Abstract
Background
Probiotic supplementation has been used to alleviate abdominal pain in children and adolescents with irritable bowel syndrome (IBS), but the evidence is not compelling. Thus, a systematic review and meta-analysis of randomized clinical trials (RCTs) were performed to investigate the effects of probiotic supplementation on abdominal pain in pediatric patients with IBS.
Methods
PubMed/MEDLINE, Web of Science, Scopus, Cochrane Library, and Embase were the available databases searched to find relevant randomized clinical trials up to April 2021. The effect size was expressed as weighted mean difference (WMD) and 95% confidence interval (CI).
Results
Seven RCTs with 441 participants were included, from which the meta-analysis demonstrated that probiotic supplementation has a significant effect on reducing abdominal pain in pediatric patients with IBS (WMD = − 2.36; 95% CI − 4.12 to − 0.60; P = 0.009). Although our study involved children and adolescents (≤ 18 years), the effects of probiotic supplementation seem to be more potent in patients under 10 years old (WMD = − 2.55; 95% CI − 2.84 to − 2.27) compared to patients aged 10–18 years (WMD = − 1.70; 95% CI − 2.18 to − 1.22). The length of supplementation longer than four weeks was more effective (WMD = − 2.43; 95% CI − 2.76 to − 2.09).
Conclusion
Probiotic supplementation can reduce abdominal pain in pediatric patients with IBS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Irritable bowel syndrome (IBS) can be considered a multifactorial disease that markedly affects the patients’ welfare [1]. Its symptoms are chronic and predominantly characterized by abdominal pain and altered bowel habits [2]. The pathophysiology and diagnosis of IBS have been widely discussed among the scientific community [3].
IBS is linked to a cluster of inflammatory, immune, and neuroendocrine mechanisms which contribute to visceral hypersensitivity and chronic inflammation of the small intestine and colon, as well as increased intestinal permeability [4]. In addition, the gut-brain axis is a target to be modulated in IBS mainly in virtue of neurotransmitter levels [5]. So much so that pain modulation, gastrointestinal dysmotility, and alteration in neurotransmitters and their receptors appear to play a pivotal role in the development of IBS [6, 7]. Not surprisingly, a scientific effort is still exercised to elucidate these mechanisms; for instance, the crosstalk between the gut microbiota and enteroendocrine and enterochromaffin cells, known as neuropods, has gained attention in understating visceral pain by yielding synaptic-like connections with local neural fibers and hence stimulating neurotransmission between the epithelial layer and the nervous system [8, 9].
The Rome IV criteria are frequently used for the clinical diagnosis of IBS, involving recurrent abdominal pain on average at least 1 day/week in the last 3 months, associated with two or more of the following items: (1) related to defecation; (2) associated with a change in frequency of stool; and (3) associated with a change in the appearance/form of stools [10]. The global burden of abdominal pain remains a challenge in the management of children’s welfare, mainly those diagnosed with IBS [11]. It is no wonder that epidemiological studies consider IBS as one of the main gastrointestinal disorders among the pediatric population, which vary according to predisposing factors (e.g., bacterial overgrowth and food-related problems) [12,13,14,15,16]. More specifically, prevalence rates of IBS among children and adolescents range between 3 and 12% worldwide, with a higher prevalence between 8 and 12 years [5, 17].
While there is robust evidence supporting probiotics for improving overall symptoms in adults with IBS, as confirmed by a network meta-analysis [18], emerging research has considered probiotic supplementation as a promising and feasible candidate to alleviate general gastrointestinal symptoms and abdominal pain in children and adolescents with IBS [19]. In this population, great interest has been directed towards abdominal pain, as shown by a couple of randomized clinical trials (RCTs) [20, 21]. In an attempt to draw firm conclusions for the circles of clinical practice, therefore, we carried out a systematic review and meta-analysis of RCTs to investigate the overall effects of probiotic supplementation in reducing abdominal pain in pediatric patients with IBS.
Methods
The Preferred Reporting Items for Systematic Review and Meta-analysis statement criteria was applied for performing the current study [22]. Also, the study protocol was ethically approved by the Regional Bioethics Committee of Shahid Beheshti University of Medical Sciences.
