Abstract
An analytical study was performed on copper alloy objects and ingots/prills from Haft Tappeh Middle Elamite site, southwestern Iran, fourteenth century BC. The samples were analysed by micro-PIXE and SEM-EDS methods to characterise chemical composition and different phases in their microstructure. The results showed that the main objects’ compositions are copper with impurities and variable-Sn containing tin bronze. Furthermore, most of the ingots/prills are composed of copper with high concentration of iron and sulphur while in two samples tin bronze ingot/prill is detected. Based on the results, the main metallurgical operation in Haft Tappeh may be matte production to make metallic copper and producing tin bronze alloy probably by cementation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is proved that the Near East is a pioneering region in emergence of early metallurgy. Based on the literature, the Iranian Plateau is an important region in the Near East for the beginning of copper metallurgy started at the 8th/7th millennium BC (Thornton 2009; Pigott 2004). From the early stages of ancient metallurgy, copper has been the main metal used by ancient metalworkers to make different objects. The copper metallurgy was started by working on native copper pieces to manufacture small objects in the Neolithic period (the 8th/7th millennium BC) and then continued by melting native copper as well as smelting copper oxidic and sulphidic ores to obtain raw material for making larger objects in the late Neolithic and the Chalcolithic periods (the 6th to the 4th millennium BC) (Thornton 2009; Helwing 2013; Pigott 2004, 1999; Smith 1967). Using copper alloys was the next step of metallurgy started during the Chalcolithic period by emerging arsenical copper and continued by an important innovation at the Bronze Age: tin bronze (Thornton 2009, 2010; Pigott 2004). Furthermore, copper metallurgy was continued during the Iron Age by using copper and its alloys to manufacture different objects (Oudbashi et al. 2012; Thornton 2009).
The Elamite kingdom was the early civilization in the Iranian Plateau that was begun at the similar time of the prehistoric period of other regions of Iran. It took long from the early Bronze Age to the end of the Iron Age (from the beginning of third to the mid of first millennium BC) that was emerged in the lowlands (southwest) of Iran. The common Elamite civilization is well recognised today by its own language, customs, and monuments, as formerly was characterised in the ancient time by the neighbouring different Mesopotamian kingdoms (Basello 2016). This civilization is categorised to four main periods: Proto-Elamite (ca 3200–2700 BC), Old Elamite (ca 2700/2400–1500 BC), Middle Elamite (ca1500–1100 BC), and Neo Elamite (ca 1100–540 BC) (Potts 2016; Carter and Stolper 1984; Vallat 1998; Carter 1998).
The borders of Elam varied during the several millennia of its history from period to period and also with the point of view of the persons described it in the ancient texts. Nevertheless, the region of Elamite civilization and its kingdom can be limited to Khuzestan Plain (southwestern Iran), Anshan at Fars and Kerman (south-central Iran) and western Iran (modern Luristan and Kurdistan) (Vallat 1998; Carter 1998; Potts 2016).
The metallurgy of Elam is significant based on metallurgical activities and objects found from different excavated sites. There are many evidences from different periods through Elamite civilization by which using copper alloys with high craftsmanship are obvious. It was an important industry to produce various artefacts especially religious and non-religious statues during the Old and Middle Elamite periods (Potts 2016). The various metallic statues from the Old Elamite period are simple and small, they are cast with arsenical copper or in some cases with tin bronze containing low amount of tin (Moorey 1994). Also, some copper alloy objects discovered from Tal-e Malyan (dated to the late 3rd/early 2nd millennium BC) present application of arsenical copper and tin bronze (Pigott et al. 2003).
One of the significant instances of metallurgy from the Middle Elamite period is the life-size statue of queen Napir-Asu discovered from the Acropole of Susa, dated to fourteenth century BC. It is a cast copper sculpture in one piece that is covered with a thick cast layer of tin bronze,129 cm in height (without head). This extraordinary statue shows the craftsmanship of Elamite metalworkers in casting. Another instance is a three-dimensional representation cast model of tin bronze so-called Sit-Shamshi (Sunrise), discovered from the Acropole of Susa, twelfth century BC, 60 cm in length (Harper et al. 1992; Potts 2016; Moorey 1994). Furthermore, excavations in the Arjan, eastern Khuzestan plain, have led to discovery of a U-shape tin bronze coffin with some tin bronze and silver objects. The tomb and its burial goods are dated to the Neo Elamite II phase (Carter 1998; Alizadeh 1985).
The aim of this paper is to study on metallurgy of copper in the Middle Elamite period by analysing materials discovered from Haft Tappeh archaeological site. This study is based on results of chemical and microstructural analysis of copper objects and ingots/prills and finding relationship between these materials to develop our knowledge about ancient metallurgy at the mid of second millennium BC in southwestern Iran.
Haft Tappeh
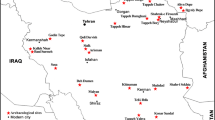
The Haft Tappeh (or Haft Tepe) archaeological site is a large area consisting of about 14 major visible archaeological mounds. It is located in lowlands of Iran at the Khuzestan plain, southwestern alluvial plains of Persia, about 10 km southeast of the famous World Heritage site of Susa and about 15 km west of the World Heritage site of Chogha Zanbil (Fig. 1a). Based on the archaeological finds, the site is consisting of remains of a Middle Elamite city that was the capital of the Elamite king, Tepti-Ahar (Negahban 2002; Mofidi-Nasrabadi 2004). However, several seal impressions and clay tablets found at the site contain the name “Ka-ap-nak” suggest the possibility that it was the original Elamite name of the Haft Tappeh (Negahban 1991).
a The map of Iran and location of Haft Tappeh and other important archaeological sites noted in this paper. b The map of excavated area of Haft Tappeh by E. O. Negahban and location of workshop and kiln (after Mofidi-Nasrabadi 2004)
Based on the archaeological excavations, it belongs to the early phase of the Middle Elamite period, dated back to the fourteenth century BC (Mofidi-Nasrabadi 2004, 2015). The massive adobe and brick buildings may show that the city has been a religious centre as well as having other public functions (Mofidi-Nasrabadi 2013). The archaeological excavations proved that the site was built during a single period at the mid of the 2nd millennium BC, the time at which Haft Tappeh was the major Elamite city, perhaps same as Susa (Negahban 1991).
