Abstract
Background
Sleep undergoes changes from birth to adulthood, while sleep disorders are associated with various cognitive deficiencies in childhood. In parallel, prematurity is known to predispose to poor neurodevelopmental outcomes. Our aim is to provide literature data about factors influencing sleep in the premature infants and sleep outcomes in this population.
Methods
A systematic review was conducted using a variety of health-related databases. Original research papers were considered and no year-of-publication restriction was placed.
Results
In total, 22 articles fulfilled our selection criteria. Available studies present remarkable heterogeneity in terms of methodological design. Compared to full term, premature infants exhibit significant differences in sleep structure, which mainly include differences in electroencephalographic spectral values, in total sleep time and in arousal threshold. Furthermore, prematurity seems to be a risk factor of sleep breathing disorders in childhood and adolescence. Data about the effect of methylxanthines and the environment of neonatal intensive care unit is controversial. With regard to the impact of prematurity-related sleep disorders on future neurodevelopment, available research papers are generally few.
Conclusions
The alterations in sleep patterns are an outcome of prematurity (immaturity of nervous system) as well as of postnatal factors and comorbidities. Sleep problems in this population of infants seems to be a missing piece of the puzzle of impaired neurodevelopment. Future studies should focus on interventions to improve sleep hygiene and limit neurodevelopmental problems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Disruption of the normal fetal organogenesis due to preterm birth (< 37+0 weeks) leads to a variety of multisystem morbidities and long-term consequences, even in adult life [1]. Prematurity (even moderate or late) has also been increasingly associated with negative neurodevelopmental outcomes, including cognitive deficits, learning difficulties and behavioral problems [2]. The disruption of normal brain growth and development is the obvious pathophysiological mechanism. Series of factors (e.g., male gender, low birth weight, intraventricular hemorrhage, bronchopulmonary dysplasia, preeclampsia, and low socioeconomic status) have been identified as independent predictors of disturbed neurodevelopment, too. At the same time, there are studies highlighting the significance of postnatal environmental factors (e.g., educational level of parents, breastfeeding) [3,4,5]. Genetic associations with atypical neurodevelopmental outcomes in preterm infants have also been considered and several candidate genes have been described [6]. As the rate of “preterm survivors” is continuously increasing, the investigation of additional factors correlated with impaired neurodevelopment (especially modifiable factors) is of great clinical significance, with sleep function being one of them.
It is well established that sleep is the predominant behavioral state in newborns and is essential for normal brain development throughout childhood, particularly for memory and learning functions [7]. More specifically, human and animal models studies have shown that sleep spindles during non-rapid eye movement (NREM) sleep are correlated with cognitive aptitude of the child, while slow oscillations promote myelin formation and anatomical connectivity of different brain parts [8,9,10]. With regard to rapid eye movement (REM) sleep, sensory feedback from myoclonic twitches during this sleep stage can trigger central neural oscillations, which in turn, promote neurodevelopmental processes (e.g., synapse formation, neuronal differentiation and migration) and permit functional connectivity in developing brain networks [11]. In other words, slow wave sleep promotes declarative memory, whereas REM stage mainly enhances procedural learning tasks. On the other hand, according to epidemiological studies, sleep deprivation or disturbed sleep is associated with significant morbidity and also predisposes to a variety of somatic and psychosocial disorders [12,13,14]. At the same time, it should be highlighted that sleep undergoes progressive changes during intrauterine and extrauterine life, which strongly depend on gestational age [15, 16] (Fig. 1).
Taking into consideration all the above mentioned, it is plausible to speculate that potential alterations in sleep structure induced by prematurity may contribute to the appearance of neurodevelopmental problems in this population (Fig. 2). Until now, this assumption has not been extensively investigated. Therefore, the aim of our study is to systematically provide literature data about (1) factors influencing sleep in the premature infants and (2) sleep outcomes in premature infants.
Literature search
Eligibility criteria
Studies fulfilling the following criteria were selected: (1) original research papers; (2) human studies conducted in preterm infants; (3) preclinical studies conducted in animal models of prematurity; (4) studies investigating factors influencing sleep in the premature infants; and (5) studies investigating sleep outcomes in premature infants.