Search strategy
A systematic search was implemented in the Scopus, Embase, PubMed, Web of Science, and Cochrane library databases from inception until April 2021. The combination of the following keywords and Medical Subject Heading (MeSH) terms were used in the search strategies: (probiotics OR lactobacillus OR bifidobacterium) AND (irritable bowel syndrome OR IBS) AND (child OR adolescent OR pediatrics). We also hand-searched the bibliographies of retrieved reviews to find further potentially relevant papers. No language or time limits were imposed in the literature search.
Eligibility criteria
After the elimination of duplicate records, titles and abstracts of identified papers were screened in detail, and studies with the following criteria were included: (1) randomized controlled trial design; (2) use of probiotics supplementation as an intervention; (3) enrolling children and adolescents (under or equal to 18 years of age) with IBS; and (4) reporting sufficient data on the severity of abdominal pain score. Studies without control groups, non-randomized trials, and studies conducted on pregnant women or adults were excluded. We also excluded studies that did not provide sufficient data on intended outcomes as well as those enrolled healthy subjects.
Data extraction
A detailed full-text review was independently performed by two authors and the following data were abstracted using standardized pre-piloted forms: first author's name, year of publication, study location, sample size, RCT design, type of probiotic supplement dose and duration of intervention, participants' characteristics (gender, mean age, mean body mass index), and the means and standard deviations (SDs) of outcome measures in both intervention and control groups.
Quality assessment
The methodological quality assessment of eligible RCTs was done in accordance with the Cochrane risk of bias criteria [23]. Studies were subjectively rated by two authors as high, low, or unclear risk of bias based on the following domains: allocation concealment, blinding of outcome assessment, blinding of participants and personnel, incomplete outcome data, random sequence generation, selective reporting, and other bias. Any disagreements were resolved by a third reviewer.
Data synthesis and statistical analysis
Data were analyzed using comprehensive meta-analysis version 2.0 and STATA version 12.0 software and results were expressed as weighted mean differences (WMDs) with a 95% confidence interval (CI). In the absence of SD of the change, we computed it using the following formula: SD change = square root [(SD baseline 2 + SD final 2) − (2 × R × SD baseline × SD final)] [24, 25]. Also, for trials that only reported standard error of the mean (SEM), SDs were computed using the following formula: SD = SEM × √n, where “n” is the number of subjects in each group. The differences in means and SDs were computed to estimate the overall effect size under the random-effects model [24, 26]. Inter-study heterogeneity was assessed using Cochrane Q statistic and quantified by I2 statistic [27]. To explore the potential sources of heterogeneity, we conducted subgroup analysis based on the mean age of participants (< 10 years or ≥ 10 years) and duration of the intervention (≤ 4 weeks or > 4 weeks) [28]. The sensitivity analysis was carried out to assess the robustness of findings by the leave-one-out method. Moreover, we examined the presence of publication bias using visual inspection of funnel plot and Egger’s regression test [29].
Results
Study selection
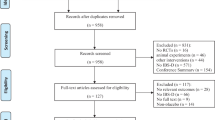
The process of extraction and exclusions is shown in Fig. 1. After searching the systematic databases, 1873 articles were selected, with 1235 articles being remained after the elimination of duplicate studies. Then, 1212 articles were deleted after reviewing the abstract or title according to the inclusion criteria, and 16 articles were discarded after retrieving the full text of the remaining 23 articles. Finally, seven articles met the eligibility and were included in our analysis [21, 30,31,32,33,34,35].
Study characteristics
The general characteristics of the eligible studies are summarized in Table 1, confirming that children and adolescents with IBS were the populations examined, in which the highest mean age among the studies was 12.5 years. Our meta-analysis demonstrated that probiotic supplementation has a significant effect on reducing abdominal pain in children and adolescents with IBS. Although our study involved children and adolescents up to 18 years old, the effects of probiotic supplementation seem to be most potent in patients younger than 10 years.
Studies were published between 2006 and 2020; two studies were conducted in Italy and other studies were performed in Poland, Iran, the USA, and India. The mean age and sample size of studies' participants varied between 6.5 and 12.5 years and 37–132, respectively, with the duration of intervention ranging from 4 to 8 weeks. In four studies, Lactobacillus rhamnosus GG probiotic was used as the intervention group and in one study 108 colony forming units Lactobacillus reuteri was evaluated. In other studies, probiotics VSL#3 and Bacillus coagulans Unique IS2 were used in the intervention group. Also, the faces pain scale, Likert scale, irritable bowel severity scoring system, subject’s global assessment of relief questionnaires and Wang-Baker faces pain rating scale were used to assess the severity of abdominal pain.