Although some evidences about the site have been noted in the publications from the end of the nineteenth century AD (e.g. de Morgan 1894–1905), Haft Tappeh was excavated in two individual periods during the twentieth and the twenty-first centuries AD. The site and architectural evidences were revealed during the construction of the new main road in 1960s, which caused to start large scale excavations (Negahban 1991). The first period of excavation was conducted from 1965 to 1978 by E. O. Negahban, and second one was resumed from 2004 and continued to 2012 by B. Mofidi-Nasrabadi (Negahban 1991; Mofidi-Nasrabadi 2010; Mofidi-Nasrabadi 2014).
One significant archaeological find in this site is evidences of metallurgical activities as well as different metal objects. Through the first period of excavations, a large room was found at the eastern side of the Central Core of the Terrace I complex, which is apparently an artist workshop (Negahban 1991). Beside it, a courtyard with a large kiln (furnace) presents at the eastern side of the Central Core (Fig. 1b). The kiln has two separate parts with a firing chamber between them and a chimney at the end of each part. The kiln had a destroyed vaulted roof. Based on the finds, the northern part was used for pottery and clay tablet baking while the southern part was used for metal smelting/melting (Negahban 1991: 18; Negahban 1979). Also as Negahban reports, many small pieces of metallic ingots and slags were found in the workshop and inside of the kiln as well as other parts of the excavated area (Negahban 1991). The metal objects consist of various types of artistic and routine objects such as small decorative pieces, tools, arms, and vessels (Negahban 1991; Mofidi-Nasrabadi 2010). Some objects now are in the National Museum of Iran, Tehran, but many metallic objects and metallurgical materials are in the Haft Tappeh museum, near the archaeological site.
Although, preliminary archeometallurgical studies are published previously on Haft Tappeh metallurgical materials (Oudbashi et al. 2009; Oudbashi and Davami 2014a), but it is necessary to do more extensive analytical study to reveal metallurgical activities at the second millennium BC at the Haft Tappeh Middle Elamite site.
Materials and methods
In order to study on chemical composition of metal objects and ingots, 52 samples were selected from materials that are maintained in the Haft Tappeh Museum (Fig. 2). The objects group include 26 objects and the ingots group also consists of 26 samples of ingots/prills. More of objects are selected from the metal collection discovered by E.O. Negahban during 1965 to 1978; nevertheless, some samples were selected from recently finds by B. Mofidi-Nasrabadi: 23 samples from the first period and 3 samples from the second period. Objects are different in type such as rods, arrowheads, vessels, and plates and were specified by a code from A1 to A26 (Table 1). Some objects belonging to both excavation periods have inventory code in Haft Tappeh museum but other samples selected as analytical ones that are not registered as museum objects.
Twenty-five ingot/prill samples are selected from the first period of excavations and are small ingots/prills with diameters about 2 to 4 cm. These are including small metallic pieces with no specific shape, seems to be small pieces that are solidified from melt, although are partially spherical in the first view. They are completely different from the metallic ingots found in some archaeological sites of Iran and Near East like bun-shaped or plano-convex ingots, such as Susa (Tallon 1987; Pigott 1999; Cuénod et al. 2015). Only one large ingot from the second excavation period was selected which is about 20 cm in diameter and about 1 kg in weight (sample I65). Only this ingot is registered as museum object and has inventory code (Fig. 2; Table 1). The ingots/prills were specified by a code from I40 to I65. It should be noted that the objects with no inventory code or having 1380 in the inventory code have not specified contextual information but as noted above, many objects and ingots/prills were found at the area of artist workshop (Negahban 1991). Thus, only four samples have specified contextual information including three objects (HT.94-39, HT.95-72, and HT.05.5-531) and the large ingot (HT.04.47-115) that are found during the second period of archaeological excavations in Haft Tappeh by Mofidi-Nasrabadi.
A small piece from each sample was cut by jeweller’s saw and a cross section was prepared from each sample by mounting in two-part epoxy adhesive and then ground with abrasive paper from 240 to 3000 grid and polished by diamond paste from 3 to 1 μm to obtain a smooth surface.
The Micro-Proton-Induced X-ray Emission (Micro-PIXE) measurement was performed with scanning proton microprobe system manufactured by Oxford Instruments (Grime and Watt 1988) using the 3 MV Van de Graaff accelerators at the Nuclear Science & Technology Research Institute in Atomic Energy Organization of Iran (AEOI).
The samples were analysed in vacuum chamber using a beam of 2.5 MeV protons focused to a diameter less than 10 μm. The beam current was in the range of 30 to 50 pA. Characteristic X-rays were detected using a Si(Li) detector with an active area of 60 mm2 positioned at an angle of 135° relative to the incident beam direction and with an energy resolution of 150 eV for Fe-Kα. The samples were scanned by a beam over a maximum area of 2.5 × 2.5 mm and digitised signals were recorded in event by event (listmode) using the OM-DAQ data acquisition system. In order to obtain accurate elemental composition of analysed samples and to omit and minimise errors and inaccuracy originated from corrosion of samples, in Copper (Cu) distribution map, the core region of the analysed area that is free from any corrosion products was chosen to extract the corresponding spectra to evaluate elemental compositions. The X-ray spectra were processed using GUPIXWIN software package (Campbell et al. 2010) to quantitative analysis and obtain elemental composition of samples.
In order to check the validity of the micro-PIXE analysis and to confirm the accuracy of the measurements, 1 Euro coin (75% Cu, 25% Ni) and a pellet of copper-phosphorous-tin powder (86% Cu, 6.5% P, and 7.5% Sn) as standard targets were analysed under the same condition. The evaluated composition of 1 Euro coin was 74.59% Cu and 24.80% Ni and copper-phosphorous-tin pellet was 84.90% Cu, 5.71% P, and 6.69% Sn. The results were in good agreement with the recommended values within 5% accuracy for major elements and 5–10% accuracy for minor elements.