Search strategy and study selection
A comprehensive search was undertaken using health-related databases: Pubmed, Embase, Scopus, Cochrane, and Web of Science. The literature search and the eligibility assessment process were performed by two reviewers independently in all stages. No year-of-publication restriction was placed. The terms that were used were: “sleep” AND [“prematurity” OR “preterm”] AND [“neurodevelopment” OR “brain development” OR “behavioral problems” OR “cognitive deficits” OR “learning disorders”].
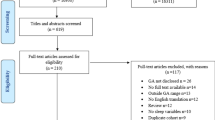
Two reviewers undertook the selection process independently. The search strategy was common for both reviewers. After searching the literature, data were abstracted and selected articles were scanned to eliminate studies with irrelevant topic, inappropriate methodology or duplicate records. A third person evaluated bias risk of studies and addressed disagreements between two reviewers. Figure 3 illustrates the flow chart of how the articles were selected.
According to our results, we have identified 22 studies fulfilling our selection criteria: 11 studies about factors influencing sleep in preterm infants (Tables 1, 2, 3) and 11 studies about sleep outcomes (Tables 4, 5). Assessment of risk of bias in studies selected was performed using the ROBINS-I tool [17]. Sources of risk of bias included confounding, selection, classification of interventions, deviation from intended interventions, missing data, measurement of outcomes, and selection of the reported results (Table 6).
Factors influencing sleep in premature infants
The role of drugs
Comorbidities associated with prematurity often demand specific pharmacological treatment. Theophylline and caffeine are widely used in preterm infants as respiratory stimulants to decrease apnea of prematurity, as they increase minute ventilation, improve CO2 sensitivity, enhance diaphragmatic activity, and reduce periodic breathing. Their mechanism of action includes antagonism of adenosine receptors phosphodiesterase inhibition and modification of dopamine binding activity at its receptors [18]. We were able to find only two studies (case–control studies) describing effects of methylxanthines on sleep structure in preterm infants [19, 20] (Table 1).
Results of these studies are conflicting. Thoman et al. report more non-alert waking activity, more drowsiness, and less active sleep among preterm infants who had received theophylline in comparison to preterm infants who had not received theophylline and normal full-term infants. It is noteworthy that the administration of theophylline had been discontinued at least 1 month prior to the beginning of the study. However, data acquisition was based on parental observations at home and no objective tools were used, a fact that reduces the integrity of the methodology. Moreover, gestational age was different between study groups, a fact that further limits the validity of the results [19]. On the other hand, Marcus et al. do not describe any significant long-term effects on sleep structure in ex-preterm infants who had been treated with caffeine after polysomnographic and actigraphic assessments at the age of 5–12 years. Nevertheless, lack of significance in this study could be due to the apparent discrepancy in total recording and sleep time between the caffeine and the placebo group [20]. Taking into account the limitations of the above two studies, no clear conclusions can be drawn with regard to the long-term impact of methylxanthines on sleep of premature infants.
The role of Neonatal Intensive Care Unit (NICU) environment
Hospitalization in an NICU is associated with a wide range of stimuli (e.g., painful experiences, and noise, light) and interventions that could potentially fragment exert a negative effect on the structure and quality of sleep in a neonate [21]. According to Zores et al. [22], changes in light (even small changes) can cause significantly more awakenings and this difference seems to be negatively correlated with the extent of the light protection in the incubator. However, a different study including less preterm infants and conducted in intermediate care unit showed that only high light levels actually affected neonatal sleep increasing wakefulness [23]. In parallel, apneic events were found to be more frequent in warm incubator conditions and also be closely related to body heat loss than body temperature [24]. Furthermore, moderate acoustic changes can disrupt sleep in very preterm infants [25]. With regard to other factors associated with NICU stay, no significant associations between phototherapy and changes in sleep structure in preterm infants have been identified in literature [26, 27] (Table 2).
Respiratory complications
Prematurity is the underlying cause of a series of pulmonary complications including apnea of prematurity and bronchopulmonary dysplasia. It is well-established that apnea of prematurity has a direct effect on sleep, as it increases arousals and can also predispose to sudden infant death syndrome [28]. On the other side, we have identified two retrospective studies linking bronchopulmonary dysplasia to sleep breathing disorders [29, 30]. More specifically, according to Ortiz et al. [29], premature infants with bronchopulmonary dysplasia exhibit a significantly higher respiratory disturbance index, especially those with more severe disease and those exposed to smoke. Furthermore, Sekar et al. [30] have shown that bronchopulmonary dysplasia predisposed to central respiratory instability in premature infants after weaning from mechanical ventilation. We were also able to find one randomized controlled trial, showing that the type of respiratory support can exert a significant effect on sleep structure of premature neonates; neonates receiving respiratory support via heated humidified high flow nasal cannulae spent less time in sleep in comparison to those receiving nasal continuous positive airway pressure [31] (Table 3). However, no other studies with the same finding have been identified.