Risk of bias assessment
The details of the study quality assessment are presented in Supplementary Table 1. As shown in the table, six studies were rated with low risk in random sequence generation, because they did not explicitly mention the random sequence generation methods and one as unclear risk. All studies were rated as unclear risk in allocation concealment. Four study reported that both the participants and the researchers were blinded and was, thus, identified as having a low risk of bias for the blinding step. Five of the trials provided a clear description of the blinding of outcome assessment. Seven studies were clear on providing incomplete outcome data and then were considered as low risk of bias. Four studies were assessed as having a low risk of bias in selective reporting, and the other article were rated as unclear risk of bias. Except for one study that was considered as high risk of bias in the quality stage, the other studies were considered as unclear risk of bias.
Meta-analysis
Effect of probiotics supplementation on the severity of abdominal pain
The quantitative meta-analysis revealed a significant effect of probiotic supplementation on the severity of abdominal pain score (WMD = − 2.36; 95% CI − 4.12 to − 0.60; P = 0.009). However, a significant heterogeneity was observed among the studies for this outcome (Cochran Q test, P < 0.001, I2 = 99.9%) (Fig. 2). Stratified analysis, according to mean age of participants and duration of the intervention, indicated a significant change in the results for the severity of abdominal pain score. Probiotic supplementation in patients < 10 years old (WMD = − 2.55; 95% CI − 2.84 to − 2.27) compared to patients aged 10–18 years (WMD = − 1.70; 95% CI − 2.18 to − 1.22). The length of supplementation longer than four weeks was more effective (WMD = − 2.43; 95% CI − 2.76 to − 2.09) (Supplementary Figs. 1, 2). None of the subgroup analyzes for the age of the participants and the duration of the intervention could find a possible source of heterogeneity.
Sensitivity analysis
The leave-one-out method was applied to assess the influence of each study on the pooled effect size. The findings remained robust after the sequential elimination of studies (Supplementary Fig. 3).
Publication bias
Visual inspection of the funnel plot revealed no evidence of publication bias regarding the impacts of probiotics supplementation on outcome measures. Additionally, the results of Egger's regression test supported the absence of significant publication bias for the severity of abdominal pain (P = 0.54) (Supplementary Fig. 4).
Discussion
This systematic review and meta-analysis of RCTs demonstrate that probiotic supplementation has a significant effect on reducing abdominal pain in children and adolescents with IBS. Although our systematic review included children and adolescents of various age (studies’ mean age ranging from 6.5 to 12.5 years), the effects of probiotic supplementation seem to be most potent for patients younger than 10 years. In addition, supplementation duration longer than four weeks was most effective (compared to ≤ 4 weeks), but it is important to note that the longest length of supplementation was 8 weeks.
Similar to our findings, another meta-analysis confirmed that probiotic supplementation for children with IBS reduces abdominal pain score, strengthening this potential by accompanying reduction in Global Assessment of Relief score and frequency of abdominal pain while increasing the rate of abdominal pain treatment success [36]. However, contrary to the above study, we also observed this relationship in different age groups and the length of treatment.
It is noteworthy that IBS is a multifactorial disease affected by epigenetics and environmental problems such as an unbalanced diet, psychological factors, and socioeconomic factors, and is associated with common childhood illnesses such as asthma and/or atopic diseases [5]. The current pharmacological arsenal for IBS consists of loperamide, lubiprostone, tricyclic antidepressants, selective serotonin receptor inhibitors, antispasmodics, rifaximin, pregabalin, gabapentin, clonidine, and octreotide [37]. Equally important, non-pharmacological strategies are other approaches to IBS, involving cognitive-behavioral therapy [38] as well as supplements and dietary changes [39].
