Abstract
Kasabach−Merritt phenomenon (KMP) is a rare disease that is characterized by severe thrombocytopenia and consumptive coagulation dysfunction caused by kaposiform hemangioendothelioma or tufted hemangioma. This condition primarily occurs in infants and young children, usually with acute onset and rapid progression. This review article introduced standardized recommendations for the pathogenesis, clinical manifestation, diagnostic methods and treatment process of KMP in China, which can be used as a reference for clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1940, Kasabach and Merritt first reported a case of severe thrombocytopenia and extensive purpura caused by a rapidly growing giant hemangioma in the left thigh. Since then, a group of diseases with giant hemangioma complicated with thrombocytopenia have been called Kasabach−Merritt syndrome (KMS) [1]. In the 1990s, Zukerber, Enjohras and Sarkar et al. [2,3,4] found that the pathological basis of KMS was not infantile hemangioma but rather kaposiform hemangioendothelioma (KHE) and tufted hemangioma (TA). Subsequently, in the same year, Sarkar suggested to refer to severe thrombocytopenia, microangiopathic hemolytic anemia, secondary fibrinogen reduction and consumptive coagulation dysfunction caused by KHE and TA as Kasabach−Merritt phenomenon (KMP) to replace KMS. The latest classification of vascular anomalies in 2018 issued by the International Society for the Study of Vascular Anomalies (ISSVA) defines KMP as a phenomenon specifically caused by KHE or TA, which does not include platelet and coagulation abnormalities caused by other vascular anomaly diseases. In view of the current controversy in the diagnosis and treatment of KMP [6], it is very important to improve the understanding, diagnosis and treatment of KMP and to standardize KMP clinical treatment. This review article introduced the currents care standards for KMP in China.
Etiology and pathogenesis
The etiology of KMP is not clear and may be related to the following factors: (1) trapping of platelets by abnormally proliferating vascular endothelial cells [7] promotes platelet adhesion, accumulation and activation, leading to activation of the coagulation cascade, deposition of a large amount of fibrin, and formation of microthrombi, causing massive platelet retention and exacerbating the consumption of the platelets and coagulation factors. The corresponding hyperactivation of the fibrinolytic system causes intratumoral hemorrhage that leads to a new round of coagulation material consumption and may eventually induce diffuse intravascular coagulation (DIC) in severe cases. However, how the platelets are trapped by the tumor remains unclear [8,9,10]; (2) the special structure of the lesion causes a combined action of several factors, including trapping of the platelets by vascular endothelial cells, exposure of endothelial cell basement membrane and local turbulent formation of the lesion, leading to the activation, accumulation and release of coagulation factors such as platelets and fibrinogen that form a coagulation cascade effect. This further induces intratumoral hemorrhage and aggravates the disease [11, 12]; (3) vascular endothelial growth factor (VEGF) mediated by the VEGF R3-PI3K-AKT-mTOR pathway promotes proliferation of KHE endothelial cells through autocrine/paracrine, thereby promoting angiogenesis and tumor growth [13, 14], and (4) impaired maturation of the megakaryocyte system in the bone marrow may be associated with tumor growth and progression [15,16,17].
Epidemiology
The incidence of KMP is not clear. KMP does not show a significant gender difference. KMP usually occurs in infants and young children within 1 year of age, among whom newborns account for about 38.5–60% of all cases. The rate in China is lower than that in other countries, possibly due to limited knowledge of KMP, leading to missed diagnosis. KMP will occur in about 50–71% of patients with KHE/TA of whom 11% have delayed KMP, with an average delay of 5 weeks [6, 18]. The incidence of KMP gradually decreases with increased age. The probability of KMP occurrence is 79% within 1 year, 47% between 1 and 5 years, 43% between 6 and 12 years and 10% between 13 and 21 years of age [19]. Tumor volume is also associated with KMP occurrence, with larger tumors corresponding to a higher incidence of KMP [20, 21]. Additionally, deep lesions are also prone to KMP. The probabilities of KMP in the retroperitoneal and thoracic cavity are as high as 85% and 100%, respectively [6]. In general, a young onset age, large tumor volume and lesions in the thoracic cavity and posterior peritoneum are risk factors for KMP in patients with KHE and TA.