Sleep outcomes in preterm infants
A series of studies have identified alterations in sleep outcomes (electroencephalographic or respiratory) in premature infants when compared to full-term infants. In total, we were able to find eight cross-sectional retrospective human studies conducted in infants or children with a history of prematurity and investigating changes in sleep electroencephalographic outcomes [32,33,34,35,36,37,38,39] (Table 4). Methods of sleep patterns assessment used by researchers were extended electroencephalographic recordings, overnight polysomnography, and actigraphy. The main outcomes of the aforementioned studies included a variety of sleep measures: total sleep duration, sleep efficiency, time spent in different sleep stages, electroencephalographic spectral values, arousal threshold, motor activity during sleep, total activity score, sleep chronotype.
According to their results, prematurity seems to exert a strong effect on maturation of sleep function. More specifically, children born preterm exhibited altered electroencephalographic spectral values, irregular sleep schedules (i.e., advanced sleep phase), increased motor activity during sleep, reduced sleep duration, and lower arousal threshold [32,33,34,35,36,37,38,39]. Differences in electroencephalographic rhythms could be either sleep stage-related or restricted to specific brain regions. Furthermore, Scher et al. [40] have interestingly shown that electroencephalographic sleep changes are most likely associated with prematurity rather than postnatal brain adaptation. It is also worth-mentioning that in 4 of the above studies, prematurity was associated with significant differences in sleep function even beyond infancy at older ages (e.g., in school-aged children, adolescents, or even young adults) [36,37,38,39] (Table 4). This finding underscores the long-term consequences that premature birth can have on sleep organization. With regard to circadian rhythm disorders, there is evidence that earlier sleep phase encountered in very preterm (born < 32nd gestational week) children can be due to possible down-regulation of hypothalamic–pituitary–adrenal axis activity [41]. Moreover, from a clearly clinical point of view, extremely preterm infants (even those with no signs of neurodevelopmental disabilities) exhibited significantly different sleep habits and behaviors at 11 years of age when compared to term-born controls [42]. On the other hand, Iglowstein et al. [43] have not detected any changes in sleep behaviors (e.g., bedsharing, night awakenings, bedtime resistance, and sleep-onset difficulties) between preterm and term children from birth to age of 10 years.
With regard to respiratory sleep outcomes, prospective and retrospective studies have revealed that prematurity has been found to be a significant predictor of sleep breathing disorders (e.g., prevalence of Obstructive Sleep Apnea Syndrome) even in older ages (i.e., school-aged children); multiple gestations and chorioamnionitis seem to further increase this risk [44,45,46] (Table 5).
It should be highlighted that all the aforementioned studies exhibit remarkable heterogeneity in terms of their methodology and design (e.g., way of sleep assessment, sample size, degree of prematurity, and birth weight). For this reason, their conclusions cannot be safely generalized. Indeed, it seems that preterm birth disturbs sleep. However, at what age and until what age? The answer is unclear, as child’s age during sleep assessment varies between different studies. Nevertheless, current available data show that prematurity can be an underlying cause of (probably long-lasting) sleep-related disorders. In other words, with regard to sleep function we could say that “the early bird does not seem to catch the worm”.
Future neurodevelopment and interventions
We were able to identify in the literature only three studies (two retrospective and one prospective) focusing on the long-term effect of sleep disorders in premature neonates on future neurodevelopment [47,48,49] (Table 7). Sample sizes ranged from 15 to 65 infants, control group was used in 1 of them, age of neurodevelopmental assessment ranged from 4 to 24 months, and their outcomes presented heterogeneity, including mental/social/motor scores, first gaze duration, and distraction episodes. It is also worth-mentioning that degree of prematurity was different in all these three studies (< 32 weeks, 30–35 weeks, < 37 weeks). According to their results, poor sleep (in terms of sleep efficiency) was associated with problems in attention orienting and distractibility disorders at 4 and 12 months of age, while low spectral beta electroencephalographic energies at neonatal age predicted lower mental scores at 12 months of age [47,48,49]. In the study by Bandyopadhyay et al. [47], high values of end-tidal CO2 during sleep (> 45 mm Hg) were positively correlated with low cognitive scores at the age of 2 years, although obstructive sleep events did not have a significant impact on neurodevelopment. These findings show that sleep disorders in premature neonates could negatively affect the development of their cognitive functions and further enhance the negative impact of prematurity on future neurodevelopment. Nevertheless, the magnitude of this effect should further investigated in more and larger prospective studies.