The SEM-EDS analyses and observations were performed on the cross sections in high vacuum using FE-SEM instrument model MIRA3 manufactured by TESCAN Company, with a SAMx backscattered electron detector (BSE) and an energy-dispersive X-ray spectrometer (EDS). The samples were inserted in the instrument and examined without any prepping procedure such as carbon or gold coating.
Results
The results of micro-PIXE analysis of samples are presented in Tables 2 and 3. The composition of 26 objects is interesting and variable. Results show that copper is the main constituent in objects. Copper varies between 79.88 and 99.80 wt% in objects. In 10 objects, tin is detected as the main alloying component, measured from 4.06 to 13.81 wt%, and is detected as minor element in two objects. These two objects can be considered as impure copper. Arsenic is detected as minor element in a lot of objects and is only detected more than 1% in 2 samples (Table 2). In sample A06, tin also is detected as major element beside arsenic but regarding to low amount of As in all samples, no object can be considered as Cu-As or Cu-Sn-As alloy. Lead is another constituent that is detected in 4 samples as major element (1.04 to 10.39 wt%). Sulphur plays an important role in the composition of the samples and is detected as major in three objects, and as minor element in composition of many objects. Iron and silver are detected in one sample > 1 wt%, Fe is measured in 17 objects as the minor element while silver is determined only in one object. Presence of Al, Cl, Mg, Si, Ca, and K is due to corrosion penetration in the metallic structure (Oudbashi 2015). Other elements such as Co, Ni, and Cr are measured as minor constituents.
Regarding to the composition of ingots/prills, copper is determined from 80.34 to 99.69 wt% as the major component (Table 3). Tin is detected only in eight samples and determined as major element in four samples. Arsenic and lead are measured in the composition of many ingots/prills, although they are detected as major element only in one ingot/prill sample. The significant subject in the composition of ingots/prills is the high concentration of iron, detected as major element in 15 samples. Moreover, sulphur is determined as major in 7 samples. Other constituents are detected such as chlorine, aluminium, silicon and magnesium same as objects.
Figure 3a presents columnar diagram showing different alloy compositions characterised in objects of Haft Tappeh. It is visible that 15 objects are made of impure copper and only 11 objects are made of different alloys including tin bronze, leaded copper and leaded tin bronze. Based on the literature, deliberate alloy composition could be proved when amount of alloying elements such as Sn and As is 2–3% and more (Coghlan 1975; Nezafati 2006; Pernicka 2014). Therefore, the alloying elements (Sn and As) were considered as deliberately added constituents regarding the composition of the samples and comparison between objects and ingots. Thus, low amounts of As and Sn are considered here as impurities that are not added to the copper deliberately. Columnar diagram in Fig. 3b shows type of the alloy in the ingots/prills. It shows that impure copper is the main compound while only in two cases tin bronze is determined. It is obvious that in some limited cases, the tin bronze ingot/prill is available with variable amount of tin (from 2.60 to 10.60 wt%).
a Columnar diagram showing distribution of different alloy compositions identified in analysed objects. b Columnar diagram of distribution of chemical composition in ingots/prills. c Scatter plot of Sn versus As in two groups of analysed materials. d Scatter plot showing Fe versus S in the composition of all samples
Figure 3c shows the scatter plot of Sn versus As in all samples. It shows that arsenic does not play an important role in the composition of samples and should be considered as impurity. The concentration of tin is variable in different tin bronze samples from 2.60 to 13.81 wt%. Furthermore, it is obviously in the plot (Fig. 3c) that objects can be divided into two groups regarding tin content, tin bronze group in which tin is added possibly deliberately because of high amount of tin in their composition, and impure copper showing objects with no tin or low amount of tin. On the other hand, tin is detected less than 3 wt% in two samples of ingots/prills showing that they cannot be considered as deliberate tin bronze ingots/prills. Also, there is no correlation between tin and arsenic in the composition of all samples. As noted above, iron is determined as an important constituent in ingots/prills. Figure 3d presents scatter plot of Fe versus S in all samples. Moreover, there is no correlation between iron and sulphur; nevertheless, two types of ingots/prills could be categorised: high-iron and low-iron, while most of objects are low in Fe and S despite of only 3 objects, and one ingot sample is determined as high-Fe and high-S sample.
The microstructure of samples was observed by SEM-EDS method. Figure 4 shows SEM-BSE micrograph of two objects (A06 and A12). A large number of dark inclusions is visible in the metallic matrix of the objects. Also, many bright phases of small dimensions are visible in the matrix of some samples. SEM-EDS analysis of the dark inclusions indicated that they are composed mainly of Cu and S and are copper sulphide compounds (Fig. 4, analyses A). SEM-EDS analyses conducted on the fine and bright phases showed that they are Pb-rich metallic phases (Fig. 4, analyses B).
SEM-BSE micrograph of two ingot/prill samples (I42 and I64) consists of metallic matrix in which some large dark phases with different grey tonalities are scattered (Fig. 5). In fact, two distinct phases (inclusions) are visible: circular pale grey and dark grey phases. SEM-EDS analysis of pale grey phases showed that they are Cu-S phases that are chemically similar to the Cu-S inclusions of objects, while the dark grey phases are Fe-rich with high amount of oxygen. Consequently, they could be specified as iron oxide. Based on compositional analysis of the samples by micro-PIXE, it was proved that iron and sulphur are detected in sample I42 as 1.95 and 1.12 wt% respectively, while sample I64 contains high amount of Fe and S in its composition. In this sample (I64), there are many iron-rich small phases and also some circular and large-sulphur rich phases. In fact, three phases could be determined in this sample: copper matrix, Fe-rich (analysis B) and S-rich (analyses A and C) phases (Fig. 5).