Clinical manifestations
KHE/TA lesions are mostly congenital, which grow rapidly in the form of dark purple masses with shiny skin on the surface, obvious edema, increased tension, touch texture and may be accompanied by petechiae or purpura around the tumor or in the whole body. The lesions usually occur in the skin and subcutaneous tissue, which can also affect muscle and skeletal tissue. The locations of KMP onset vary greatly, with the four limbs being most common, followed by the trunk and the head and neck region; the ratio of the three can reach as high as 72% [22]. Additionally, lesions in deep locations such as the mediastinum, retroperitoneum and pelvic cavity are not rare, with the ratio reaching 15–23% [19, 22].
Laboratory and imaging examinations
Routine blood examination shows a decreased platelet count, which is usually less than 50 × 109/L and is often combined with reduced hemoglobin. Coagulation function examination shows reduced fibrinogen and elevated D-dimer. Two-dimensional ultrasound shows mixed echoes, mainly low echo and unclear boundary. Color doppler shows that the lesion blood flow signal is abundant or extremely abundant, usually with a blood vessel density > 6 streams/cm2. Spectral doppler ultrasound indicates blood flow of the arterial spectrum characterized by high velocity and low resistance.
CT shows uniform or uneven low-density foci, which is significantly intensified after enhancement. MRI primarily manifests an iso- or hypo-intense signal on T1 weighted imaging, an iso- or hyperintense signal on T2 weighted imaging, and a hyperintense signal on fat suppression imaging. The signals are unevenly and significantly intensified after enhancement. And the tumor does not have clear boundaries, with adjacent skin, subcutaneous tissue and muscle layer all being affected. Emptying of the nutrient or drainage blood vessels is not obvious.
Histopathological manifestations
KHE cells grow invasively, often reaching deep into the subcutaneous adipose tissue and forming multiple nodules or diffuse hyperplastic areas surrounded by a massive amount of dilated lymphatic vessels. The tumor is composed of a large number of spindle cells. Epithelioid endothelial cells can be detected inside the tumor. The tumor is also accompanied by fissure-like or crescent-shaped lumen differentiation, while red blood cells can be seen inside. Some nodules form glomerulus-like structures with the surrounding dilated lymphatic vessels. When accompanied by KMP, a large number of red blood cells accumulate inside the tumor which may even extravasate.
Immunohistochemistry staining shows that KHE cells express vascular endothelial cell markers cluster of differentiation 31 (CD31) and CD34. The lymphatic markers prospero homeobox (PROX1), lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1), and D2-40 are expressed in varying degrees. Mature vascular endothelial cells express smooth muscle actin (SMA). And tumor cells are negative for glucose transporter 1 (GLUT-1).
Histologically, TA is similar to KHE. Morphologically, TA is mainly confined to the dermis; and tumor nodules show the characteristic bullet-like change.
Diagnosis and differential diagnosis
The diagnosis of this disease is usually not difficult and is based on the typical medical history, clinical examination, significant tendency of bleeding, reduced platelets, and corresponding laboratory examinations. Furthermore, lesion biopsy often indicates KHE/TA. In some cases, platelets are not reduced in the early stage. However, KMP should be highly suspected in patients with characteristic lesions, abnormal elevation of D-dimers and in those who are complicated with hypofibrinogenemia. Platelet changes should be dynamically monitored. The degree of lesion involvement can be determined and monitored by ultrasound, CT or MRI.
KHE/TA should be differentiated from vascular diseases, such as congenital hemangioma, venous malformation and Kaposi’s sarcoma, and from other diseases such as cellulitis, neonatal subcutaneous gangrene, neonatal scleredema, lymphoma and childhood soft tissue tumors. In particular, transient thrombocytopenia may also occur in rapidly involuting congenital hemangioma (RICH). Notably, venous malformation complicated with localized intravascular coagulopathy (LIC) may also lead to elevated D-dimers and the consumption of coagulation factors, with some cases having thrombocytopenia, which is easily confused with KMP in the clinic. Compared with KMP, LIC patients are mainly older children or adults, with higher levels of D-dimer (more than two times that of the normal level and can be higher than 10,000 µg/L in severe cases) and often not accompanied with thrombocytopenia or only mild platelet reduction [23, 24].
Clinical classification
Clinical classification is the basis for formulating treatment principles. KMP can further be divided into the coagulopathy phase and the residual lesion phase according to changes in the disease condition. The coagulopathy phase is manifested by characteristic lesions complicated with thrombocytopenia, coagulation factor consumption and anemia. After intervention, the coagulation dysfunction is usually corrected after one year of age which enters the residual lesion phase. At this point, the platelets return to normal, but D-dimer levels remains elevated at varying degrees until it gradually returns to normal.
Treatments
Treatments mainly include systemic drug application, local compression therapy, mesh suture, intraluminal intervention, surgical resection, and blood product infusion.