Although prematurity is sometimes unavoidable, there are interventions which can provide preterm infants with a favorable antenatal environment and rescue sleep function and future neurodevelopment. Recent studies using videotaping of sleep–wake states of preterm infants in NICU have revealed that supine position predisposes to more frequent awakenings, whereas prone position (under close supervision) facilitates sleep reducing at the same time stress levels [50]. Furthermore, Jarus et al. report a greater variety of sleep patterns (e.g., deep or light sleep and drowsiness) in prone position, while in supine position, awake patterns (quiet or awake, agitated fussy) dominated. In the same study, the prone position was found to have a positive neurodevelopmental impact, as it was associated with more approach reactions in contrast to withdrawal reactions while in supine position [51]. On the other side, it should also be mentioned that according to polysomnographic recordings, prone position seems to shift respiratory events during sleep from obstructive to central apneas, although the importance of this finding has not been clarified, yet [52].
In parallel, skin-to-skin contact with the mother has been found to decrease arousals during sleep and also promotes sleep organization while nesting and swaddling seem to increase total and quiet sleep time when applied to preterm infants [53, 54]. With regard to other interventions, cycled light simulates a day–night environment and increases total sleep time. Sleep duration and active sleep efficiency are also increased by mattresses interventions, which permit a similar position as in the uterus [55, 56]. Earmuffs use has also been found to reduce the level of noise in NICUs and improve preterm neonates’ light sleep stability, while quiet time is a nursing intervention, which increases total sleep time, too [57, 58]. On the other hand, cobedding, music interventions, swaddled tub baths, and non-nutrition sucking were not found to have any significant effects. It is clear that there are plenty of modifiable factors in the NICU environment that can affect infants’ sleep structure and hygiene [59, 60]. More randomized clinical trials are undoubtedly needed to confirm the impact of such non-pharmacological interventions on sleep stabilization of preterm infants.
Future research
Given the burden of prematurity along with poor neurodevelopmental outcomes on quality of life, the identification of modifiable risk factors is a crucial topic of future research. More and larger prospective clinical studies are needed to assess the true effect of drugs, environmental stressors in NICU, and medical interventions (e.g., ventilatory support) on sleep structure. Mapping of pathways of sleep maturation could also permit a better prediction of disorders in sleep function induced by prematurity, as well as early and targeted implementation of therapeutic strategies. Furthermore, long-term neurodevelopmental outcomes of ex-preterm children with sleep disorders need to be further investigated and the follow-up period of these patients needs to be extended to older ages, so that neurodevelopmental complications are placed in their true dimensions.
Research interest has also emerged in the field of non-pharmacological interventions in NICU that could improve sleep quality of preterm infants and reverse negative impact on brain development. Therefore, the effects of cycled light, skin-to-skin contact and position during sleep should be further analyzed, as they consist simple, safe, and costless measures that can be easily taken in any NICU.
Conclusions
Sleep function exhibits a variety of special features at the early extreme of age and undergoes significant progressive changes across infancy and childhood. Immaturity of nervous system induced by preterm birth along with postnatal factors and comorbidities seem to have an effect on sleep outcomes of this population of infants. Nevertheless, the real burden of prematurity-related sleep disorders on future brain development is yet to be clarified. Future research needs to search ways to alleviate any negative impact of preterm birth on sleep maturation and help neonatal brain to reach the maximum potential of its neuroplasticity and its competencies.
References
Roggero P, Giannì ML, Garbarino F, Mosca F. Consequences of prematurity on adult morbidities. Eur J Intern Med. 2013;24:624–6.
Bröring T, Oostrom KJ, van Dijk-Lokkart EM, Lafeber HN, Brugman A, Oosterlaan J. Attention deficit hyperactivity disorder and autism spectrum disorder symptoms in school-age children born very preterm. Res Dev Disabil. 2018;74:103–12.