Discussion
Micro-PIXE analysis of metal objects showed that many of samples are consisting of copper with impurities. Of course, tin bronze is the main alloy that is identified among Haft Tappeh objects. Based on analytical studies on prehistoric objects from Iran, tin bronze is emerged in western Iran at the Early Bronze Age (the late 4th millennium BC/early 3rd millennium BC) (Fleming et al. 2005; Thornton 2009). There are limited evidences of application of tin bronze during the third millennium BC in the Iranian Plateau, such as Luristan, Susa, Tappeh Giyan, Tal-e Malyan, and Sialk (Moorey 1982; Nezafati 2006; Ghirshman 1938; Pigott et al. 2003). Of course, this alloy was more common in western Iran than other regions of the Iranian Plateau such as eastern or central Iran (Oudbashi et al. 2012; Pigott 2004; Thornton 2009; Nezafati et al. 2006). Nevertheless, bronze was not a commonplace material for object’s production until the mid of 2nd millennium BC (end of the Bronze Age) at all the Iranian Plateau (Thornton and Lamberg-Karlovsky 2004; Thornton 2009; Moorey 1969; Pigott et al. 1982; Thornton et al. 2002). Finally, bronze was the main material to manufacture different objects during the Iron Age (1500–550 BC) (Moorey 1982; Oudbashi et al. 2012; Haerinck 1988).
Haft Tappeh is dated to early phase of the Middle Elamite period at the same time of the beginning of the Iron Age (ca fourteenth century BC). In that time, tin bronze was the important alloy composition used in metallurgical activities. Surprisingly, several metallic objects are consisting of copper with impurities as the main compound. Of course, bronze with variable tin content is next important material in the metallurgy of Haft Tappeh. Also, the metal ingots/prills are made of impure copper with low amounts of other elements such as tin as well as high Sn-bearing copper (tin bronze) in two samples with different amount of tin.
Presence of variable tin in the composition of bronze objects has been observed during the Bronze Age and the Iron Age of Iran. Analysis of bronze objects of several archaeological sites from northern and western Iran revealed that they are made of variable-tin bronze alloy and there is no correlation between function/typology and composition of the objects (Oudbashi and Davami 2014a; 2014b; Oudbashi et al. 2016; Oudbashi and Hessari 2017; Vatandoost-Haghighi 1977; Fleming et al. 2006). This statement is also visible in the bronze objects from the Haft Tappeh Elamite site. In fact, the ancient Elamite metalworkers produced tin bronze with an uncontrolled alloying process or it has not been important to make tin bronze with specific Cu/Sn proportion. Some reports stated that specific alloying recipes have been used to produce tin bronze in the Near East (Joannes 1997; Muhly 1973). However, there is no evidence of using alloying recipes in the prehistoric metallurgy of Iran such as the Bronze and Iron Age objects from Luristan and northern Iran (Oudbashi and Hessari 2017; Oudbashi et al. 2016; Oudbashi and Davami 2014b). Thus, the analytical results well indicate that controlling Sn content in tin bronze alloy has not been done by the Elamite metalworkers in Haft Tappeh.
Also, lead is detected in two objects; it is more likely that it does not play an important role in the metallurgy of this site. Of course, the concentration of an element in the composition of ancient copper alloys may be strongly related to its concentration in the original ore. Presence of lead in two samples may be due to deliberately addition of lead to copper or high concentration of lead in the ore used for smelting such as fahlerz or grey copper (e.g. tennantite (Cu,Fe)12As4S13) that is an As-bearing copper ore and is usually associate with lead-rich minerals (Fleming et al. 2006; Coghlan 1975), that may result to make Pb-rich copper alloys. Therefore, lead has been detected in other samples as impurity.
Presence of copper and bronze ingots/prills and objects in Haft Tappeh reveals that two metallurgical processes should be considered in this site: copper smelting and its alloying with tin. Two bronze ingots/prills beside copper ingots/prills may be due to application of an alloying process such as co-smelting or cementation. In co-smelting, the copper (sulphidic or sulphidic/oxidic) and tin ores (cassiterite, SnO2) are smelted directly together to make bronze while cementation is the process of adding cassiterite to metallic copper in crucible in a reducing atmosphere to make bronze (Rovira et al. 2009; Oudbashi et al. 2016, Pigott 2004). Erb-Satullo et al. (2015) studied some metallurgical materials such as slag and crucible from South Caucasus and showed application of cementation process by adding tin ore directly to a crucible charge to make bronze. Of course other alloying operations are noted in literature including melting metallic copper and tin together, recycling or using Cu-Sn bearing complex ores (Coghlan 1975; Pigott 2004; Oudbashi et al. 2016; Oudbashi and Hessari 2017). It is worthy to note that no evidence has been found of tin smelting or presence of metallic tin in Haft Tappeh site (as well as other prehistoric sites of Iran), which it may prove that using metallic tin to produce bronze has not been undertaken in that time. On the other hand, recycling or re-melting tin bronze pieces to make new objects has been a commonplace method in the ancient time (Henderson 2000). Producing tin bronze with this process may lead to a significant decrease of tin amount in the final product (Figueiredo et al. 2010). Nevertheless, presenting copper-tin ingots/prills in Haft Tappeh shows that this process may not be used (at least as a common/routine method of alloying) to make tin bronze alloy in the Middle Elamite period in this site as will be explained in more details in next pages.
Considerable amount of Fe and S is another significant subject in the composition of objects and specifically ingots/prills. Sulphur and iron are detected as minor constituents in the composition of many archaeological copper alloy objects from the Bronze and Iron Ages of Iran, and many Cu-S (with variable amount of Fe) inclusions are observed in the microstructure of these objects (Oudbashi and Davami 2014a; 2014b; Oudbashi et al. 2016; Oudbashi and Hessari 2017). Based on the literature, it reveals that sulphidic copper or a mixture of sulphidic/oxidic copper ores are used in the prehistoric time leading to enter low amounts of S and Fe in copper alloy objects (Rostoker et al. 1989). Furthermore, high amount of iron in the composition of some copper ingots/prills and low iron concentration in some objects may be due to the presence of iron in smelted copper ore or due to the use of an iron–copper sulphide ore such as chalcopyrite (CuFeS2) to smelt copper (Craddock and Meeks 1987; Erb-Satullo et al. 2014; Van Brempt and Kassianidou 2016). Figure 6 shows scatter plot of Cu versus S and Cu versus Fe in the composition of all samples. Cu versus S scatter plot shows that sulphur has more significant role in the composition of ingots in comparison with objects although S is fairly detected as minor element in more samples (Fig. 6a). Furthermore, scatter plot of Cu versus Fe presents important role of iron in composition of ingots/prills in comparison with objects (Fig. 6b). Of course there is a correlation between copper and iron only in composition of ingots. Thus, as noted earlier, it is possible to classify copper ingots/prils as high-Fe and low-Fe.