Systemic drug treatment
Glucocorticoids
For half a century, glucocorticoids have been the main drug for treating KHE/TA by inhibiting the abnormal proliferation of vascular endothelial cells and by reducing the inflammatory response. However, a single steroid therapy is sometimes ineffective, in which some patients may experience relapse. Meta-analysis and systematic literature reviews have shown that the effective rates of glucocorticoid treatment are 35.8% and 54.7%, respectively [25]. Steroids remain to be primary drug of choice for the treatment of KMP due to their low cost, ease of management, and rapid response [26]. The recommended regimen is intravenous or oral prednisone 3–5 mg/kg daily. And the clinical efficacy should be evaluated after two weeks of continuous drug treatment to determine whether other drugs are needed. For patients who respond well to the glucocorticoid treatment, the drug dosage should be reduced gradually but not withdrawn immediately. It is generally recommended that the dose is gradually reduced of the course of 3–4 months until the drug is completely withdrawn.
Sirolimus
Sirolimus inhibits the expression of many cytokines, including VEGF, by blocking the mammalian target of rapamycin (mTOR) signaling pathway and has anti-angiogenic, pro-apoptotic, and autophagy effects. In the latest review about the treatment of KHE and TA, among the 29 cases treated with sirolimus alone and 5 cases treated with sirolimus together with other drugs, the tumor volume of all cases was reduced, and the effective rate of 31 patients with KMP was 96.8% [19]. An increasing number of clinical studies have shown that rapamycin is effective for the majority of KMP cases and which is expected to be a compelling potential drug for the treatment of KMP. The dose of sirolimus for treating KMP is 0.8 mg/m2 taken orally twice a day. And the drug concentration in the blood should be maintained at 8–15 ng/mL. When steroids are taken simultaneously, the compound sulfamethoxazole should also be taken at the same time at a dose of 25 mg/kg, twice daily for 3 days per week. The main short-term side effects reported in the literature include immunosuppression, elevated transaminases, hyperlipidemia, mucositis, failed Bacillus Calmette-Guerin (BCG) vaccination, mild thrombocytosis, neutropenia, and headache. During the treatment, attention should be given to the tendency of infection in the patients. Preventive administration of antibiotics and monitoring the drug concentration in the blood are important measures to prevent serious complications. Further evaluation is needed to assess the long-term safety and treatment specifications of sirolimus.
Vincristine
Vincristine can inhibit endothelial cell proliferation and can significantly promote apoptosis of vascular endothelial cells and tumor cells [30,31,32,33]. Intravenous vincristine can be used for KMP patients who are not sensitive to steroids. The common regimen for children less than 10 kg is 0.05 mg/kg and 1.5 mg/m2 for more than 10 kg, once every week for 4–6 weeks. Following that, the dose interval can be extended to once per month for six months. The drug onset time, usually 2–4 weeks, is slower than steroids, the effective rate of which is over 80%. It is important to know that vincristine has an inhibitory effect on bone marrow in newborns, but its side effects are mild in infants and young children. Common complications mainly include peripheral neuropathy, abdominal pain, constipation, and mild elevation of liver enzymes in some children. Additionally, vincristine is a foaming agent, extravasation of which may lead to tissue necrosis and secondary infection.
Propranolol
Propranolol is a nonselective ß-adrenergic receptor blocker which has different efficacies in the treatment of KHE compared to the treatment of KHE complicated with KMP. It is only effective in some cases, who can easily recur after 2–3 weeks. Therefore, propranolol has limited efficacy for KMP. The recommended dose for propranolol is 1–2 mg/kg/day divided into 1–2 oral administrations. The dose for patients younger than 1.5 months is 1 mg/kg in one oral administration and is 2 mg/kg divided into two oral administrations with an interval of 6–8 hours for patients older than 1.5 months. Common adverse reactions of propranolol include sinus bradycardia, diarrhea or constipation, sleep changes, hypotension, and hypoglycemia.
Interferon-α (IFN-α)
IFN-α used to be a potential therapy [38,39,40] but is now replaced by other drugs due to concerns about its toxicity. Published studies show that the effective rate of IFN-α in treating KHE is approximately 50%. IFN-α-2a has a therapeutic efficacy similar to IFN-α-2b, with a conventional dose of three million IU/m2 by subcutaneous injection over four weeks. IFN has the risk of causing irreversible spastic diplegia, especially when used in infants under one year old. Therefore, it should be used carefully and monitored closely.
