V New Horizon Cruise “QUAGMIRE II”: 23 April - 17 May 1990. Post Cruise Rep SIO Ref Ser 90:1–43
Wilson GDF (1990b) Biological evaluation of a preservational reserve area “BEPRA I”; cruise report and interim report on laboratory analysis. An oceanographic cruise from Puntarenas, Costa Rica to Honolulu, Hawaii on the R/V Moana Wave (University Of Hawaii) during 22 September To 14 October, 1989 SIO Ref Ser 90:1–34
Wilson GDF (1990c) The biological impact of deep ocean manganese nodule mining - a review of current programs and future directions. Report on a Workshop held on July 6-7, 1989 at the Scripps Institution of Oceanography SIO Ref Ser 90:1–73. doi:10.13140/RG.2.1.2417.5521
Wilson GDF (1991) Functional morphology and evolution of isopod genitalia. In: Bauer RT, Martin JW (eds) Crustacean sexual biology. Columbia University Press, New York, pp 228–245
Wilson GDF (1992) Biological evaluation of a preservational reserve area; faunal data and comparative analysis. Australian Museum, Sydney. doi:10.13140/RG.2.1.2416.8484
Wilson GDF, Hessler RR (1987) The effects of manganese nodule test mining on the benthic fauna in the North equatorial pacific. In: Spiess FN, Hessler RR, Wilson GDF, Weydert M (eds) Environmental effects of deep-sea dredging. Final report to the national oceanic and atmospheric administration on contract NA83-SAC-00659, vol SIO Reference 87–5. SIO Reference, vol 87. Scripps Institution of Oceanography, La Jolla, pp 24–86. doi:10.13140/RG.2.1.1024.2080, appendices A-H
Woolley SNC et al (2016) Deep-sea diversity patterns are shaped by energy availability. Nature 533:393–396. doi:10.1038/nature17937
Young CM (2003) Reproduction, development and life history traits. In: Tyler PA (ed) Ecosystems of the deep oceans. Ecosystems of the world, vol 28. Elsevier, Amsterdam, pp 381–426
Acknowledgements
Peter A. Jumars, Robert R. Hessler and Fred N. Spiess directed the initial NOAA programs and provided much of the intellectual overview to the ongoing programs. The captains, chief engineers, crews, and scientific parties of the research vessels Melville, Oceanographer, New Horizon and Moana Wave tirelessly provided their expertise to collect the samples on which this research is based. Taxonomic expertise for the macrofauna was provided by Jurgen Sieg, Kristian Fauchald, Kirk Fitzhugh, Timothy Ragen, and Elana Varnum. Susan Garner and coworkers in my Scripps lab, and Liko Self and coworkers in the Jumars lab at the University of Washington were invaluable in sorting the samples and organizing the large datasets that resulted from the field programs. George Sugihara (SIO) provided the original Fortran of the lognormal estimation algorithm that I extended with a bootstrap estimator. Eugene D. Gallagher (University of Massachusetts, Boston) provided the corrected Fortran NEWNESS program and advised on various analytical issues. I am grateful to Dwight Trueblood, who found Jumars (1980) in his NOAA office files. This research was funded by NOAA contracts 03-78-B01-17, 83-SAC-00659, NA-84-ABH-0300, 40-AANC-701124, 50-DGNC-8-00113, 50-DSNC-9-00108. Two anonymous referees kindly provided helpful advice, who requested clarifications on the methods employed, productivity estimates and probable disperal modes. I am grateful to these people and institutions for their contributions to this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. S. M. Kaiser
Rights and permissions
About this article
Cite this article
Wilson, G.D.F. Macrofauna abundance, species diversity and turnover at three sites in the Clipperton-Clarion Fracture Zone. Mar Biodiv 47, 323–347 (2017). https://doi.org/10.1007/s12526-016-0609-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-016-0609-8