Regarding dietary aspects, fermentable carbohydrates called fermentable oligo-, di- and monosaccharides, and polyols (FODMAPs), along with different proportions and types of proteins and fats, as well as the intake of coffee and hot spices, are generally linked to abdominal pain [40]. These foodstuffs can imply gastrointestinal problems even in healthy people, while further intensifying the bowel motility and abdominal distention in patients with IBS who naturally have visceral hypersensitivity [40]. Nevertheless, given that decreasing FODMAPs, especially in childhood, can induce nutrient restriction, our meta-analysis examined interventions based only on probiotics to generate results with greater clinical adhesion, as probiotic supplements are administrated via capsules or sachets while dietary interventions are more susceptible to biases due to different habits and incorrect eating choices. Furthermore, despite the pharmacological efficacy in IBS [41], the investigation of probiotic supplements in our study is relevant to provide the magnitude of an adjuvant therapy or even a major strategy in the management of children and adolescents since two or more concurrent medications may be considered pediatric polypharmacy and it is often difficult to administer medicines for children [42], whereas probiotics have no residual flavor and can be easily diluted in liquids. Indeed, it is essential to investigate in scrutiny the types of probiotic supplements in pediatric digestive disorders, as the gut microbiota modulation cannot be neglected in children and adolescents with IBS [43]. So much so that dysbiosis is associated with increased abdominal pain in this population, whereby an unhealthy gut microbiota leads to growth in pathogenic bacteria and a decrease in commensal bacteria [44]. Before considering the supplementation of probiotics as a means of mitigating these harmful effects, it is worth mentioning the bacteria species present in the pathophysiology of IBS so that a clinical rationale can be done.
Saulnier et al. observed that children with IBS had higher proportions of the phylum Proteobacteria (the class γ-Proteobacteria), genera such as Dorea (member of Firmicutes), Haemophilus (member of γ-Proteobacteria), as well as Haemophilus parainfluenzae and a novel Ruminococcus-like organism, while a greater status of the genus Eubacterium and the species Bacteroides vulgatus were noted in healthy children [45]. In addition, decreased bacterial diversity in the gut, such as some species like Bifidobacterium, Collinsella, and Clostridiales can also be detected in IBS [46]. Such a disturbance in the gut microbiota of patients with IBS may be related to impaired signal transduction pathways, with ensuing inflammatory state in the intestinal mucosa caused by Th17-related pro-inflammatory cytokines [e.g., interleukin (IL)-17A, IL-17F, and IL-22] [47,48,49]. History of previous gastrointestinal infections (e.g., infection by Giardia lamblia, Salmonella species, and Campylobacter species) seem to be involved in the etiology of IBS as well [19].
Regarding mechanisms, probiotics can enhance gut barrier function, inhibit pathogen binding, and modulate gut inflammatory response [50]. More specifically, as abdominal pain in IBS is related to elevated pro-inflammatory cytokine levels such as tumor necrosis factor-alpha, IL-1β, IL-6, and IL-12, increased intestinal mucosal permeability, and imbalance between commensal and pathogenic bacteria [44], probiotics could mitigate abdominal pain by restoring the gut microbiota and hence stabilizing colonic fermentation, whose actions physiologically decrease the inflammatory response and strengthen the intestinal mucosal barrier [41].
The studies selected in our meta-analysis ought to be individually discussed in view of providing particular attention to the types of probiotics used. Lactobacillus rhamnosus GG was the strain more used among the studies [21, 32,33,34]. For instance, Francavilla et al., working on 141 children with IBS, found that those who underwent L. rhamnosus GG had decreased frequency and intensity of abdominal pain compared to placebo, whose length of duration was 8 weeks [33]. The authors suggested that the improvement in symptomatology is secondary to an enhanced gut barrier [33]. Gawrońska et al. tested the effects of L. rhamnosus GG supplementation in children with IBS, functional dyspepsia, or functional abdominal pain (n = 104), observing moderate amelioration in abdominal pain symptoms for those who received the supplement [32]. Sudha et al. showed benefits in supplementing B. coagulans Unique IS2 for children with IBS, showing a decline in pain intensity, abdominal discomfort, bloating, staining, urgency, incomplete evacuation, and passage of gas, as well as improved stool consistency when compared to placebo [30]. This bacterial strain is used for many adult conditions [51, 52], and Sudha et al. [30] help in expanding the evidence for treating children.