Antiplatelet drugs
Platelet activation and release of pro-angiogenic mediators are critical to the pathogenesis of KMP. As a result, antiplatelet drugs can be used to treat KMP. Aspirin, ticlopidine and dipyridamole can be used alone or in combination [41]. Although it is still controversial to use antiplatelet drugs for treating KHE, there are also successful case reports. The Spanish Society of Pediatric Oncology has included the vincristine-aspirin-ticlopidine (VAT) regimen in the guidelines for KMP treatment [33, 42], in which the dose of vincristine is 0.05 mg/kg and the dose of both aspirin and ticlopidine is 10 mg/kg/d. However, this regimen has not been used in China.
Local compression therapy
Local compression therapy includes elastic compression and air pressure therapy [43,44,45]. Elastic compression uses elastic bandages or specially made pneumatic cuffs lined with cotton pads to continuously apply local pressure in the treatment area. Its possible mechanism is to block some tumor cavities through physical compression and to induce apoptosis through local ischemia and hypoxia. This method is convenient, noninvasive, safe and effective and is suitable for areas that can be compressed, including the limbs, trunk, and scalp. However, insufficient pressure will not be effective, in which too much pressure may cause complications such as local congestion and ischemia. Therefore, pressure control is essential for the efficacy of this treatment which will reduce complications. During pressure treatment using specially made cuffs, the therapeutic pressure is 20–25 mmHg for patients of 1–6 months old, 25–30 mmHg for patients of 7–12 months old, and 30–35 mmHg for patients over 1 year. When an elastic bandage is used for compression treatment, it is best when the blood supply to the hind limb artery is normal and there is mild venous congestion after compression. Mild edema may occur locally after a few days of compression which is a normal reaction. When compression therapy is used for chest lesions, the patient’s breathing, heart rate, complexion, and nursing activity should be closely monitored. In the beginning of compression, the tumor is usually checked once every 2–3 hours until the tumor shrinks, platelets increase, and the blood coagulation indices recover.
Mesh suture
Mesh suture treatment uses an absorbable suture with a needle, which is inserted into the boundary between the periphery of the lesion and the normal skin. The needle penetrates in a curve deep into the base of the lesion and exits through the surface of the lesion skin. The needle is then inserted again at the same point deep into the tumor and exited through boundary between the opposite side of the tumor and normal tissue. Finally, the needle is inserted from the opposite direction into the shallow subcutaneous area of the lesion and exited through the initial starting point. The thread is tightened and knotted. Usually, at one day after the suture, the color on the lesion surface gradually changes from dark purple to light red, the tension decreases, edema is relieved and the skin color gradually returns to normal after 1–2 weeks. This method causes little trauma which is easy to carry out. However, its application is restricted in regions with important nerves and blood vessels and in deep lesions with large areas.
Surgical treatment [46,47,48,49,50]
Surgery is not recommended as the first-line treatment and is only used as a treatment alternative if systemic medication is not effective or the waiting period is long. During the surgery, suture can be performed around the lesion to reduce the blood supply of the tumor to facilitate tumor resection. Surgical resection can also be performed when the platelets return to normal after drug treatment and the tumor shrinks.
Intervenient embolism therapy
Digital subtraction angiography (DSA)-guided intervenient embolism is an important therapeutic strategy when the lesion is extensive, cannot be surgically resected, the drug treatment effect is not obvious, or the coagulation function is not improved and continues to deteriorate [52]. The advantage of intervenient embolism is its rapid onset. Embolization of the blood-supplying artery causes ischemia, degeneration, and necrosis of the tumor so that the tumor shrinks, reducing the capture and destruction of platelets in the tumor. Intervenient embolism also has a good effect on reducing the dose and course of steroid use [51,52,53,54]. Commonly used embolic agents include pingyangmycin (bleomycin), iodine oil, polyvinyl alcohol particles (PVA) and absolute ethanol [51,52,53]. The specific embolization procedure is as follows. After confirming the lesion and the blood-supplying artery by angiography, a microcatheter of 1.7 F or smaller is inserted into the blood supplying artery, and a mixture of 3 mg pingyangmycin (bleomycin) + 3 mg dexamethasone + 2 mL iodine oil + 3–7 mL contrast agent is injected into the artery. The injection pressure is adjusted according to the tumor range. The embolic agent is injected in bolus. Injection is stopped when the embolic agent is refluxed. The position of the microcatheter is kept unchanged. And PVA is injected to further embolize the blood supply artery. If the efficacy is poor, vincristine (0.050–0.065 mg/kg) can be infused transarterially [53]. Postoperative complications include blistering, ulceration, and tumor necrosis of the lesion, which can be healed by local dressing. It is rare to have myelosuppression, neurotoxicity, and serious complications involved in heart, brain, and lung [52, 53].