Johnson S, Evans TA, Draper ES, Field DJ, Manktelow BN, Marlow N, et al. Neurodevelopmental outcomes following late and moderate prematurity: a population-based cohort study. Arch Dis Child Fetal Neonatal Ed. 2015;100:301–8.
Candel-Pau J, Perapoch López J, Castillo Salinas F, Sánchez Garcia O, Pérez Hoyos S, Llurba Olivé E. Neurodevelopment in preterm infants with and without placenta-related intrauterine growth restriction and its relation to perinatal and postnatal factors. J Matern Fetal Neonatal Med. 2016;29:2268–74.
Vieira ME, Linhares MB. Developmental outcomes and quality of life in children born preterm at preschool- and school-age. J Pediatr (Rio J). 2011;87:281–91.
Blair LM, Pickler RH, Anderson C. Integrative review of genetic factors influencing neurodevelopmental outcomes in preterm infants. Biol Res Nurs. 2016;18:127–37.
Hill CM, Hogan AM, Karmiloff-Smith A. To sleep, perchance to enrich learning? Arch Dis Child. 2007;92:637–43.
Del Rio-Bermudez C, Blumberg MS. Active sleep promotes functional connectivity in developing sensorimotor networks. Bioessays. 2018;40:1700234.
Clawson BC, Durkin J, Aton SJ. Form and function of sleep spindles across the lifespan. Neural Plast. 2016;2016:6936381.
Kurth S, Olini N, Huber R, LeBourgeois M. Sleep and early cortical development. Curr Sleep Med Rep. 2015;1:64–73.
Kurth S, Riedner BA, Dean DC, O’Muircheartaigh J, Huber R, Jenni OG, et al. Traveling slow oscillations during sleep: a marker of brain connectivity in childhood. Sleep. 2017. https://doi.org/10.1093/sleep/zsx121.
Simola P, Liukkonen K, Pitkaranta A, Pirinen T, Aronen ET. Psychosocial and somatic outcomes of sleep problems in children: a 4-year follow-up study. Child Care Health Dev. 2014;4:60–7.
Touchette E, Cote SM, Petit D, Liu X, Boivin M, Falissard B, et al. Short nighttime sleep-duration and hyperactivity trajectories in early childhood. Pediatrics. 2009;124:985–93.
Gosselin N, Baril AA, Osorio R, Kaminska M, Carrier J. Obstructive sleep apnea and the risk of cognitive decline in older adults. Am J Respir Crit Care Med. 2019;199:142–8.
MacLean JE, Fitzgerald DA, Waters KA. Developmental changes in sleep and breathing across infancy and childhood. Paediatr Respir Rev. 2015;16:276–84.
Urquhart DS, Tan HL. Sleep disordered breathing at the extremes of age: infancy. Breathe (Sheff). 2016;12:1–11.
Hinneburg I. ROBINS-1: a tool for asssessing risk of bias in non-randomised studies of interventions. Med Monatsschr Pharm. 2017;40:175–7.
Funk GD. Losing sleep over the caffeination of prematurity. J Physiol. 2009;587:5299–300.
Thoman EB, Davis DH, Raye JR, Philipps AF, Rowe JC, Denenberg VH. Theophylline affects sleep–wake state development in premature infants. Neuropediatrics. 1985;16:13–8.
Marcus CL, Meltzer LJ, Roberts RS, Traylor J, Dix J, D’ilario J, et al. Long-term effects of caffeine therapy for apnea of prematurity on sleep at school age. Am J Respir Crit Care Med. 2014;190:791–9.
Bertelle V, Sevestre A, Laou-Hap K, Nagahapitiye MC, Sizun J. Sleep in the neonatal intensive care unit. J Perinat Neonatal Nurs. 2007;21:140–8.
Zores C, Dufour A, Pebayle T, Dahan I, Astruc D, Kuhn P. Observational study found that even small variations in light can wake up very preterm infants in a neonatal intensive care unit. Acta Paediatr. 2018;107:1191–7.
Orsi KC, Avena MJ, de Cacia Lurdes, Pradella-Hallinan M, da Luz Gonçalves Pedreira M, Tsunemi MH, Machado Avelar AF, et al. Effects of handling and environment on preterm newborns sleeping in incubators. J Obstet Gynecol Neonatal Nurs. 2017;46:238–47.
Tourneux P, Cardot V, Museux N, Chardon K, Léké A, Telliez F, et al. Influence of thermal drive on central sleep apnea in the preterm neonate. Sleep. 2008;31:549–56.