Interestingly, however, not only isolated bacterial strain but also some studies examined the effects of commercial probiotic mixture. Guandalini et al. evaluated the efficacy of VSL#3 supplementation in children with IBS compared to placebo for 6 weeks, and those who supplemented with VSL#3 reported decreased abdominal pain, as well as improved abdominal bloating, gas, and subjective assessment of symptoms [31]. VSL#3 is a commercial probiotic mixture consisting of eight bacterial strains: four strains of Lactobacillus (Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei, and Lactobacillus delbrueckii subspecies bulgaricus), three strains of Bifidobacterium (Bifidobacterium breve, Bifidobacterium longum, and Bifidobacterium infantis), and one strain of Streptococcus (Streptococcus salivarius subspecies thermophilus) [53]. Collectively, synergic mechanisms are conceivable among these bacterial strains whereby the gene clusters of Bifidobacterium may enhance the intestinal barrier integrity by coding tight adherence pili, while several surface layer proteins of Lactobacilli display essential roles in the modulation of the host immune response by acting on the adhesion to host epithelium and extracellular matrix components [53,54,55]. In addition, the gene clusters of Streptococcus thermophiles have abilities in coding most of the defense systems [53].
Our meta-analysis has strengths and limitations. This work brings practical data and enlarges the evidence geared toward the management of children and adolescents, whose is a population that can suffer from IBS and cannot be ignored. Along these lines, this research may assist the practice of general practitioners, pediatricians, and dietitians who deal with the pediatric population.
Moreover, in addition to improving the well-being of IBS patients, commercial probiotic supplements are apparently safe in general [56]; however, caution should be exercised to critically sick infants and patients with immune-compromised complexity due to the association between bacteremia and fungemia with some probiotic strains used as supplements, such as Lactobacillus species (mainly L. rhamnosus) and Saccharomyces boulardii [57]. As for limitations, the small number of high-quality RCTs on probiotics for IBS in various pediatric populations and the limited numbers of subjects recruited into many trials are the main limitations. Besides, due to insufficient study to obtain sufficient results, it was not possible to refer to other aspects IBS severity, e.g., stool ethnicity and frequency or any division based on subtype of IBS, as well as to perform subanalyses for probiotic strains.
In conclusion, this meta-analysis supports the favorable benefits of probiotic supplementation as a means of reducing the severity of abdominal pain in children and adolescents with IBS. However, a dose–response effect cannot be established so far, as the bacterial strain, dose, and duration of treatment vary substantially between studies. Therefore, further research is warranted, while practitioners (e.g., physicians and registered dietitians) should consider personalized dosing regimens based on their experience and the cost-effectiveness of the available products for current advice.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014.
Mayer EA. Clinical practice. Irritable bowel syndrome. New Engl J Med. 2008;358:1692–9.
Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:6759–73.
Ng QX, Soh AYS, Loke W, Lim DY, Yeo WS. The role of inflammation in irritable bowel syndrome (IBS). J Inflamm Res. 2018;11:345–9.
Devanarayana NM, Rajindrajith S. Irritable bowel syndrome in children: current knowledge, challenges and opportunities. World J Gastroenterol. 2018;24:2211–35.
Gros M, Gros B, Mesonero JE, Latorre E. Neurotransmitter dysfunction in irritable bowel syndrome: emerging approaches for management. J Clin Med. 2021;10:3429.
Bednarska O, Icenhour A, Tapper S, Witt ST, Tisell A, Lundberg P, et al. Reduced excitatory neurotransmitter levels in anterior insulae are associated with abdominal pain in irritable bowel syndrome. Pain. 2019;160:2004–12.
El-Salhy M, Hausken T, Gilja OH, Hatlebakk JG. The possible role of gastrointestinal endocrine cells in the pathophysiology of irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. 2017;11:139–48.
Najjar SA, Albers KM. Pain in inflammatory bowel disease: optogenetic strategies for study of neural–epithelial signaling. Crohn’s Colitis 360. 2021;3:otab040.
Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. 2017;6:99.
Bielefeldt K, Davis B, Binion DG. Pain and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:778–88.
Hyams JS, Treem WR, Justinich CJ, Davis P, Shoup M, Burke G. Characterization of symptoms in children with recurrent abdominal pain: resemblance to irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 1995;20:209–14.
Devanarayana NM, Adhikari C, Pannala W, Rajindrajith S. Prevalence of functional gastrointestinal diseases in a cohort of Sri Lankan adolescents: comparison between Rome II and Rome III criteria. J Trop Pediatr. 2011;57:34–9.