Ablation therapy
Ablation includes cryoablation [54] and radio frequency ablation [55]. Ablation therapy achieves the effect similar to surgical resection through the action of cold and heat which is especially suitable for deep lesion. It can be used as an alternative treatment when other treatments are ineffective. However, the ablation damage is not tissue selective. And its application is restricted in positions with important nerves and blood vessels.
Blood product infusion
Blood products include fresh frozen plasma, cryoprecipitate, and fibrinogen. Clinical observation shows that some patients have low platelet tolerance. These patients do not have skin purpura when the platelets are below the level of 10 × 109/L. It is currently believed that frequent infusion of platelets may aggravate the pathological process of KMP [42, 56, 57]. Therefore, platelet levels should not be used as the only reference standard for blood transfusions. Platelet transfusion is not recommended under the following situations: fibrinogen is less than 1 g/L; skin or visceral bleeding occurs. At this point cryoprecipitate, fresh frozen plasma, and concentrated fibrinogen can be infused.
Treatment selection
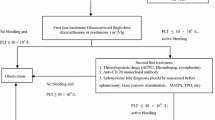
If the disease condition allows, a biopsy should be acquired prior to treatment to confirm the diagnosis. The treatment principle during the coagulation impairment phase is to confirm diagnosis and start treatment as early as possible to control lesion progression, correct coagulation disorders, and prevent the occurrence of DIC. Individualized, stepwise, sequential therapy should be adopted according to the age, lesion location, tumor area, and the extent of thrombocytopenia and coagulation dysfunction. It is recommended to first use systemic drug treatment or noninvasive compression therapy for the extremities, trunk, and scalp and to observe changes in the disease condition. Glucocorticoids, local compression therapy, or mesh sutures are recommended as first-line regimens. If these treatments are ineffective, rapamycin alone or in combination with glucocorticoids, or glucocorticoids together with vincristine can be used as a second-line regimen. The appropriate treatment method can be selected according to the noninvasive–minimally invasive–invasive treatment principle. Coagulation disorders should be closely monitored and corrected during the treatment, including appropriate use of blood products and symptomatic supportive care (Fig. 1).
The key to the residual lesion phase is to prevent functional impairment and improve appearance. The “cure” of KMP lesions is not completely permanent [58] and is characterized by progressive fibrosis. Residual lesion in the muscles, bones, or joints will lead to joint contracture or subluxation, muscle atrophy, scoliosis, and limited mobility, among which the most common symptoms include local pain, itching, excessive sweating, and limb edema. The disease condition may aggravate by rapidly developing the stage during puberty, after infection or trauma, and in the period after surgery [59]. It is recommended that children with KMP be followed up at least once per year to monitor pain and recurrence. Local injection of suspension glucocorticoids or surgical correction should be performed when necessary.
Conclusions
This review article is a summary of the experience of KMP experts and provides standardized recommendations for the pathogenesis, clinical manifestation, diagnostic methods and treatment process of KMP, which can be used as a reference for clinical practice.
References
Kasabach HH, Merritt KK. Capillary hemangioma with extensive purpura: report of a case. Arch Pediatr Adolesc Med. 1940;59:1063–70.
Zukerberg LR, Nickoloff BJ, Weiss SW. Kaposiform hemangioendothelioma of infancy and childhood. An aggressive neoplasm associated with Kasabach-Merritt syndrome and lymphangiomatosis. Am J Surg Path. 1993;17:321–8.
Enjolras O, Wassef M, Mazoyer E, Frieden IJ, Rieu PN, Drouet L, et al. Infants with Kasabach-Merritt syndrome do not have “true” hemangiomas. J Pediatr. 1997;130:631–40.
Sarkar M, Mulliken JB, Kozakewich HP, Robertson RL, Burrows PE. Thrombocytopenic coagulopathy (Kasabach-Merritt phenomenon) is associated with Kaposiform hemangioendothelioma and not with common infantile hemangioma. Plast Reconstr Surg. 1997;100:1377–86.
Sun YH, Che ZG, Zheng JW. 2018 newly revised ISSVA classification for vascular anomalies. China J Oral Maxillofac Surg. 2019;17:13–9 (in Chinese).