Lackinger et al. (2013) reported results of an experimental study on co-smelting of copper and tin ores to obtain bronze ingots/prills. They stated that it is easily occurred to produce bronze alloy by using the co-smelting of copper and tin ores in a crucible in an open fire (bowl) furnace. The metallic ingots/prills that they are obtained are very similar to the small ingots/prills of Haft Tappeh.
As noted, the small ingots/prills contain high amount of iron in some cases. Rostoker (1975), Rostoker et al. (1989), and Killick (2014) present a multi-stage process to obtain metallic copper from sulphide ores or chalcopyrite (CuFeS2). It could be classified as following stages:
-
Roasting chalcopyrite in oxidising environment resulting partially roasted chalcopyrite with red surface.
-
Reheating roasted product with silica in 1100 °C resulting in iron-rich slag and matte (a mixture of metallic copper and iron sulphides)
-
Melting copper matte to separate residual iron in the form of iron oxide in slag matrix and to enrich matte with copper.
-
Re-roasting the copper matte to purify final copper product.
The following reactions may simplify the multi-stage process (Killick 2014):
Copper ingots/prills enriched in iron (and in some cases, sulphur) are very similar to the copper matte: the product of the multi-stage smelting process. Of course, some copper and bronze ingots/prills are not rich in iron and sulphur and their composition is fairly similar to metallic objects, especially bronze ingots/prills. Thus, the ingots/prills could be classified in two groups: Fe-rich copper mattes and purified copper/bronze ingots/prills. Of course, it is proved that copper sulphidic (or mixed with copper oxidic) ores can be smelted directly by another process consisting of completely and directly oxidising copper sulphides (dead roasting) and then smelting the ore (Rostoker 1975; Rostoker et al. 1989; Rostoker and Dvorak 1991; Killick 2014). Of course, the metallic copper produced needs to refine before using to make objects (Killick 2014). Presence of copper and bronze objects in the Haft Tappeh also may be explained by direct smelting tin-bearing complex copper ores. Evidence of this process is observed in Deh Hosein, an ancient mine in western Iran, that complex copper-tin ore is used directly to make bronze alloy during the Bronze Age and Iron Age (Nezafati 2006; Nezafati et al. 2006). Therefore, it is possible that the bronze ingots/prills and subsequently bronze objects are made by direct smelting of Cu-Sn complex ores such as stannite (Cu2FeSnS4) (Radivojević et al. 2013). Nevertheless, it needs to use two different copper ore resources including mines with copper sulphidic ores and mines with Cu-Sn complex ores. It is less likely that the different copper and bronze objects in Haft Tappeh were produced from different ore resources.
One large ingot was analysed among the samples (Sample I65). It is a copper ingot with low amount of sulphur and show that the small ingots/prills may be melted together to make large copper ingots to manufacture large objects.
Metallic objects from two important Elamite site of Susa (Susa I to Susa VB levels, the 4th to 2nd millennium BC) and Tal-e Malyan (Kaftari phase, the late 3rd and early 2nd millennium BC) are analysed previously (Malfoy and Menu 1987; Pigott et al. 2003). The results of analysis of Susa objects show that copper and arsenical copper (with arsenic concentration < 5 wt%) were used during the entire time span, while arsenical tin bronzes start to use in Susa II–IIIa period. Also, tin bronze with low arsenic content (< 2 wt%) appeared from the end of Susa IVa period although some evidences of arsenical copper and arsenical tin bronze were observed in the 3rd millennium BC of Susa. Furthermore, tin bronze has been used as the main alloy at the beginning of 2nd millennium BC of Susa (Malfoy and Menu 1987; Pigott et al. 2003; De Ryck et al. 2005). Also, Pigott et al. (2003) present results of analysis of some objects from Tal-e Malyan (Kaftari phase) showing that the tin bronze is used in some objects while one arsenical copper object is found. Based on the results of analysis of objects from Susa, Tal-e Malyan, and Haft Tappeh, it is obviously visible that the copper metallurgy of Elamite period started by using arsenical copper at the late 4th millennium BC (end of Susa I) and continued to Susa IIIb (the mid of third millennium BC). The early evidences of tin bronze (Cu-Sn) and arsenical tin bronze (Cu-As-Sn) are visible at the end of Susa IVa phase (about 2400 BC) beside Cu-As objects. Tin bronze was used widely during the second half of third millennium BC (Susa IVb and V phases) with variable-tin bronze objects while Cu-As and Cu-Sn-As objects are also indicated in these phases (De Ryck et al. 2005). Nevertheless, tin bronze was main material to make objects during the Susa VB phase and Tal-e Malyan at the beginning of the second millennium BC (Pigott et al. 2003; Malfoy and Menu 1987). The results of current study on Haft Tappeh show that the copper and tin bronze are the main materials in the metallurgy of the Middle Elamite period and Cu-As alloy was not commonplace in that time, although low amount of arsenic (about 1 wt%) is determined in some objects and ingots/prills. Luristan, the neighbour highland region of the Elam kingdom, is an important region in tin bronze metallurgy of the Iranian Plateau. Archaeometallurgical studies on emerging of tin bronze in prehistoric Luristan shows that this alloy has been familiar at beginning of the third millennium BC (Fleming et al. 2005), earlier than Elamite lowlands. It is obvious that arsenical copper has been used during the Bronze Age of Luristan (3300–1500 BC) beside tin bronze while it has not been observed during the Iron Age (1500–550 BC) (Oudbashi and Hessari 2017; Oudbashi et al. 2016; Fleming et al. 2005). In fact, tin bronze was known by Elamite metalworkers later than Luristan but was widespread during the second millennium BC. It is worthy notable that presence of tin bronze beside copper and arsenical copper in both regions during the 3rd and 2nd millennium BC may be due to the following reasons:
-
Using different ore resources by local metalworkers; some of the resources may contained As-bearing ores leading to produce some objects with high amount of arsenic,
-
Trading copper ingots as another source to obtain raw material beside using local resources leading to produce different compositions in metallic objects.