Kuhn P, Zores C, Langlet C, Escande B, Astruc D, Dufour A. Moderate acoustic changes can disrupt the sleep of very preterm infants in their incubators. Acta Paediatr. 2013;102:949–54.
Cremer M, Jost K, Gensmer A, Pramana I, Delgado-Eckert E, Frey U, et al. Immediate effects of phototherapy on sleep in very preterm neonates: an observational study. J Sleep Res. 2016;25:517–23.
Shimada M, Segawa M, Higurashi M, Kimura R, Oku K, Yamanami S, et al. Effects of phototherapy in neonates on circadian sleep–wake and saliva cortisol level rhythms. J Perinat Neonatal Nurs. 2003;17:222–31.
Eichenwald EC. Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea of prematurity. Pediatrics. 2016;137:e20153757. https://doi.org/10.1542/peds.2015-3757.
Ortiz LE, McGrath-Morrow SA, Sterni LM, Collaco JM. Sleep disordered breathing in bronchopulmonary dysplasia. Pediatr Pulmonol. 2017;52:1583–91.
Sekar KC, Duke JC. Sleep apnea and hypoxemia in recently weaned premature infants with and without bronchopulmonary dysplasia. Pediatr Pulmonol. 1991;10:112–6.
Collins CL, Barfield C, Davis PG, Horne RS. Randomized controlled trial to compare sleep and wake in preterm infants less than 32 weeks of gestation receiving two different modes of non-invasive respiratory support. Early Hum Dev. 2015;91:701–4.
Scher MS, Sun M, Steppe DA, Banks DL, Guthrie RD, Sclabassi RJ. Comparisons of EEG sleep state-specific spectral values between healthy full-term and preterm infants at comparable postconceptional ages. Sleep. 1994;17:47–51.
Peirano P, Curzi-Dascalova L. Modulation of motor activity patterns and sleep states in low-risk prematurely born infants reaching normal term: a comparison with full-term newborns. Neuropediatrics. 1995;26:8–13.
Horne RS, Sly DJ, Cranage SM, Chau B, Adamson TM. Effects of prematurity on arousal from sleep in the newborn infant. Pediatr Res. 2000;47:468–74.
Asaka Y, Takada S. Activity-based assessment of the sleep behaviors of VLBW preterm infants and full-term infants at around 12 months of age. Brain Dev. 2010;32:150–5.
Björkqvist J, Paavonen J, Andersson S, Pesonen AK, Lahti J, Heinonen K, et al. Advanced sleep–wake rhythm in adults born prematurely: confirmation by actigraphy-based assessment in the Helsinki Study of Very Low Birth Weight Adults. Sleep Med. 2014;15:1101–6.
Biggs SN, Meltzer LJ, Tapia IE, Traylor J, Nixon GM, Horne RS, et al. Sleep/wake patterns and parental perceptions of sleep in children born preterm. J Clin Sleep Med. 2016;12:711–7.
Yiallourou SR, Arena BC, Wallace EM, Odoi A, Hollis S, Weichard A, et al. Being born too small and too early may alter sleep in childhood. Sleep. 2017. https://doi.org/10.1093/sleep/zsx193.
Wehrle FM, Latal B, O’Gorman RL, Hagmann CF, Huber R. Sleep EEG maps the functional neuroanatomy of executive processes in adolescents born very preterm. Cortex. 2017;86:11–21.
Scher MS, Steppe DA, Banks DL. Postnatal adaptation of brain function in full-term neonates as assessed by EEG sleep analyses. Sleep. 1995;18:531–5.
Maurer N, Perkinson-Gloor N, Stalder T, Hagmann-von Arx P, Brand S, Holsboer-Trachsler E, et al. Salivary and hair glucocorticoids and sleep in very preterm children during school age. Psychoneuroendocrinology. 2016;72:166–74.
Stangenes KM, Fevang SK, Grundt J, Donkor HM, Markestad T, Hysing M, et al. Children born extremely preterm had different sleeping habits at 11 years of age and more childhood sleep problems than term-born children. Acta Paediatr. 2017;106:1966–72.
Iglowstein I, Latal Hajnal B, Molinari L, Largo RH, Jenni OG. Sleep behaviour in preterm children from birth to age 10 years: a longitudinal study. Acta Paediatr. 2006;95:1691–3.