Croteau SE, Liang MG, Kozakewich HP, Alomari AI, Fishman SJ, Mulliken JB, et al. Kaposiform hemangioendothelioma: atypical features and risks of Kasabach-Merritt phenomenon in 107 referrals. J Pediatr. 2013;162:142–7.
Seo SK, Suh JC, Na GY, Kim IS, Sohn KR. Kasabach-Merritt syndrome: identification of platelet trapping in a tufted angioma by immunohistochemistry technique using monoclonal antibody to CD61. Pediatr Dermatol. 1999;16:392–4.
Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, Stockinger H, et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115:3997–4005.
Suzukiinoue K, Kato Y, Inoue O, Olcaydu D, Chrenek P, Stockinger H, et al. Involvement of the snake toxin receptor CLEC-2, in Podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993–6001.
O'Rafferty C, O'Regan GM, Irvine AD, Smith OP. Recent advances in the pathobiology and management of Kasabach-Merritt phenomenon. Br J Haematol. 2015;171:38–51.
Drolet BA, Trenor CC, Brandão LR, Chiu YE, Chun RH, Dasgupta R, et al. Consensus-derived practice standards plan for complicated Kaposiform hemangioendothelioma. J Pediatr. 2013;163:285–91.
Rodriguez V, Witman PM, Anderson PA. Kasabach-Merritt phenomenon: case series and retrospective review of the mayo clinic experience. J Pediatr Hematol Oncol. 2009;31:522–6.
Adams DM, Trenor CC, Hammill AM, Vinks AA, Patel MN, Chaudry G, et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. 2016;137:1–10.
Nadal M, Giraudeau B, Tavernier E, Almeida R, Caldeira D, Rosa M, et al. Efficacy and safety of mammalian target of rapamycin Inhibitors in vascular anomalies: a systematic review. Acta Derm Venereol. 2016;96:448–52.
Zhou DK. Hemangioma and vascular malformation. In: Dong Q, Jing Q, Gao JC, editors. Pediatric surgical oncology. Beijing: People's Medical Publishing House; 2009. p. 271–86 (in Chinese).
Zhou DK, Liu WY, Liu Q. Hemangioma and vascular malformation in children. In: Jing XQ, Shi CR, editors. Guidelines for diagnosis and treatment of solid tumors in children. Beijing: People's Medical Publishing House; 2011. p. 154–175 (in Chinese).
Wang S. Hemangioma and vascular malformation. In: Cai W, Sun N, Wei GH, editors. Pediatric Srugery,5th edition. Beijing: People's Medical Publishing House; 2014. p. 126–135 (in Chinese).
Ren SL, Qi HY. Retrospective analysis of Kasabach-Merritt phenomenon: a report of 39 cases. Chin J Pediatr Surg. 2018;39:757–61 (in Chinese).
Schmid I, Klenk AK, Sparber-Sauer M, Koscielniak E, Maxwell R, Häberle B. Kaposiform hemangioendothelioma in children: a benign vascular tumor with multiple treatment options. World J Pediatr. 2018;14:322–9.
Gruman A, Liang MG, Mulliken JB, Fishman SJ, Burrows PE, Kozakewich HP, et al. Kaposiform hemangioendothelioma without Kasabach-Merritt phenomenon. J Am Acad Dermatol. 2005;52:616–22.
Wong BLK, Lee VNY, Tikka T, Kim D, Dwivedi RC. Kaposiform haemangioendothelioma of the head and neck. Crit Rev Oncol Hematol. 2016;104:156–68.
Chinello M, Di Carlo D, Olivieri F, Balter R, De Bortoli M, Vitale V, et al. Successful management of Kaposiform hemangioendothelioma with long-term sirolimus treatment: a case report and review of the literature. Mediterr J Hematol Infect Dis. 2018;10:e2018043.
Mazoyer E, Enjolras O, Laurian C, Houdart E, Drouet L. Coagulation abnormalities associated with extensive venous malformations of the limbs: differentiation from Kasabach-Merritt syndrome. Clin Lab Haematol. 2002;24:243–51.
Mulliken JB, Anupindi S, Ezekowitz RA. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 13–2004 A newborn girl with a large cutaneous lesion, thrombocytopenia, and anemia. N Engl J Med. 2004;350:1764–75.
Yao W, Li K, Wang Z, Pei J, Liu X, Zheng S, et al. Comparison of corticosteroid and vincristine in treating Kaposiform hemangioendothelioma and tufted angioma: a systematic review and meta-analysis. Eur J Pediatr Surg. 2019;29:401–7.