There is no certain evidence about copper and tin resources used during the Bronze Age and Iron Age as well as the Elamite period. Nevertheless, Deh Hosein ancient mine is introduced as a probable resource for the Luristan bronze metallurgy especially during the Bronze Age (Nezafati 2006; Nezafati et al. 2006). Also, some resources from Oman are suggested as probable copper ore resources for the late 3rd/early 2nd millennium BC of Susa and Tal-e Malyan (Pigott et al. 2003). Presence of As, Ni, and Co as minor element in many objects and ingots/prills of Haft Tappeh may be related to presence of them in the Omani copper resources that may be used during the third and second millennium BC by Elamite metalworkers (Malfoy and Menu 1987). However, it requires to undertake a provenance study on the Haft Tappeh metallurgical materials by using isotopic and trace element analyses and comparative study to find ore resources and relationships between this site and other Elamite metals as well as Iranian and neighbour ore resources.
Consequently, based on compositional study on two groups of metallic materials from the Haft Tappeh Elamite site, a complicated scenario could be explained about copper metallurgy here:
-
Copper has been produced by one of two processes: matte production or smelting. Regarding to the composition of the copper ingots/prills it is more likely to use matte production process. Variable concentration of iron in ingots/prills may be due to the refining process after matte production.
-
The produced copper ingots/prills are used to manufacture copper objects. Low amounts of other elements in copper objects are related to the composition of the original ores.
-
About the bronze objects, there are three probabilities: cementation, co-smelting of copper and tin ores, or smelting a complex copper-tin ore. Therefore, it is more likely to use cementation by adding cassiterite to melted copper. This may be proved by low Fe and S concentration in bronze ingots/prills because using a complex Cu-Sn ore may lead to high concentration of Fe and S in bronze ingot/prill. On the other hand, co-smelting is less probable because of many impure copper ingots/prills found in this site, although it should be considered as a probable alloying process. Also, other method known as recycling/re-melting of used tin bronze objects have not been the main alloying methods in Haft Tappeh metallurgical activities during the Middle Elamite period.
Conclusion
This study presents new insights on copper archaeometallurgy of the Middle Elamite period (ca 1500–1100 BC) based on analysis of some metallic objects and ingots/prills by micro-PIXE and SEM-EDS method. Results of analysis of Haft Tappeh metallurgical finds allowed the identification of metallurgical processes used to smelting copper as well as alloy production providing new information about technology performed by Elamite metalworkers and smelters to produce metal objects by copper and its alloys. Analysis of 52 samples (objects and ingots/prills) suggested that the main material to make metal objects is copper with some impurities. On the Other hand, variable-tin bronze has been the main alloy produced by Elamite metalworkers in Haft Tappeh. Other alloying elements such as arsenic or lead have not been added to the composition deliberately and more likely are high concentration impurities that came from the original ores. Iron has been detected as a major element in composition of some ingots/prills revealing that the main metallurgical process to obtain metallic copper has been matte production in the form of small Fe-rich (and in some cases S-rich) copper ingots/prills. Presence of many Fe-rich and S-rich phases in the microstructure of copper ingots/prills stated that these are made by the matte production process. The produced matte has been refined to achieve impure copper ingots/prills. Finally, cementation has been probably used to produce tin bronze by adding cassiterite to melted copper. In fact, Elamite metalworkers applied a complex metallurgical operation including matte production, matte refining and bronze alloying to manufacture metal objects. Thus, Haft Tappeh should be considered as an important archaeometallurgical centre of the Elamite period.
References
Alizadeh A (1985) A tomb of the Neo-Elamite Period at Arjān, near Behbahān. AMI, N.F. 18, pp. 49–73
Basello GP (2016) Elamite Kingdom. The Encyclopedia of Empire 1–10
Campbell JL, Boyd NI, Grassi N, Bonnick P, Maxwell JA (2010) The Guelph PIXE software package IV. Nucl Inst Methods Phys Res B 268(20):3356–3363
Carter E (1998) ELAM II. The archaeology of Elam, Encyclopaedia Iranica, VIII/3, pp. 313–325
Carter E, Stolper MW (1984) Elam: survey of political history and archaeology, University of California Press, near eastern Studies Vol. 25, Berkeley and Los Angeles
Cuénod A, Bray P, Pollard AM (2015) The “tin problem” in the prehistoric near east: further insights from a study of chemical datasets on copper alloys from Iran and Mesopotamia. Iran 53:29–48
Coghlan HH (1975) Notes on the prehistoric metallurgy of copper and bronze in the old world, occasional paper on technology 4, 2nd edn. Pitt Rivers Museum, Oxford
Craddock PT, Meeks ND (1987) Iron in ancient copper. Archaeometry 29:187–204
de Morgan J (1894-1905) Mission scientifique en Perse. E. Leroux, Paris
De Ryck I, Adriaens A, Adams F (2005) An overview of Mesopotamian bronze metallurgy during the 3rd millennium BC. J Cult Herit 6:261–268