Rosen CL, Larkin EK, Kirchner HL, Emancipator JL, Bivins SF, Surovec SA, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–9.
Manuel A, Witmans M, El-Hakim H. Children with a history of prematurity presenting with snoring and sleep-disordered breathing: a cross-sectional study. Laryngoscope. 2013;123:2030–4.
Tapia IE, Shults J, Doyle LW, Nixon GM, Cielo CM, Traylor J, et al. Perinatal risk factors associated with the obstructive sleep apnea syndrome in school-aged children born preterm. Sleep. 2016;39:737–42.
Bandyopadhyay A, Harmon H, Slaven JE, Daftary AS. Neurodevelopmental outcomes at two years of age for premature infants diagnosed with neonatal obstructive sleep apnea. J Clin Sleep Med. 2017;13:1311–7.
Geva R, Yaron H, Kuint J. Neonatal sleep predicts attention orienting and distractibility. J Atten Disord. 2016;20:138–50.
Scher MS, Steppe DA, Banks DL. Prediction of lower developmental performances of healthy neonates by neonatal EEG-sleep measures. Pediatr Neurol. 1996;14:137–44.
Peng NH, Chen LL, Li TC, Smith M, Chang YS, Huang LC. The effect of positioning on preterm infants’ sleep–wake states and stress behaviours during exposure to environmental stressors. J Child Health Care. 2014;18:314–25.
Jarus T, Bart O, Rabinovich G, Sadeh A, Bloch L, Dolfin T, et al. Effects of prone and supine positions on sleep state and stress responses in preterm infants. Infant Behav. 2011;34:257–63.
Bhat RY, Hannam S, Pressler R, Rafferty GF, Peacock JL, Greenough A. Effect of prone and supine position on sleep, apneas, and arousal in preterm infants. Pediatrics. 2006;118:101–7.
Ludington-Hoe SM, Johnson MW, Morgan K, Lewis T, Gutman J, Wilson PD, et al. Neurophysiologic assessment of neonatal sleep organization: preliminary results of a randomized, controlled trial of skin contact with preterm infants. Pediatrics. 2006;117:909–23.
Abdeyazdan Z, Mohammadian-Ghahfarokhi M, Ghazavi Z, Mohammadizadeh M. Effects of nesting and swaddling on the sleep duration of premature infants hospitalized in neonatal intensive care units. Iran J Nurs Midwifery Res. 2016;21:552–6.
Lacina L, Casper T, Dixon M, Harmeyer J, Haberman B, Alberts JR, et al. Behavioral observation differentiates the effects of an intervention to promote sleep in premature infants: a pilot study. Adv Neonatal Care. 2015;15:70–6.
Guyer C, Huber R, Fontijn J, Bucher HU, Nicolai H, Werner H, et al. Cycled light exposure reduces fussing and crying in very preterm infants. Pediatrics. 2012;130:145–51.
Khalesi N, Khosravi N, Ranjbar A, Godarzi Z, Karimi A. The effectiveness of earmuffs on the physiologic and behavioral stability in preterm infants. Int J Pediatr Otorhinolaryngol. 2017;98:43–7.
Pugliesi RR, Campillos MS, Calado Orsi KCS, Avena MJ, Pradella-Hallinan MLC, Tsunemi MH, et al. Correlation of premature infant sleep/wakefulness and noise levels in the presence or absence of “Quiet Time”. Adv Neonatal Care. 2018;18:393–9.
Liao JH, Hu RF, Su LJ, Wang S, Xu Q, Qian XF, et al. nonpharmacological interventions for sleep promotion on preterm infants in neonatal intensive care unit: a systematic review. Worldviews Evid Based Nurs. 2018;15:386–93.
de Freitas P, Bueno M, Holditch-Davis D, Santos HP, Kimura AF. Biobehavioral responses of preterm infants to conventional and swaddled tub baths: a randomized crossover trial. J Perinat Neonatal Nurs. 2018;32:358–65.
Funding
None.
Author information
Authors and Affiliations
Contributions
MG performed literature search, analyzed data, and wrote the manuscript. KH performed literature search and analyzed data. EP designed the study, analyzed data and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not required for this review article.
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gogou, M., Haidopoulou, K. & Pavlou, E. Sleep and prematurity: sleep outcomes in preterm children and influencing factors. World J Pediatr 15, 209–218 (2019). https://doi.org/10.1007/s12519-019-00240-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-019-00240-8