Kim T, Roh MR, Cho S, Chung KY. Kasabach-merritt syndrome arising from tufted angioma successfully treated with systemic corticosteroid. Ann Dermatol. 2010;22:426–30.
Arunachalam P, Kumar VR, Swathi D. Kasabach-Merritt syndrome with large cutaneous vascular tumors. J Indian Assoc Pediatr Surg. 2012;17:33–6.
Triana P, Dore M, Cerezo VN, Cervantes M, Sánchez AV, Ferrero MM, et al. Sirolimus in the treatment of vascular anomalies. Eur J Pediatr Surg. 2017;27:86–90.
Wang HJ, Guo XK, Duan YT, Zheng BJ, Gao Y. Sirolimus as initial therapy for Kaposiform hemangioendothelioma and tufted angioma. Pediatr Dermatol. 2018;35:635–8.
Haisley-Royster C, Enjolras O, Frieden IJ, Garzon M, Lee M, Oranje A. Kasabach-merritt phenomenon: a retrospective study of treatment with vincristine. J Pediatr Hematol Oncol. 2002;24:459–62.
Fahrtash F, Mccahon E, Arbuckle S. Successful treatment of kaposiform hemangioendothelioma and tufted angioma with vincristine. J Pediatr Hematol Oncol. 2010;32:506–10.
Wang Z, Li K, Yao W, Dong K, Xiao X, Zheng S. Steroid-resistant kaposiform hemangioendothelioma: a retrospective study of 37 patients treated with vincristine and long-term follow-up. Pediatr Blood Cancer. 2015;62:577–80.
Fernandez-Pineda I, Lopez-Gutierrez JC, Chocarro G, Bernabeu-Wittel J, Ramirez-Villar GL. Long-term outcome of vincristine-aspirin-ticlopidine (VAT) therapy for vascular tumors associated with Kasabach-Merritt phenomenon. Pediatr Blood Cancer. 2013;60:1478–81.
Radović SV, Kolinović M, Ljubić D. Propranolol in the preoperative treatment of Kasabach-Merritt syndrome: a case report. J Med Case Rep. 2017;11:308.
Hermans DJ, van Beynum IM, van der Vijver RJ, Kool LJ, de Blaauw I, van der Vleuten CJ. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:e171–3.
Mizutani K, Umaoka A, Tsuda K, Kakeda M, Habe K, Yamanaka K, et al. Successful combination therapy of propranolol and prednisolone for a case with congenital Kasabach-Merritt syndrome. J Dermatol. 2017;44:1389–91.
Chiu YE, Drolet BA, Blei F, Carcao M, Fangusaro J, Kelly ME, et al. Variable response to propranolol treatment of kaposiform hemangioendothelioma, tufted angioma, and Kasabach-Merritt phenomenon. Pediatr Blood Cancer. 2012;59:934–48.
Acharya S, Pillai K, Francis A, Criton S, Parvathi VK. Kasabachmerritt syndrome: management with interferon. Indian J Dermatol. 2010;55:281–3.
Deb G, Jenkner A, De Sio L, Boldrini R, Bosman C, Standoli N, et al. Spindle cell (Kaposiform) hemangioendothelioma with Kasabach-Merritt syndrome in an infant: successful treatment with alpha-2A interferon. Med Pediatr Oncol. 1997;28:358–61.
Duclaux-Loras R, Lachaux A, Guibaud L, Bertrand Y. Is alfa-interferon still current in the management of Kasabach-Merritt syndrome. Arch Pediatr. 2015;22:523–7.
O’Rafferty G, O’Regan GM, Irvine AD, Smith OP. Recent advances in the pathobiology and management of Kasabach-Merritt phenomenon. Br J Haematol. 2015;171:38–51.
Vivascolmenares GV, Ramirezvillar GL, Bernabeuwittel J, Matute de Cardenas JA, Fernandez-Pineda I. The importance of early diagnosis and treatment of Kaposiform hemangioendothelioma complicated by Kasabach-Merritt phenomenon. Dermatol Prac Concept. 2015;5:91–3.
Qin ZP, Ren L, Liu XJ, Wu B, Liu XJ, Li KL. Treatment of pediatric hemangioma by barometric therapy. Chin J Surg. 2001;39:93–4 (in Chinese).
Liu XJ, Tai MZ, Tian MX, Qin ZP. Clinical analysis of 17 eases of pneumatic compression therapy in infants with Kasabach-Merritt phenomenon. Natl Med J China. 2009;89:1830–3 (in Chinese).