Erb-Satullo NL, Gilmour BJJ, Khakhutaishvili N (2015) Crucible technologies in the Late Bronze Early Iron Age South Caucasus: copper processing, tin bronze production, and the possibility of local tin ores. J Archaeol Sci 61:260–276
Erb-Satullo NL, Gilmour BJJ, Khakhutaishvili N (2014) Late Bronze and early Iron Age copper smelting technologies in the South Caucasus: the view from ancient Colchis c. 1500-600 BC. J Archaeol Sci 49:147–159
Figueiredo E, Silva RJC, Senna-Martinez SC, Araújo MF, Fernandes FMB, Inês Vaz JL (2010) Smelting and recycling evidences from the late Bronze Age habitat site of Baiões (Viseu, Portugal). J Archaeol Sci 37:1623–1634
Fleming SJ, Pigott VC, Swann CP, Nash SK, Haerinck E, Overlaet B (2006) The Archaeometallurgy of War Kabud. Western Iran, Iranica Antiqua XLI, pp 31–57
Fleming SJ, Pigott VC, Swann CP, SK Nash (2005) Bronze in Luristan: preliminary analytical evidence from copper/bronze artifacts excavated by the Belgian mission in Iran, Iranica Antiqua, XL: 35-64
Ghirshman R (1938) Fouilles de Sialk. Librairie Orientaliste Paul Guenthner, Paris
Grime GW, Watt F (1988) Focusing protons and light ions to micron and submicron dimensions. Nucl Inst Methods Phys Res B 30(3):227–234
Haerinck E (1988) The iron age in Guilan: proposal for a chronology. In: Bronze working Centres of Western Asia 1000–539 B.C. Curtis J (ed.), London, pp: 63–78
Harper PO, Aruz J, Tallon F (1992) The Royal City of Susa: ancient near eastern treasures in the Louvre. The Metropolitan Museum of Art, New York
Helwing B (2013) Early metallurgy in Iran- an innovative region as seen from the inside, in metal matters: innovative technologies and social change in prehistory and antiquity. In: Burmeister S, Hansen S, Kunst M, Müller-Scheeßel N (eds) Menschen-Kulturen-Traditionen, Forschungs Cluster 2, Band 12. DEUTSCHES ARCHAOLOGISCHES INSTITUT, Berlin, pp 105–136
Henderson J (2000) The science and archaeology of materials: an investigation of inorganic materials. Routledge, Abingdon
Joannes F (1997) Metalle und Metallurgie, A. I. In Mesopotamien. Reallexikon der Assyriologie und vorderasiatischen Archäologie 8, pp. 96–112
Killick D (2014) From ores to metals. In: Roberts BW, Thornton CP (eds) Archaeometallurgy in global perspective: methods and syntheses. Springer, New York, pp 11–46
Lackinger A, Figueredo E, Fátima Araújo F, Silva R, Rovira S (2013) Copper + tin + people: public co-smelting experimentation in Northwestern Iberia, EXARC journal, 2013/3, http://journal.exarc.net/issue-2013-3/ea/copper-tin-people-public-co-smelting-experimentation-northwestern-iberia
Malfoy JM, Menu M (1987) La métallurgie du cuivre à Suse aux IVe et IIIe millénaires: analyses en laboratoire. In: Tallon J-CF (ed) Métallurgie susienne I : de la fondation de Suse au XVIIIe avant. Editions de la Réunion des Musées Nationaux, Paris, pp 355–373
Mofidi-Nasrabadi B (2015) Ergebnisse der C14-Datierung der Proben aus Haft Tappeh. Elamica 5:7–36
Mofidi-Nasrabadi B (2014) Vorbericht der archäologischen Ausgrabungen der Kampagnen 2012-2013 in Haft Tappeh (Iran). Elamica 4:67–167
Mofidi-Nasrabadi B (2013) Some chronological aspects of the building structures at Haft Tappeh. In: De Graef K, Tavernier J (eds) Susa and Elam, archaeological, philological, historical and geographical perspectives, Proceedings of the International Congress. 14-17, Dec. 2009. Ghent. Brill, Leiden, pp 161–172
Mofidi-Nasrabadi B (2010) Vorbericht der archäologischen Ausgrabungen der Kampagnen 2005–2007 in Haft Tappeh (Iran). Münster
Mofidi-Nasrabadi B (2004) Elam: archaeology and history. In: Stöllner T, Slotta R, Vatandoust A (eds) Persiens Antike Pracht, Bergbau Handwerk Archäologie, exhibition catalogue. Deutsches Bergbau-Museum, Bochum, pp 294–309
Moorey PRS (1994) Ancient Mesopotamian materials and industries, the archaeological evidence. Clarendon Press, Oxford
Moorey PRS (1982) Archaeology and pre-Achaemenid metalworking in Iran: a fifteen year retrospective. Iran 20:81–101
Moorey PRS (1969) Prehistoric copper and bronze metallurgy in western Iran (with special reference to Lūristān). Iran 7:131–153
Muhly JD (1973) Copper and tin: the distribution of mineral resources and the nature of the metals trade in the bronze age. Connecticut Academy of Arts and Sciences, New Haven
Negahban EO (2002) Haft Tepe, Encyclopaedia Iranica, Vol. XI, Fasc 5:526–530
Negahban EO (1991) Excavation at Haft Tepe, Iran, The University Museum of Archaeology and Anthropology, University Museum Monograph 70. University of Pennsylvania, Philadelphia
Negahban EO (1979) Architecture of Haft Tepe. In Akten des VII Internationalen Kongresses für Iranische Kunst und Archäologie, München, 7-10 September 1976. Archaeologische Mitteilungen aus Iran 6:9–29
Nezafati N (2006) Au-Sn-W-Cu-mineralization in the Astaneh-Sarband area, west Central Iran, including a comparison of the ores with ancient bronze artifacts from western Asia, PhD Dissertation, Der Geowissenschaftlichen Fakultät, Der Eberhard-Karls-Universität Tübingen, Germany, Unpublished
Nezafati N, Pernicka E, Momenzadeh M (2006) Ancient tin: old question and a new answer. Antiquity 80:308
Oudbashi O (2015) Multianalytical study of corrosion layers in some archaeological copper alloy artefacts. Surf Interface Anal 47:1133–1147
Oudbashi O, Hessari M (2017) Iron age tin bronze metallurgy at Marlik, northern Iran: an analytical investigation. Archaeol Anthropol Sci 9:233–249
Oudbashi O, Naseri R, Malekzadeh M (2016) Technical studies on the bronze age metal artefacts from the graveyard of Deh Dumen, south-western Iran (third millennium BC). Archaeometry 58:947–965