Bouzios I, Chouliaras G, Chrousos GP, Roma E, Gemou-Engesaeth V. Functional gastrointestinal disorders in Greek children based on ROME III criteria: identifying the child at risk. Neurogastroenterol Motil. 2017. https://doi.org/10.1111/nmo.12951.
Korterink JJ, Diederen K, Benninga MA, Tabbers MM. Epidemiology of pediatric functional abdominal pain disorders: a meta-analysis. PLoS One. 2015;10:e0126982.
Zablah R, Velasco-Benítez CA, Merlos I, Bonilla S, Saps M. Prevalence of functional gastrointestinal disorders in school-aged children in El Salvador. Rev Gastroenterol Mex. 2015;80:186–91.
Devanarayana NM, Rajindrajith S, Pathmeswaran A, Abegunasekara C, Gunawardena NK, Benninga MA. Epidemiology of irritable bowel syndrome in children and adolescents in Asia. J Pediatr Gastroenterol Nutr. 2015;60:792–8.
Liang D, Longgui N, Guoqiang X. Efficacy of different probiotic protocols in irritable bowel syndrome: a network meta-analysis. Medicine (Baltimore). 2019;98:e16068.
Sandhu BK, Paul SP. Irritable bowel syndrome in children: pathogenesis, diagnosis and evidence-based treatment. World J Gastroenterol. 2014;20:6013–23.
Giannetti E, Maglione M, Alessandrella A, Strisciuglio C, De Giovanni D, Campanozzi A, et al. A mixture of 3 bifidobacteria decreases abdominal pain and improves the quality of life in children with irritable bowel syndrome: a multicenter, randomized, double-blind, placebo-controlled, crossover trial. J Clin Gastroenterol. 2017;51:e5-10.
Kianifar H, Jafari SA, Kiani M, Ahanchian H, Ghasemi SV, Grover Z, et al. Probiotic for irritable bowel syndrome in pediatric patients: a randomized controlled clinical trial. Electron Physician. 2015;7:1255–60.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Hoboken: Wiley; 2019.
Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration. https://www.cochrane-handbook.org. 2011.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Borenstein M, Higgins JPT. Meta-analysis and subgroups. Prev Sci. 2013;14:134–43.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Sudha MR, Jayanthi N, Aasin M, Dhanashri RD, Anirudh T. Efficacy of Bacillus coagulans Unique IS2 in treatment of irritable bowel syndrome in children: a double blind, randomised placebo controlled study. Benef Microbes. 2018;9:563–72.
Guandalini S, Magazzù G, Chiaro A, La Balestra V, Di Nardo G, Gopalan S, et al. VSL# 3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24–30.
Gawrońska A, Dziechciarz P, Horvath A, Szajewska H. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther. 2007;25:177–84.
Francavilla R, Miniello V, Magistà AM, De Canio A, Bucci N, Gagliardi F, et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics. 2010;126:e1445–52.
Bausserman M, Michail S. The use of Lactobacillus GG in irritable bowel syndrome in children: a double-blind randomized control trial. J Pediatr. 2005;147:197–201.
Rahmani P, Ghouran-Orimi A, Motamed F, Moradzadeh A. Evaluating the effects of probiotics in pediatrics with recurrent abdominal pain. Clin Exp Pediatr. 2020;63:485–90.
Xu HL, Zou LL, Chen MB, Wang H, Shen WM, Zheng QH, et al. Efficacy of probiotic adjuvant therapy for irritable bowel syndrome in children: a systematic review and meta-analysis. PLoS One. 2021;16:e0255160.
Trinkley KE, Nahata MC. Treatment of irritable bowel syndrome. J Clin Pharm Ther. 2011;36:275–82.
Everitt HA, Landau S, O’Reilly G, Sibelli A, Hughes S, Windgassen S, et al. Cognitive behavioural therapy for irritable bowel syndrome: 24-month follow-up of participants in the ACTIB randomised trial. Lancet Gastroenterol Hepatol. 2019;4:863–72.
Dai YK, Wu YB, Li RL, Chen WJ, Tang CZ, Lu LM, et al. Efficacy and safety of non-pharmacological interventions for irritable bowel syndrome in adults. World J Gastroenterol. 2020;26:6488–509.