Li K, Tai M, Qin Z, Ge C. Clinical observations in mesh suture treatment for infants of Kasabach-Merritt phenomenon. J Paediatr Child Health. 2015;51:529–33.
Drolet BA, Scott LA, Esterly NB, Gosain AK. Early surgical intervention in a patient with Kasabach-Merritt phenomenon. J Pediatr. 2001;138:756–8.
Leung M, Chao NS, Tang PM, Liu K, Chung K. Pancreatic Kaposiform hemangioendothelioma presenting with duodenal obstruction and Kasabach-Merritt phenomenon: a neonate cured by whipple operation. European J Pediatr Surg Rep. 2014;2:7–9.
Pascal S, Bettex Q, Andre N, Petit P, Casanova D, Degardin N. Successful surgical manageent of congenital Kasabach-Merritt syndrome. Pediatr Int. 2017;59:89–92.
Lei HZ, Huang J, Meng XF, Ma YC, Sun B, Qiao JB, et al. Management of Kasabach-Merritt syndrome by drug therapy and surgery. Chin J Plast Surg. 2013;29:104–8 (in Chinese).
Zou R, Peng F, Yu T, Zeng S, You Y, Chen K, et al. Kasabach-Merritt syndrome combined with hypercalcemia: a case repor. Exp Ther Med. 2017;14:6164–8.
Su L, Wang D, Fan X. Comprehensive therapy for hemangioma presenting with Kasabach-Merritt syndrome in the maxillofacial region. J Oral Maxillofac Surg. 2015;73:92–8.
Zhou SY, Zhang J. Transarterial embolization in treatment of Kasabach Merritt Syndrome. Chin J Interv Imaging Ther. 2014;11:415–8 (in Chinese).
Tan XY, Zhang J, Zhou SY, Sheng G, Liu L, Chen KS, et al. Successful treatment of steroid-resistant Kasabach-Merritt syndrome in infants with transcatheter arterial scleroembolization and vincristine. Chin J Radio. 2014;48:934–7 (in Chinese).
Thompson SM, Callstrom MR, McKusick MA, Woodrum DA. Initial results of image-guided percutaneous ablation as second-line treatment for symptomatic vascular anomalies. Cardiovasc Intervent Radiol. 2015;38:1171–8.
Ge CX, Chen T, Tai MZ, Li KL, Qin ZP. Percutaneous radiofrequency ablation for children with Kasabach -Merritt phenomenon: a preliminary report. China J Oral Maxillofac Surg. 2019;17:175–80 (in Chinese).
Phillips WG, Marsden JR. Kasabach-Merritt syndrome exacerbated by platelet transfusion. J R Soc Med. 1993;86:231–2.
Korsaga-Somé N, Maruani A, Abdo I, Favrais G, Lorette G. Kasabach-Merritt phenomenon (KMP) exacerbated by platelet transfusions. Ann Dermatol Venereol. 2015;142:578–80.
Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105–24.
Schaefer BA, Wang D, Merrow AC, Dickie BH, Adams DM. Long-term outcome for kaposiform hemangioendothelioma: a report of two cases. Pediatr Blood Cancer. 2016;64:284–6.
Funding
None.
Author information
Authors and Affiliations
Contributions
YW and LKL contributed equally to this paper. They design, acquisition, analysis and draft the manuscript; QZP contributed to conception, design, acquisition data of the manuscript; LK contributed to conception, design, acquisition data of the manuscript; ZJW contributed to conception, design, acquisition data of the manuscript; FXD contributed to revise the manuscript contributed to acquisition data of the manuscript; ML contributed to analysis and interpretation of data and revise the manuscript; ZDK contributed to analysis and interpretation of data and revise the manuscript; LXJ contributed to acquisition data of the manuscript; WL contributed to acquisition data of the manuscript; LL contributed to acquisition data of the manuscript; TMZ contributed to acquisition data of the manuscript; WJH contributed to acquisition data of the manuscript; JY contributed to analysis and interpretation of data; ZL contributed to analysis and interpretation of data; HHJ contributed to analysis and interpretation of data; GXY contributed to analysis and interpretation of data; HZJ contributed to analysis and interpretation of data; GS contributed to analysis and interpretation of data; YHY contributed to analysis and interpretation of data.
Corresponding author
Ethics declarations
Ethical approval
Not required for this review paper.
Conflict of interest
No financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yao, W., Li, KL., Qin, ZP. et al. Standards of care for Kasabach−Merritt phenomenon in China. World J Pediatr 17, 123–130 (2021). https://doi.org/10.1007/s12519-020-00379-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-020-00379-9