Oudbashi O, Davami P (2014a) Investigation on manufacturing process in some Elamite copper alloy artefacts from Haft Tappeh, South-west Iran. Microsc Microanal 20:2034–2035
Oudbashi O, Davami P (2014b) Metallography and microstructure interpretation of some archaeological tin bronze vessels from Iran. Mater Charact 97:74–82
Oudbashi O, Emami SM, Davami P (2012) Bronze in archaeology: a review of the archaeometallurgy of bronze in ancient Iran. In: Collini L (ed) Copper alloys-early applications and current performance-enhancing processes. InTech Open Access Publication, Rijeka, pp 153–178
Oudbashi O, Emami SM, Bakhshandehfard H (2009) Preliminary Archaeometallurgical studies on mineralogical Structureand chemical composition of ancient metal objects and slag from haft Tepe, Southwest Iran, Khuzestan (middle Elamite period), proceedings of 36th international symposium on Archaeometry, ISA 2006, 2-6 may 2006, Quebec City, Can Underwrit:407-412
Pernicka E (2014) Provenance determination of archaeological metal objects. In: Roberts BW, Thornton CP (eds) Archaeometallurgy in global perspective, methods and syntheses. Springer, New York, pp 239–268
Pigott, V. C., 2004, On the importance of Iran in the study of prehistoric copper-base metallurgy, In Persia’s Ancient splendour, mining, handicraft and archaeology, Stöllner T., Slotta R. & Vatandoust A. (eds.), pp. (28–43), Deutsches Bergbau- Museum, Bochum
Pigott VC (1999) The development of metal production in Iranian plateau: an archaeometallurgical perspective. In: Pigott VC (ed) The archaeometallurgy of Asian old world. University Museum Monograph, 89, University of Pennsilvania Museum, Philadephia, pp 73–106
Pigott VC, Rogers HC, Nash SK (2003) Archaeometallurgical investigations at Tal-e Malyan: the evidence for tin-bronze in the Kaftari phase. In: Miller NF, Abdi K (eds) Yeki Bud, Yeki Nabud: essays on the archaeology of Iran in honor of William M. Sumner. University of Pennsylvania Museum of Archaeology and Anthropology, Philadelphia, pp 161–175
Pigott VC, Howard SM, Epstein SM (1982) Pyrotechnology and culture change at bronze age Tepe Hisar (Iran). In: Early pyrotechnology, the evolution of the first fire- using industries. Wertime TA and Wertime SA (Eds.), Washington D. C. pp: 215–236
Potts DT (2016) The archaeology of Elam: formation and transformation of an ancient Iranian state, 2nd edn. Cambridge University Press, Cambridge
Radivojević M, Rehren T, Kuzmanović-Cvetković J, Jovanović M, Northover JP (2013) Tainted ores and the rise of tin bronzes in Eurasia, c. 6500 years ago. Antiquity 87:1030–1045
Rostoker W (1975) Some experiments in prehistoric copper smelting. Paléorient 3:311–315
Rostoker W, Pigott V, Dvorak J (1989) Direct reduction of copper metal by oxide-sulphide mineral interaction. Archaeomaterials 3:69–87
Rostoker W, Dvorak J (1991) Some experiments with co-smelting to copper alloys. Archaeomaterials 5:5–20
Rovira S, Montero-Ruiz I, Renzi M (2009) Experimental co-smelting to copper-tin alloys. In: Metals and societies studies in honour of Barbara S. Ottaway. TL Kienlin, B Roberts (eds.) 407–414
Smith CS (1967) The interpretation of microstructures of metallic artifacts. In: Application of science in the examination of works of art. Young WJ (ed.), September 7-16, 1965, Research Laboratory of Museum of Fine Arts: Boston 20-52
Tallon F (1987) Métallurgie susienne I : de la fondation de Suse au XVIIIe avant J.-C., Volume 15 of Noteset documents des musées de France.
Thornton CP (2010) The rise of arsenical copper in Southeastern Iran, Iranica Antiqua, XLV, pp: 31–50
Thornton CP (2009) The emergence of complex metallurgy on the Iranian plateau: escaping the Levantine paradigm. J World Prehist 22:301–327
Thornton CP, Lamberg-Karlovsky CC (2004) A new look at the prehistoric metallurgy of southeastern Iran. Iran 42:61–76
Thornton CP, Lamberg-Karlovsky CC, Liezers M, Young MM (2002) On pins and needles: tracing the evolution of copper-base alloying at Tepe Yahya, Iran, via ICP-MS analysis of common-place items. J Archaeol Sci 29:1451–1460
Vallat F (1998) ELAM I. The history of Elam, Encyclopaedia Iranica, VIII/3, pp. 301–313
Van Brempt L, Kassianidou V (2016) Facing the complexity of copper sulphide ore smelting and assessing the role of copper in southcentral Cyprus: a comparative study of the slag assemblage from late bronze age Kalavasos-Ayios Dhimitrios. J Archaeol Sci Rep 7:539–553
Vatandoost-Haghighi AR (1977) Aspects of prehistoric Iranian copper and bronze technology. Ph.D. dissertation, Institute of Archaeology: London
Acknowledgements
The authors are thankful to Dr. Alireza Razeghi and Atefeh Rashnooei, former and current Heads of Chogha Zanbil and Haft Tappeh World Heritage Research Center, Dr. Behzad Mofidi-Nasrabadi, Head of Haft Tappeh excavations, Ali Chaharlang, Akram Soltani and Alireza Faraji, Chogha Zanbil and Haft Tappeh World Heritage Research Center and Dr. Mohammad Lamehi Rashti, Nuclear Science & Technology Research Institute (NSTRI). The experimental work presented in this paper has been carried out in the framework of the research project rent No. 951/1, financed by the Research Office of Art University of Isfahan, 2016–2018, and supported by Chogha Zanbil and Haft Tappeh World Heritage Research Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oudbashi, O., Agha-Aligol, D., Mishmastnehi, M. et al. The Elamite metalworkers: multianalytical study on copper objects and ingots from second millennium BC of southwestern Iran. Archaeol Anthropol Sci 11, 2059–2072 (2019). https://doi.org/10.1007/s12520-018-0636-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12520-018-0636-4