Simrén M, Månsson A, Langkilde AM, Svedlund J, Abrahamsson H, Bengtsson U, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63:108–15.
Chiou E, Nurko S. Functional abdominal pain and irritable bowel syndrome in children and adolescents. Therapy. 2011;8:315–31.
Bakaki PM, Horace A, Dawson N, Winterstein A, Waldron J, Staley J, et al. Defining pediatric polypharmacy: a scoping review. PLoS One. 2018;13:e0208047.
Ding JH, Jin Z, Yang XX, Lou J, Shan WX, Hu YX, et al. Role of gut microbiota via the gut-liver-brain axis in digestive diseases. World J Gastroenterol. 2020;26:6141–62.
Chumpitazi BP, Shulman RJ. Underlying molecular and cellular mechanisms in childhood irritable bowel syndrome. Mol Cell Pediatr. 2016;3:11.
Saulnier DM, Riehle K, Mistretta TA, Diaz MA, Mandal D, Raza S, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. 2011;141:1782–91.
Distrutti E, Monaldi L, Ricci P, Fiorucci S. Gut microbiota role in irritable bowel syndrome: new therapeutic strategies. World J Gastroenterol. 2016;22:2219–41.
Jalanka-Tuovinen J, Salojärvi J, Salonen A, Immonen O, Garsed K, Kelly FM, et al. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut. 2014;63:1737–45.
Sundin J, Rangel I, Repsilber D, Brummer RJ. Cytokine response after stimulation with key commensal bacteria differ in post-infectious irritable bowel syndrome (PI-IBS) patients compared to healthy controls. PLoS One. 2015;10:e0134836.
Kamada N, Núñez G. Role of the gut microbiota in the development and function of lymphoid cells. J Immunol. 2013;190:1389–95.
Spiller R. Review article: probiotics and prebiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:385–96.
Madempudi RS, Neelamraju J, Ahire JJ, Gupta SK, Shukla VK. Bacillus coagulans Unique IS2 in constipation: a double-blind, placebo-controlled study. Probiot Antimicrob Proteins. 2020;12:335–42.
Ratna Sudha M, Yelikar KA, Deshpande S. Clinical study of Bacillus coagulans Unique IS-2 (ATCC PTA-11748) in the treatment of patients with bacterial vaginosis. Indian J Microbiol. 2012;52:396–9.
Cheng FS, Pan D, Chang B, Jiang M, Sang LX. Probiotic mixture VSL#3: an overview of basic and clinical studies in chronic diseases. World J Clin Cases. 2020;8:1361–84.
Muscariello L, De Siena B, Marasco R. Lactobacillus cell surface proteins involved in interaction with mucus and extracellular matrix components. Curr Microbiol. 2020;77:3831–41.
O’Connell Motherway M, Zomer A, Leahy SC, Reunanen J, Bottacini F, Claesson MJ, et al. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc Natl Acad Sci U S A. 2011;108:11217–22.
Vandenplas Y, Veereman-Wauters G, De Greef E, Peeters S, Casteels A, Mahler T, et al. Probiotics and prebiotics in prevention and treatment of diseases in infants and children. J Pediatr Rio J. 2011;87:292–300 (in English, Portuguese).
Didari T, Solki S, Mozaffari S, Nikfar S, Abdollahi M. A systematic review of the safety of probiotics. Expert Opin Drug Saf. 2014;13:227–39.
Funding
None.
Author information
Authors and Affiliations
Contributions
FS and HA contributed equally to this study. FS carried out the concept, design and drafting of this study, and performed the acquisition, analysis, and interpretation of data. SMH carried out the concept, design and drafting of this study, searched databases, screened articles and extracted data, and critically revised the manuscript. SA and SIGO performed the acquisition, analysis, and interpretation of data. SHO searched databases, screened articles and extracted data. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Ethical approval for this research was waived by its nature.
Conflict of interest
None of the authors had any personal or financial conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fatahi, S., Hosseini, A., Sohouli, M.H. et al. Effects of probiotic supplementation on abdominal pain severity in pediatric patients with irritable bowel syndrome: a systematic review and meta-analysis of randomized clinical trials. World J Pediatr 18, 320–332 (2022). https://doi.org/10.1007/s12519-022-00516-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-022-00516-6